The Molecular Mechanisms Associated with Aerobic Exercise-Induced Cardiac Regeneration
Abstract
1. Introduction
2. Regeneration Capacity of the Heart Differs by Species and Life Stage
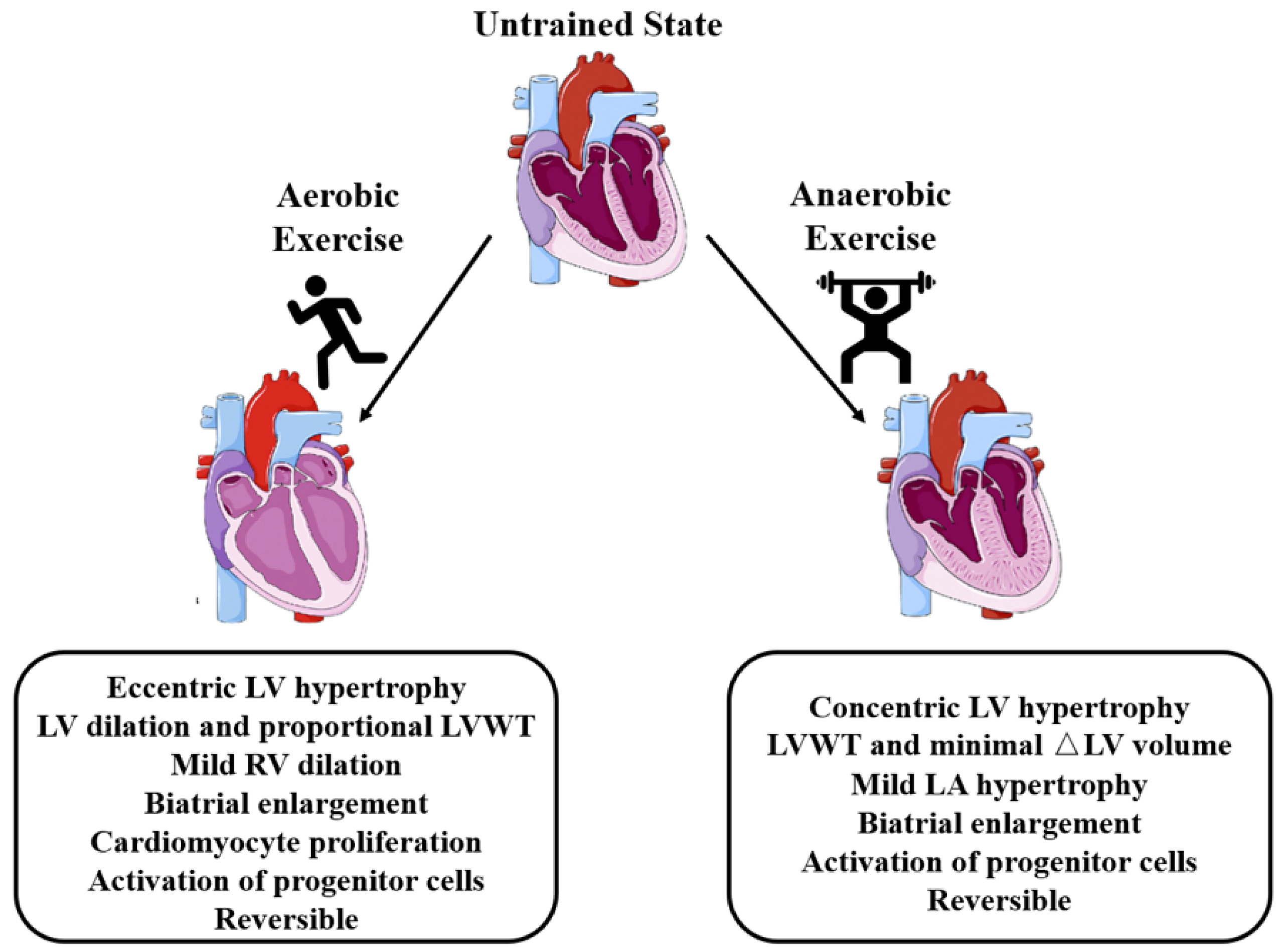
3. Effects of Aerobic Exercise on the Heart
4. Molecular Mechanisms Related to Aerobic Exercise-Induced Cardiac Regeneration
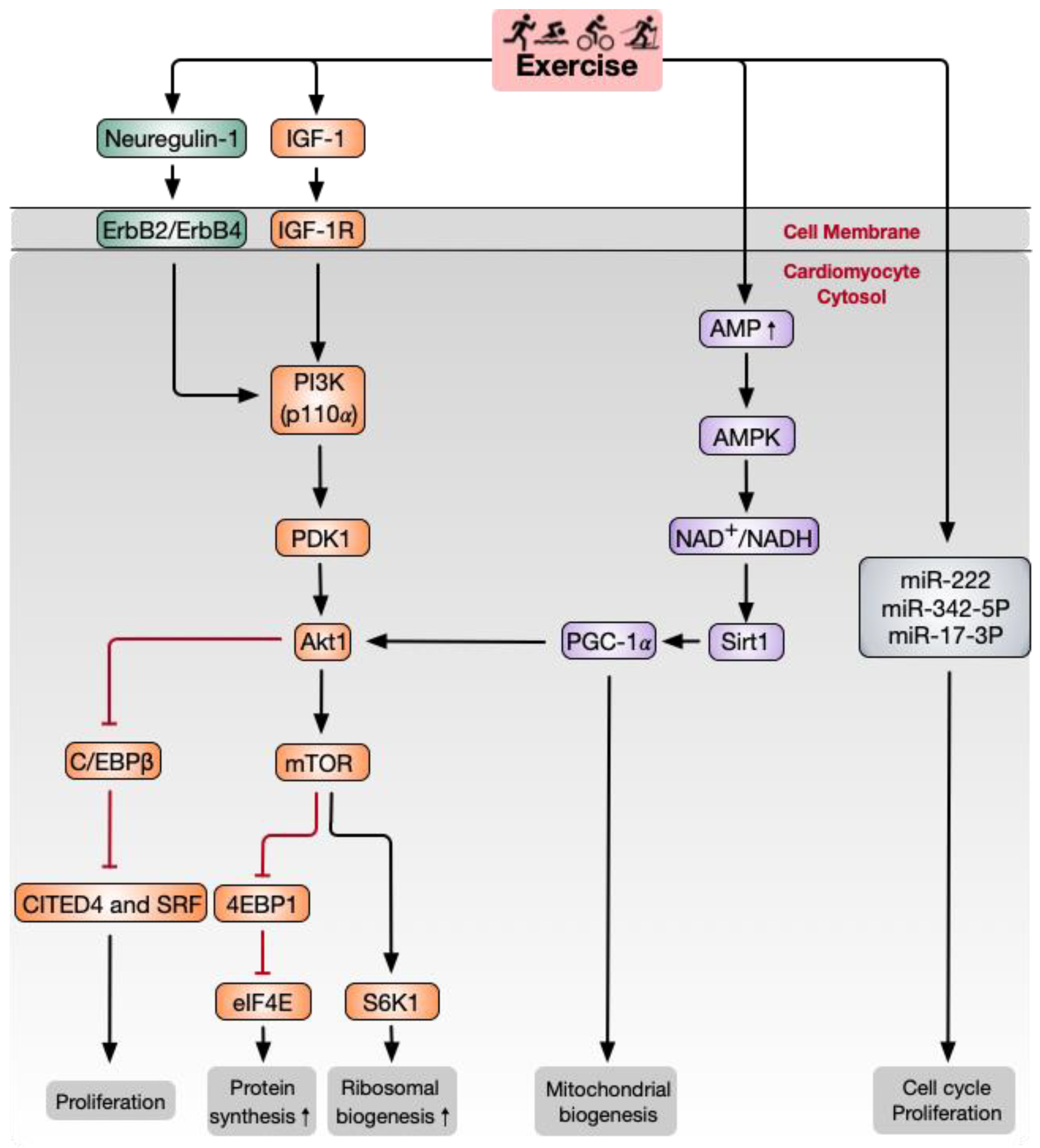
4.1. Paracrine Factors
4.2. PI3K/Akt/Mammalian Target of Rapamycin (mTOR) Signaling Pathway
4.3. NAD-Dependent Deacetylase Sirtuin1 (Sirt1)/PGC-1α/Akt Signaling Pathway
4.4. miRNAs
5. Potential for Exercise to Trigger Cardiac Regeneration in Humans
6. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Gal, T.B.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart failure in cardiomyopathies: A position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail 2019, 21, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Bohm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Askoxylakis, V.; Thieke, C.; Pleger, S.T.; Most, P.; Tanner, J.; Lindel, K.; Katus, H.A.; Debus, J.; Bischof, M. Long-term survival of cancer patients compared to heart failure and stroke: A systematic review. BMC Cancer 2010, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of exercise to improve cardiovascular health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef]
- Vagnozzi, R.J.; Molkentin, J.D.; Houser, S.R. New myocyte formation in the adult heart: Endogenous sources and therapeutic implications. Circ. Res. 2018, 123, 159–176. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Huang, X.; Bhaloo, S.I.; Zhao, H.; Zhang, S.; Pu, W.; Tian, X.; Li, Y.; Liu, Q.; et al. Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation 2018, 138, 793–805. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Choudhury, R.P.; Riley, P.R. Heart regeneration and repair after myocardial infarction: Translational opportunities for novel therapeutics. Nat. Rev. Drug Discov. 2017, 16, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Lázár, E.; Sadek, H.A.; Bergmann, O. Cardiomyocyte renewal in the human heart: Insights from the fall-out. Eur. Heart J. 2017, 38, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Verdoorn, K.S.; Matsuura, C.; Borges, J.P. Exercise for cardiac health and regeneration: Killing two birds with one stone. Ann. Transl. Med. 2017, 5 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Fiuza-Luces, C.; Lucia, A. Exercise as the master polypill of the 21st century for the prevention of cardiovascular disease. Int. J. Cardiol. 2015, 181, 360–361. [Google Scholar] [CrossRef]
- Maessen, M.F.H.; Eijsvogels, T.M.H.; Stevens, G.; van Dijk, A.P.J.; Hopman, M.T.E. Benefits of lifelong exercise training on left ventricular function after myocardial infarction. Eur. J. Prev. Cardiol. 2017, 24, 1856–1866. [Google Scholar] [CrossRef]
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the heart: The good, the bad, and the ugly. Eur. Heart J. 2015, 36, 1445–1453. [Google Scholar] [CrossRef]
- Coats, A.J.S.; Forman, D.E.; Haykowsky, M.; Kitzman, D.W.; McNeil, A.; Campbell, T.S.; Arena, R. Physical function and exercise training in older patients with heart failure. Nat. Rev. Cardiol. 2017, 14, 550–559. [Google Scholar] [CrossRef]
