Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Bacterial Strains
2.3. Eukaryotic Cell Culture Preparation
2.4. Infection of MG-63 Cells
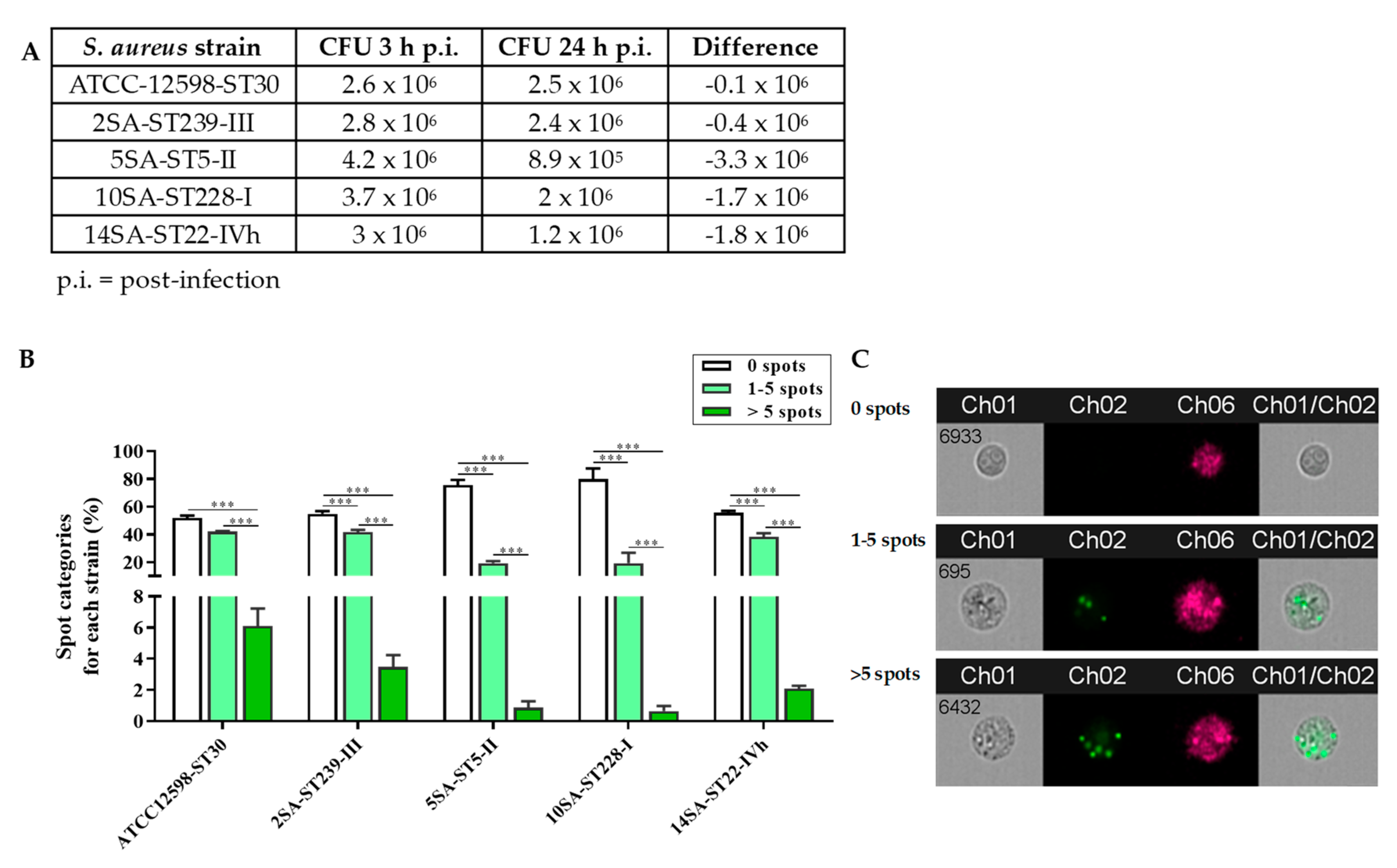
2.5. Evaluation of the Frequency of Internalization and Intracellular Persistence by Colony-Forming Units (CFUs) and Spot Counting
2.6. Evaluation of Cell Viability by MTT Assay
2.7. Gene Expression Analysis by Quantitative Real-Time PCR (qRT-PCR)
2.8. Cytokine Secretion Analysis by Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
3.1. The Intracellular Persistence and Number of Bacteria in MG-63 Osteoblast-Like Cells Vary Significantly among the Different Strains
3.2. Infection with ST30, ST239, ST5, ST228, or ST22 Strains Differently Affected MG-63 Osteoblast-Like Cell Viability
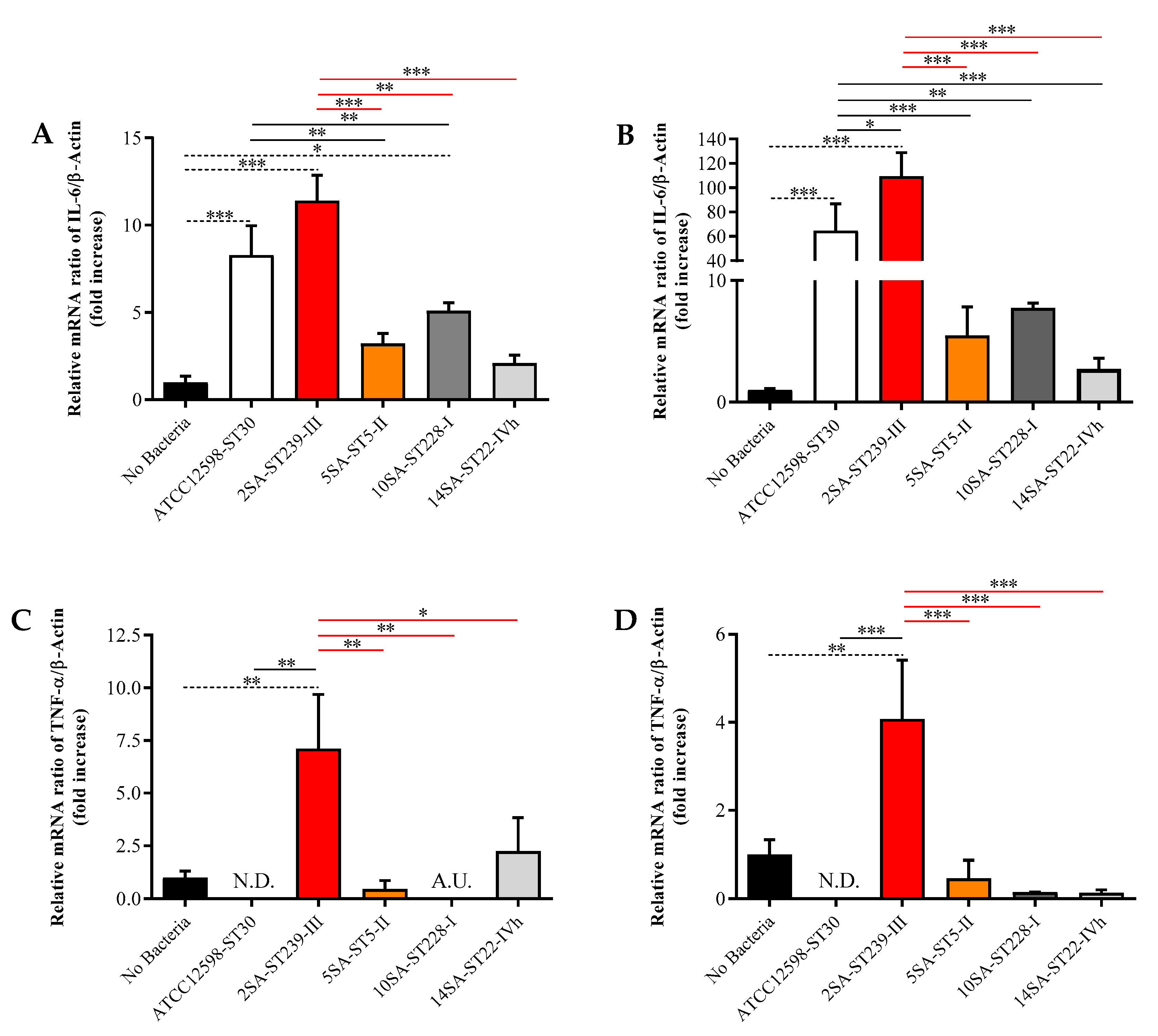
3.3. The Longer Infection Time with ST239 Strain Leads to the Up-Regulation of IL-6, TNF-α, TGF-β1, and GAPDH in MG-63 Osteoblast-Like Cells
3.4. The ST239 Strain Is the Least Effective in Modulating Nrf2 and HO-1 Gene Expression in MG-63 Osteoblast-Like Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular pathogens: Host immunity and microbial persistence strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef]
- Mitchell, G.; Isberg, R.R. Innate immunity to intracellular pathogens: Balancing microbial elimination and inflammation. Cell Host Microbe 2017, 22, 166–175. [Google Scholar] [CrossRef]
- Rasigade, J.P.; Vandenesch, F. Staphylococcus aureus: A pathogen with still unresolved issues. Infect. Genet. Evol. 2014, 21, 510–514. [Google Scholar] [CrossRef]
- Moldovan, A.; Fraunholz, M.J. In or out: Phagosomal escape of Staphylococcus aureus. Cell. Microbiol. 2019, 21, e12997. [Google Scholar] [CrossRef] [Green Version]
- Alva-Murillo, N.; Lopez-Meza, J.E.; Ochoa-Zarzosa, A. Nonprofessional phagocytic cell receptors involved in Staphylococcus aureus internalization. BioMed Res. Int. 2014, 2014, 538546. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Zhang, X. Interaction of Staphylococcus aureus with osteoblasts (review). Exp. Ther. Med. 2012, 3, 367–370. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Loffler, B. Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection. Curr. Genet. 2016, 62, 15–17. [Google Scholar] [CrossRef]