- Boström, P.; Mann, N.; Wu, J.; Quintero, P.A.; Plovie, E.R.; Panáková, D.; Gupta, R.K.; Xiao, C.; MacRae, C.A.; Rosenzweig, A.; et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 2010, 143, 1072–1083. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Zhu, H.; Wei, X.; Platt, C.; Damilano, F.; Xiao, C.; Bezzerides, V.; Boström, P.; Che, L.; et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015, 21, 584–595. [Google Scholar] [CrossRef]
- Guida, M.C.; Birse, R.T.; Dall’Agnese, A.; Toto, P.C.; Diop, S.B.; Mai, A.; Adams, P.D.; Puri, P.L.; Bodmer, R. Intergenerational inheritance of high fat diet-induced cardiac lipotoxicity in Drosophila. Nat. Commun. 2019, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.C.; Lam, N.T.; Savla, J.J.; Nakada, Y.; Pereira, A.H.M.; Elnwasany, A.; Menendez-Montes, I.; Ensley, E.L.; Petric, U.B.; Sharma, G.; et al. Mitochondrial Substrate Utilization Regulates Cardiomyocyte Cell Cycle Progression. Nat. Metab. 2020, 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Epstein, P.N.; Uchida, S.; Zheng, Y.; McNally, L.A.; Obal, D.; Katragadda, K.; Trainor, P.; Conklin, D.J.; Brittian, K.R.; et al. Exercise-Induced Changes in Glucose Metabolism Promote Physiological Cardiac Growth. Circulation 2017, 136, 2144–2157. [Google Scholar] [CrossRef] [PubMed]
- Hafstad, A.D.; Boardman, N.T.; Lund, J.; Hagve, M.; Khalid, A.M.; Wisloff, U.; Larsen, T.S.; Aasum, E. High intensity interval training alters substrate utilization and reduces oxygen consumption in the heart. J. Appl. Physiol. (1985) 2011, 111, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Vujic, A.; Lerchenmüller, C.; Wu, T.-D.; Guillermier, C.; Rabolli, C.P.; Gonzalez, E.; Senyo, S.E.; Liu, X.; Guerquin-Kern, J.-L.; Steinhauser, M.L.; et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat. Commun. 2018, 9, 1659. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-X.; Shi, X.-C.; Chen, T.; Tan, Z.-N.; Lin, Q.-Q.; Du, S.-J.; Tian, Z.-J. Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci. 2016, 149, 1–9. [Google Scholar] [CrossRef]
- Broughton, K.M.; Sussman, M.A. Adult cardiomyocyte cell cycle detour: Off-ramp to quiescent destinations. Trends Endocrinol. Metab. 2019, 30, 557–567. [Google Scholar] [CrossRef]
- Porrello, E.R.; Olson, E.N. A neonatal blueprint for cardiac regeneration. Stem Cell Res. 2014, 13 Pt B, 556–570. [Google Scholar] [CrossRef]
- Poss, K.D. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.-I.; Penninger, J.M. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef] [PubMed]
- van Berlo, J.H.; Molkentin, J.D. An emerging consensus on cardiac regeneration. Nat. Med. 2014, 20, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte regeneration: A consensus statement. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef]
- He, L.; Li, Y.; Li, Y.; Pu, W.; Huang, X.; Tian, X.; Wang, Y.; Zhang, H.; Liu, Q.; Zhang, L.; et al. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat. Med. 2017, 23, 1488–1498. [Google Scholar] [CrossRef]
- Wang, W.E.; Li, L.; Xia, X.; Fu, W.; Liao, Q.; Lan, C.; Yang, D.; Chen, H.; Yue, R.; Zeng, C.; et al. Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation 2017, 136, 834–848. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.-C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef]
- Levine, B.D.; Baggish, A.L.; Kovacs, R.J.; Link, M.S.; Maron, M.S.; Mitchell, J.H. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 1: Classification of sports: Dynamic, static, and impact: A scientific statement from the american heart association and american college of cardiology. Circulation 2015, 132, e262–e266. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task Force 8: Classification of sports. J. Am. Coll. Cardiol. 2005, 45, 1364–1367. [Google Scholar] [CrossRef]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef]
- Poole, D.C.; Hirai, D.M.; Copp, S.W.; Musch, T.I. Muscle oxygen transport and utilization in heart failure: Implications for exercise (in)tolerance. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1050–H1063. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Alkhawam, H.; Madanieh, R.; Shah, N.; Kosmas, C.E.; Vittorio, T.J. Aerobic vs. anaerobic exercise training effects on the cardiovascular system. World J. Cardiol. 2017, 9, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Atalay, M.; Laaksonen, D.E. Diabetes, oxidative stress and physical exercise. J. Sports Sci. Med. 2002, 1, 1–14. [Google Scholar] [PubMed]
- Acierno, L.J. Adolph Fick: Mathematician, physicist, physiologist. Clin. Cardiol. 2000, 23, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Adolph fick (1829–1901) mathematician, physicist, physiologist. JAMA J. Am. Med. Assoc. 1967, 202, 1100–1101. [CrossRef]
- Palabiyik, O.; Tastekin, E.; Doganlar, Z.B.; Tayfur, P.; Dogan, A.; Vardar, S.A. Alteration in cardiac PI3K/Akt/mTOR and ERK signaling pathways with the use of growth hormone and swimming, and the roles of miR21 and miR133. Biomed. Rep. 2019, 10, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Ooi, J.Y.Y.; Weeks, K.L.; Patterson, N.L.; McMullen, J.R. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: Current knowledge and emerging concepts. Physiol. Rev. 2018, 98, 419–475. [Google Scholar] [CrossRef]
- Strain, T.; Wijndaele, K.; Dempsey, P.C.; Sharp, S.J.; Pearce, M.; Jeon, J.; Lindsay, T.; Wareham, N.; Brage, S. Wearable-device-measured physical activity and future health risk. Nat. Med. 2020, 26, 1385–1391. [Google Scholar] [CrossRef]
- Merghani, A.; Malhotra, A.; Sharma, S. The U-shaped relationship between exercise and cardiac morbidity. Trends Cardiovasc. Med. 2016, 26, 232–240. [Google Scholar] [CrossRef]
- Villelabeitia-Jaureguizar, K.; Vicente-Campos, D.; Senen, A.B.; Jiménez, V.H.; Bautista, L.R.; Garrido-Lestache, M.E.B.; Chicharro, J.L. Mechanical efficiency of high versus moderate intensity aerobic exercise in coronary heart disease patients: A randomized clinical trial. Cardiol. J. 2019, 26, 130–137. [Google Scholar] [CrossRef]
- Wewege, M.A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-intensity interval training for patients with cardiovascular disease-is it safe? A systematic review. J. Am. Heart Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef] [PubMed]