- Gould, I.M.; Cauda, R.; Esposito, S.; Gudiol, F.; Mazzei, T.; Garau, J. Management of serious meticillin-resistant Staphylococcus aureus infections: What are the limits? Int. J. Antimicrob. Agents 2011, 37, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Chowdhury, R.; Datta, M.; Chowdhury, G.; Mukhopadhyay, A.K. Characterization of the clonal profile of methicillin resistant Staphylococcus aureus isolated from patients with early post-operative orthopedic implant based infections. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 8. [Google Scholar] [CrossRef]
- Campanile, F.; Bongiorno, D.; Perez, M.; Mongelli, G.; Sessa, L.; Benvenuto, S.; Gona, F.; Varaldo, P.E.; Stefani, S. Epidemiology of Staphylococcus aureus in italy: First nationwide survey, 2012. J. Glob. Antimicrob. Resist. 2015, 3, 247–254. [Google Scholar] [CrossRef]
- Chamon, R.C.; Ribeiro, S.D.; da Costa, T.M.; Nouér, S.A.; Dos Santos, K.R. Complete substitution of the brazilian endemic clone by other methicillin-resistant Staphylococcus aureus lineages in two public hospitals in rio de janeiro, brazil. Braz. J. Infect. Dis. 2017, 21, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; Mackenzie, F.M. Meticillin-resistant Staphylococcus aureus (mrsa): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Campanile, F.; Bongiorno, D.; Falcone, M.; Vailati, F.; Pasticci, M.B.; Perez, M.; Raglio, A.; Rumpianesi, F.; Scuderi, C.; Suter, F.; et al. Changing italian nosocomial-community trends and heteroresistance in Staphylococcus aureus from bacteremia and endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 739–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldan, R.; Testa, F.; Lorè, N.I.; Bragonzi, A.; Cichero, P.; Ossi, C.; Biancardi, A.; Nizzero, P.; Moro, M.; Cirillo, D.M. Factors contributing to epidemic mrsa clones replacement in a hospital setting. PLoS ONE 2012, 7, e43153. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Mora, C. Diabetes, inflammation, proinflammatory cytokines, and diabetic nephropathy. ScientificWorldJournal 2006, 6, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [Green Version]
- Lopalco, G.; Lucherini, O.M.; Vitale, A.; Talarico, R.; Lopalco, A.; Galeazzi, M.; Lapadula, G.; Cantarini, L.; Iannone, F. Putative role of serum amyloid-a and proinflammatory cytokines as biomarkers for behcet’s disease. Medicine 2015, 94, e1858. [Google Scholar] [CrossRef]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory process in parkinson’s disease. J. Neural Transm. Suppl. 2006, 373–381. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.F.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine prevents abeta-induced oxidative stress and inflammation in microglial cells: A key role of tgf-beta1. Cells 2019, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Imanishi, J. Expression of cytokines in bacterial and viral infections and their biochemical aspects. J. Biochem. 2000, 127, 525–530. [Google Scholar] [CrossRef]