- Waring, C.D.; Vicinanza, C.; Papalamprou, A.; Smith, A.J.; Purushothaman, S.; Goldspink, D.F.; Nadal-Ginard, B.; Torella, D.; Ellison, G.M. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur. Heart J. 2014, 35, 2722–2731. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.F. Rodent models for human diseases. Eur. J. Pharmacol. 2015, 759, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Rhee, J.; Chaudhari, V.; Rosenzweig, A. The role of exercise in cardiac aging: From physiology to molecular mechanisms. Circ. Res. 2016, 118, 279–295. [Google Scholar] [CrossRef]
- Riehle, C.; Bauersachs, J. Small animal models of heart failure. Cardiovasc. Res. 2019, 115, 1838–1849. [Google Scholar] [CrossRef]
- Bernardo, B.C.; McMullen, J.R. Molecular aspects of exercise-induced cardiac remodeling. Cardiol. Clin. 2016, 34, 515–530. [Google Scholar] [CrossRef]
- Kemi, O.J.; Loennechen, J.P.; Wisløff, U.; Ellingsen, Ø. Intensity-controlled treadmill running in mice: Cardiac and skeletal muscle hypertrophy. J. Appl. Physiol. 2002, 93, 1301–1309. [Google Scholar] [CrossRef]
- Yeh, J.K.; Aloia, J.F.; Chen, M.; Ling, N.; Koo, H.C.; Millard, W.J. Effect of growth hormone administration and treadmill exercise on serum and skeletal IGF-I in rats. Am. J. Physiol. Endocrinol. Metab. 1994, 266, E129–E135. [Google Scholar] [CrossRef]
- Koziris, L.P.; Hickson, R.C.; Chatterton, R.T.; Groseth, R.T.; Christie, J.M.; Goldflies, D.G.; Unterman, T.G. Serum levels of total and free IGF-I and IGFBP-3 are increased and maintained in long-term training. J. Appl. Physiol. 1999, 86, 1436–1442. [Google Scholar] [CrossRef]
- Neri Serneri, G.G.; Boddi, M.; Modesti, P.A.; Cecioni, I.; Coppo, M.; Padeletti, L.; Michelucci, A.; Colella, A.; Galanti, G. Increased cardiac sympathetic activity and insulin-like growth factor-i formation are associated with physiological hypertrophy in athletes. Circ. Res. 2001, 89, 977–982. [Google Scholar] [CrossRef]
- Ikeda, H.; Shiojima, I.; Ozasa, Y.; Yoshida, M.; Holzenberger, M.; Kahn, C.R.; Walsh, K.; Igarashi, T.; Abel, E.D.; Komuro, I. Interaction of myocardial insulin receptor and IGF receptor signaling in exercise-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2009, 47, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Qi, X.; McAnally, J.; Schwartz, R.J.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 2011, 4, ra70. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wende, A.R.; Sena, S.; Theobald, H.A.; Soto, J.; Sloan, C.; Wayment, B.E.; Litwin, S.E.; Holzenberger, M.; LeRoith, D.; et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol. Endocrinol. 2008, 22, 2531–2543. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.R.; Shioi, T.; Huang, W.-Y.; Zhang, L.; Tarnavski, O.; Bisping, E.; Schinke, M.; Kong, S.; Sherwood, M.C.; Brown, J.; et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110alpha) pathway. J. Biol. Chem. 2004, 279, 4782–4793. [Google Scholar] [CrossRef]
- Boudina, S.; Bugger, H.; Sena, S.; O’Neill, B.T.; Zaha, V.G.; Ilkun, O.; Wright, J.J.; Mazumder, P.K.; Palfreyman, E.; Tidwell, T.J.; et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 2009, 119, 1272–1283. [Google Scholar] [CrossRef]
- Riehle, C.; Wende, A.R.; Zhu, Y.; Oliveira, K.J.; Pereira, R.O.; Jaishy, B.P.; Bevins, J.; Valdez, S.; Noh, J.; Kim, B.J.; et al. Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol. Cell. Biol. 2014, 34, 3450–3460. [Google Scholar] [CrossRef]
- McMullen, J.R.; Shioi, T.; Zhang, L.; Tarnavski, O.; Sherwood, M.C.; Kang, P.M.; Izumo, S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2003, 100, 12355–12360. [Google Scholar] [CrossRef]