- Holub, M.; Lawrence, D.A.; Andersen, N.; Davidova, A.; Beran, O.; Maresova, V.; Chalupa, P. Cytokines and chemokines as biomarkers of community-acquired bacterial infection. Mediat. Inflamm. 2013, 2013, 190145. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, L.; Cooper, A.; Fantino, C.; Altmann, D.M.; Sriskandan, S. The mechanism of superantigen-mediated toxic shock: Not a simple th1 cytokine storm. J. Immunol. 2005, 175, 6870–6877. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Levast, B.; Madrenas, J. Staphylococcus aureus downregulates ip-10 production and prevents th1 cell recruitment. J. Immunol. 2017, 198, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Spampinato, S.F.; Cardaci, V.; Caraci, F.; Sortino, M.A.; Merlo, S. Beta-amyloid and oxidative stress: Perspectives in drug development. Curr. Pharm. Des. 2019, 25, 4771–4781. [Google Scholar] [CrossRef]
- Caruso, G.; Benatti, C.; Blom, J.M.C.; Caraci, F.; Tascedda, F. The many faces of mitochondrial dysfunction in depression: From pathology to treatment. Front. Pharmacol. 2019, 10, 995. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Dill, C.; Bonay, M. Regulation of oxidative stress by nrf2 in the pathophysiology of infectious diseases. Médecine et Mal. Infect. 2013, 43, 100–107. [Google Scholar] [CrossRef]
- Horn, J.; Stelzner, K.; Rudel, T.; Fraunholz, M. Inside job: Staphylococcus aureus host-pathogen interactions. Int. J. Med. Microbiol. 2018, 308, 607–624. [Google Scholar] [CrossRef]
- Josse, J.; Velard, F.; Gangloff, S.C. Staphylococcus aureus vs. Osteoblast: Relationship and consequences in osteomyelitis. Front. Cell Infect. Microbiol 2015, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- Lüthje, F.L.; Jensen, L.K.; Jensen, H.E.; Skovgaard, K. The inflammatory response to bone infection-a review based on animal models and human patients. Apmis 2020, 128, 275–286. [Google Scholar] [CrossRef]