- D’Uva, G.; Aharonov, A.; Lauriola, M.; Kain, D.; Yahalom-Ronen, Y.; Carvalho, S.; Weisinger, K.; Bassat, E.; Rajchman, D.; Yifa, O.; et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 2015, 17, 627–638. [Google Scholar] [CrossRef]
- Bersell, K.; Arab, S.; Haring, B.; Kühn, B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 2009, 138, 257–270. [Google Scholar] [CrossRef]
- Sebastian, T.; Johnson, P.F. Stop and go: Anti-proliferative and mitogenic functions of the transcription factor C/EBPβ. Cell Cycle 2006, 5, 953–957. [Google Scholar] [CrossRef]
- DeBosch, B.; Treskov, I.; Lupu, T.S.; Weinheimer, C.; Kovacs, A.; Courtois, M.; Muslin, A.J. Akt1 is required for physiological cardiac growth. Circulation 2006, 113, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Qi, J.; Meng, S.; Wen, B.; Zhang, J. Swimming exercise training-induced left ventricular hypertrophy involves microRNAs and synergistic regulation of the PI3K/AKT/mTOR signaling pathway. Eur. J. Appl. Physiol. 2013, 113, 2473–2486. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.; Frederico, M.J.; da Luz, G.; Pauli, J.R.; Silva, A.S.R.; Pinho, R.A.; Velloso, L.A.; Ropelle, E.R.; De Souza, C.T. Exercise training reduces insulin resistance and upregulates the mTOR/p70S6k pathway in cardiac muscle of diet-induced obesity rats. J. Cell. Physiol. 2011, 226, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Shioi, T.; Kang, P.M.; Douglas, P.S.; Hampe, J.; Yballe, C.M.; Lawitts, J.; Cantley, L.C.; Izumo, S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO 2000, 19, 2537–2548. [Google Scholar] [CrossRef]
- Shiojima, I.; Walsh, K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006, 20, 3347–3365. [Google Scholar] [CrossRef]
- Jia, S.; Liu, Z.; Zhang, S.; Liu, P.; Zhang, L.; Lee, S.H.; Zhang, J.; Signoretti, S.; Loda, M.; Roberts, T.M.; et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 2008, 454, 776–779. [Google Scholar] [CrossRef]
- Parekh, P.; Motiwale, L.; Naik, N.; Rao, K.V.K. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cells. Exp. Toxicol. Pathol. 2011, 63, 167–173. [Google Scholar] [CrossRef]
- Shiojima, I.; Sato, K.; Izumiya, Y.; Schiekofer, S.; Ito, M.; Liao, R.; Colucci, W.S.; Walsh, K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Investig. 2005, 115, 2108–2118. [Google Scholar] [CrossRef]
- Coronado, M.; Fajardo, G.; Nguyen, K.; Zhao, M.; Kooiker, K.; Jung, G.; Hu, D.-Q.; Reddy, S.; Sandoval, E.; Stotland, A.; et al. Physiological mitochondrial fragmentation is a normal cardiac adaptation to increased energy demand. Circ. Res. 2018, 122, 282–295. [Google Scholar] [CrossRef]
- Ruan, Y.; Dong, C.; Patel, J.; Duan, C.; Wang, X.; Wu, X.; Cao, Y.; Pu, L.; Lu, D.; Shen, T.; et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell. Physiol. Biochem. 2015, 35, 1116–1124. [Google Scholar] [CrossRef]
- Bayod, S.; del Valle, J.; Lalanza, J.F.; Sanchez-Roige, S.; de Luxán-Delgado, B.; Coto-Montes, A.; Canudas, A.M.; Camins, A.; Escorihuela, R.M.; Pallàs, M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp. Gerontol. 2012, 47, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Wende, A.R.; Schaeffer, P.J.; Parker, G.J.; Zechner, C.; Han, D.-H.; Chen, M.M.; Hancock, C.R.; Lehman, J.J.; Huss, J.M.; McClain, D.A.; et al. A role for the transcriptional coactivator PGC-1α in muscle refueling. J. Biol. Chem. 2007, 282, 36642–36651. [Google Scholar] [CrossRef] [PubMed]