- Delpino, M.V.; Fossati, C.A.; Baldi, P.C. Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with brucella spp. Infect. Immun. 2009, 77, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Bachtiar, B.M.; Bachtiar, E.W. Proinflammatory mg-63 cells response infection with enterococcus faecalis cps2 evaluated by the expression of tlr-2, il-1beta, and inos mrna. BMC Res. Notes 2017, 10, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selan, L.; Papa, R.; Ermocida, A.; Cellini, A.; Ettorre, E.; Vrenna, G.; Campoccia, D.; Montanaro, L.; Arciola, C.R.; Artini, M. Serratiopeptidase reduces the invasion of osteoblasts by Staphylococcus aureus. Int. J. Immunopathol. Pharmacol. 2017, 30, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Clover, J.; Gowen, M. Are mg-63 and hos te85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Young, J. Regulation of alkaline phosphatase by 1,25-dihydroxyvitamin d3 and ascorbic acid in bone-derived cells. J. Bone Miner. Res. 1990, 5, 1157–1167. [Google Scholar] [CrossRef]
- Lajeunesse, D.; Frondoza, C.; Schoffield, B.; Sacktor, B. Osteocalcin secretion by the human osteosarcoma cell line mg-63. J. Bone Miner. Res. 1990, 5, 915–922. [Google Scholar] [CrossRef]
- Bongiorno, D.; Mongelli, G.; Stefani, S.; Campanile, F. Burden of rifampicin- and methicillin-resistant Staphylococcus aureus in italy. Microb Drug Resist. 2018, 24, 732–738. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of pro-oxidant and pro-inflammatory activities of m1 macrophages by the natural dipeptide carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef] [Green Version]
- McPherson, J.C., 3rd; Runner, R.R.; Shapiro, B.; Walsh, D.S.; Stephens-DeValle, J.; Buxton, T.B. An acute osteomyelitis model in traumatized rat tibiae involving sand as a foreign body, thermal injury, and bimicrobial contamination. Comp. Med. 2008, 58, 369–374. [Google Scholar]
- Campoccia, D.; Montanaro, L.; Ravaioli, S.; Cangini, I.; Testoni, F.; Visai, L.; Arciola, C.R. New parameters to quantitatively express the invasiveness of bacterial strains from implant-related orthopaedic infections into osteoblast cells. Materials 2018, 11, 550. [Google Scholar] [CrossRef] [Green Version]
- Bongiorno, D.; Musso, N. Detection of methicillin-resistant Staphylococcus aureus persistence in osteoblasts using imaging flow cytometry. MicrobiologyOpen 2020, 9, e1017. [Google Scholar] [CrossRef] [Green Version]
- Valour, F.; Trouillet-Assant, S.; Riffard, N.; Tasse, J.; Flammier, S.; Rasigade, J.P.; Chidiac, C.; Vandenesch, F.; Ferry, T.; Laurent, F. Antimicrobial activity against intraosteoblastic Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 2029–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valour, F.; Rasigade, J.P.; Trouillet-Assant, S.; Gagnaire, J.; Bouaziz, A.; Karsenty, J.; Lacour, C.; Bes, M.; Lustig, S.; Bénet, T.; et al. Delta-toxin production deficiency in Staphylococcus aureus: A diagnostic marker of bone and joint infection chronicity linked with osteoblast invasion and biofilm formation. Clin. Microbiol. Infect. 