- Zechner, C.; Lai, L.; Zechner, J.F.; Geng, T.; Yan, Z.; Rumsey, J.W.; Collia, D.; Chen, Z.; Wozniak, D.F.; Leone, T.C.; et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010, 12, 633–642. [Google Scholar] [CrossRef]
- Pilegaard, H.; Saltin, B.; Neufer, P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J. Physiol. 2003, 546, Pt 3, 851–858. [Google Scholar] [CrossRef]
- O’Neill, B.T.; Kim, J.; Wende, A.R.; Theobald, H.A.; Tuinei, J.; Buchanan, J.; Guo, A.; Zaha, V.G.; Davis, D.K.; Schell, J.C.; et al. A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab. 2007, 6, 294–306. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhu, S.; Wu, J.; Zhang, M.; Xu, Y.; Xu, W.; Cui, J.; Yu, B.; Cao, W.; Liu, J. Exercise enhances cardiac function by improving mitochondrial dysfunction and maintaining energy homoeostasis in the development of diabetic cardiomyopathy. J. Mol. Med. 2020, 98, 245–261. [Google Scholar] [CrossRef]
- Vega, R.B.; Horton, J.L.; Kelly, D.P. Maintaining ancient organelles: Mitochondrial biogenesis and maturation. Circ. Res. 2015, 116, 1820–1834. [Google Scholar] [CrossRef]
- Finck, B.N.; Lehman, J.J.; Leone, T.C.; Welch, M.J.; Bennett, M.J.; Kovacs, A.; Han, X.; Gross, R.W.; Kozak, R.; Lopaschuk, G.D.; et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J. Clin. Investig. 2002, 109, 121–130. [Google Scholar] [CrossRef]
- Gilde, A.J.; van der Lee, K.A.J.M.; Willemsen, P.H.M.; Chinetti, G.; van der Leij, F.R.; van der Vusse, G.J.; Staels, B.; van Bilsen, M. Peroxisome proliferator-activated receptor (PPAR) α and PPARβ/δ, but not PPARγ, modulate the expression of genes involved in cardiac lipid metabolism. Circ. Res. 2003, 92, 518–524. [Google Scholar] [CrossRef]
- Bellafiore, M.; Battaglia, G.; Bianco, A.; Palma, A. Expression pattern of angiogenic factors in healthy heart in response to physical exercise intensity. Front. Physiol. 2019, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mühlfeld, C.; Niemann, B.; Pan, R.; Li, R.; Hilfiker-Kleiner, D.; Chen, Y.; Rohrbach, S. Mitochondrial biogenesis and PGC-1α deacetylation by chronic treadmill exercise: Differential response in cardiac and skeletal muscle. Basic Res. Cardiol. 2011, 106, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Riehle, C.; Abel, E.D. PGC-1 proteins and heart failure. Trends Cardiovasc. Med. 2012, 22, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J. Cell. Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef]
- Ghiasi, R.; Mohammadi, M.; Ashrafi Helan, J.; Jafari Jozani, S.R.; Mohammadi, S.; Ghiasi, A.; Naderi, R. Influence of two various durations of resistance exercise on oxidative stress in the male rat’s hearts. J. Cardiovasc. Thorac. Res. 2015, 7, 149–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, J.; Bei, Y.; Kong, X.; Liu, X.; Lei, Z.; Xu, T.; Wang, H.; Xuan, Q.; Chen, P.; Xu, J.; et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics 2017, 7, 664–676. [Google Scholar] [CrossRef]
- Fernandes, T.; Baraúna, V.G.; Negrão, C.E.; Phillips, M.I.; Oliveira, E.M. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H543–H552. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, X.; Hu, Y.; Zhang, X.; Li, G.; Wu, J.; Li, J.; Sha, J.; Chen, J.; Xia, J.; et al. Longterm exercise-derived exosomal miR-342-5p. Circ. Res. 2019, 124, 1386–1400. [Google Scholar] [CrossRef]
- Gao, F.; Kataoka, M.; Liu, N.; Liang, T.; Huang, Z.-P.; Gu, F.; Ding, J.; Liu, J.; Zhang, F.; Ma, Q.; et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat. Commun. 2019, 10, 1802. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Ferro, M.D.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ho, B.X.; Pang, J.K.S.; Pek, N.M.Q.; Hor, J.H.; Ng, S.-Y.; Soh, B.-S. Wnt/β-catenin-mediated signaling re-activates proliferation of matured cardiomyocytes. Stem Cell Res. Ther. 2018, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- MacGrogan, D.; Münch, J.; de la Pompa, J.L. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat. Rev. Cardiol. 2018, 15, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Viana, V.A.R.; Grassmann, V.; Santos, R.T.; Santos, R.F.; Tufik, S.R.; Mello, M.T. The impact of resistance exercise on the cognitive function of the elderly. Med. Sci. Sports Exerc. 2007, 39, 1401–1407. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Wang, C.-H.; Pan, C.-Y.; Chen, F.-C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and, G.H.; IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Patil, H.R.; Lavie, C.J.; Magalski, A.; Vogel, R.A.; McCullough, P.A. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin. Proc. 2012, 87, 587–595. [Google Scholar] [CrossRef]
- Fukazawa, R.; Miller, T.A.; Kuramochi, Y.; Frantz, S.; Kim, Y.D.; Marchionni, M.A.; Kelly, R.A.; Sawyer, D.B. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J. Mol. Cell. Cardiol. 2003, 35, 1473–1479. [Google Scholar] [CrossRef]
- Liao, P.H.; Hsieh, D.J.; Kuo, C.H.; Day, C.H.; Shen, C.Y.; Lai, C.H.; Chen, R.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. Moderate exercise training attenuates aging-induced cardiac inflammation, hypertrophy and fibrosis injuries of rat hearts. Oncotarget 2015, 6, 35383–35394. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, C.C.; Ting, W.J.; Pai, P.Y.; Kuo, C.H.; Ho, T.J.; Kuo, W.W.; Chang, C.H.; Huang, C.Y.; Lin, W.T. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age 2014, 36, 9705. [Google Scholar] [CrossRef]
| Classification | Factor | Aerobic Exercise Model | Species | Duration | Regenerative Label | Observation | Reference No. |
|---|---|---|---|---|---|---|---|
| Paracrine factor | IGF-1 | Swimming exercise: ramp protocol started from 10 min to 90 min, with 20 min increased each day, twice/day | Mice | 4 weeks 7 days/week | None | IGF-1R- and IR-mediated signals in the development of exercise-induced physiological cardiac hypertrophy | [61] |
| IGF-1R | Swimming exercise: ramp protocol started from 10 min to 90 min, with 10 min increased each day, twice/day | Mice | 5 weeks 7 days/week | None | Cardiac hypertrophy growth induced by exercise blunted in IGF-1R KO mice | [63] | |
| IGF-1, Neuregulin 1 | Running exercise: low intensity (55–60% of individual VO2max) and high intensity (85–90% of | Rat | 4 weeks 4 days/week | BrdU, Ki67 | IGF-1, Neuregulin 1, TGF-β1 ↑ Newly formed cardiomyocytes ↑ | [52] | |
| Neuregulin 1/ErbB/PI3K | Running exercise: ramp protocol started from 10 m/min for 10 min, progressively increased to 16 m/min, 50 min/day | Rat | 4 weeks 5 days/week | BrdU, PCNA | Neuregulin 1 expression ↑ Activity of ErbB2, ErbB4, and PI3K/Akt pathway ↑ | [26] | |
| CITED4, C/EBPβ | Swimming exercise: ramp protocol started from 10 min to 90 min, with 10 min increased each day, twice/day | Mice | 2 weeks 7 days/week | BrdU, Ki67, AuroraB, pH3 | C/EBPβ expression ↓ CITED4 expression ↑ | [19] | |
| Signaling pathway | PI3K/Akt/mTOR | Swimming exercise: ramp protocol followed by 60 min sessions with 5% body overload, twice/day | Rat (Female) | 8 weeks 5 times/week | None | PIK3/Akt/mTOR pathway gene expression ↑ PTEN gene expression ↓ | [72] |
| Akt-1 | Swimming exercise: each session lasted 90 min, twice/day | Mice | 4 weeks 5 days/week | None | Exercise-induced hypertrophy attenuated in Akt1-/- mice | [71] | |
| mTOR/p70S6K | Swimming exercise: each session lasted 60 min, with 5% body overload, twice/day | Rat (obese) | 12 weeks 5 days/week | None | Activity of mTOR/p70S6k pathway ↑ | [73] | |
| Sirt1/PGC-1α | Running exercise: endurance training ramp protocol increased from 4.2 m/min up to 12 m/min for 30 min/day | Rat | 36 weeks 4–5 days/week | None | Sirt1 and PGC-1α protein expression ↑ | [81] | |
| Sirt1/PGC-1α/Akt | Running exercise: ramp protocol started from 10 m/min for 30 min/day, progressively increased to 16 m/min, 60 min/day | Rat | 4 weeks 7 days/week | None | Sirt1/PGC-1α/PI3K/Akt pathway ↑ | [94] | |
| microRNA | miR-222 | Running exercise: voluntary wheel running [20,25] Swimming exercise: ramp protocol followed by 90 min, twice/day [20] | Mice | 4 weeks 7 days/week [20] 8 weeks 5 days/week [25] | EdU, Ki67, pH3 [20] 15N-thymidinel [25] | miR-222 expression ↑ Inhibition of miR-222 blocks the cardiomyogenic exercise response | [20,25] |
| miR-17-3p | Swimming exercise: ramp protocol followed by 90 min, twice/day for 3 weeks Running exercise: voluntary wheel running for 3 weeks | Mice | 3 weeks 7 days/week | EdU, Ki67, pH3 | Inhibition of miR-17-3p attenuates exercise-induced cardiac growth in vivo; Mice injected with miR-17-3p agonist are protected from adverse remodeling after cardiac ischemia/reperfusion injury | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, B.; Zhou, Y.; Zheng, Q.; Wang, G.; Zhou, K.; Wei, J. The Molecular Mechanisms Associated with Aerobic Exercise-Induced Cardiac Regeneration. Biomolecules 2021, 11, 19. https://doi.org/10.3390/biom11010019
Bo B, Zhou Y, Zheng Q, Wang G, Zhou K, Wei J. The Molecular Mechanisms Associated with Aerobic Exercise-Induced Cardiac Regeneration. Biomolecules. 2021; 11(1):19. https://doi.org/10.3390/biom11010019
Chicago/Turabian StyleBo, Bing, Yang Zhou, Qingyun Zheng, Guandong Wang, Ke Zhou, and Jianshe Wei. 2021. "The Molecular Mechanisms Associated with Aerobic Exercise-Induced Cardiac Regeneration" Biomolecules 11, no. 1: 19. https://doi.org/10.3390/biom11010019
APA StyleBo, B., Zhou, Y., Zheng, Q., Wang, G., Zhou, K., & Wei, J. (2021). The Molecular Mechanisms Associated with Aerobic Exercise-Induced Cardiac Regeneration. Biomolecules, 11(1), 19. https://doi.org/10.3390/biom11010019