2015, 21, 568.e1–568.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupieux, C.; Trouillet-Assant, S.; Camus, C.; Abad, L.; Bes, M.; Benito, Y.; Chidiac, C.; Lustig, S.; Ferry, T.; Valour, F.; et al. Intraosteoblastic activity of daptomycin in combination with oxacillin and ceftaroline against mssa and mrsa. J. Antimicrob. Chemother. 2017, 72, 3353–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, G.; Distefano, D.A.; Parlascino, P.; Fresta, C.G.; Lazzarino, G.; Lunte, S.M.; Nicoletti, V.G. Receptor-mediated toxicity of human amylin fragment aggregated by short- and long-term incubations with copper ions. Mol. Cell. Biochem. 2017, 425, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Lazzarino, G.; Distefano, D.A.; Parlascino, P.; Lunte, S.M.; Lazzarino, G.; Caraci, F. Sub-toxic human amylin fragment concentrations promote the survival and proliferation of sh-sy5y cells via the release of vegf and hspb5 from endothelial rbe4 cells. Int. J. Mol. Sci. 2018, 19, 3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine decreases pma-induced oxidative stress and inflammation in murine macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrisi, S.A.; Geraci, F.; Tropea, M.R.; Grasso, M.; Caruso, G.; Fidilio, A.; Musso, N.; Sanfilippo, G.; Tascedda, F.; Palmeri, A.; et al. Fluoxetine and vortioxetine reverse depressive-like phenotype and memory deficits induced by abeta1-42 oligomers in mice: A key role of transforming growth factor-beta1. Front. Pharmacol 2019, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines il-6 and tnf-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15 (Suppl. S2), 120–122. [Google Scholar] [CrossRef]
- Ji, T.; Li, G.; Chen, J.; Zhao, J.; Li, X.; Lin, H.; Cai, X.; Cang, Y. Distinct role of interleukin-6 and tumor necrosis factor receptor-1 in oval cell- mediated liver regeneration and inflammation-associated hepatocarcinogenesis. Oncotarget 2016, 7, 66635–66646. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Fresta, C.G.; Grasso, M.; Santangelo, R.; Lazzarino, G.; Lunte, S.M.; Caraci, F. Inflammation as the common biological link between depression and cardiovascular diseases: Can carnosine exert a protective role? Curr. Med. Chem. 2020, 27, 1782–1800. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Xu, P.; Lu, J.; Wang, R.; Mu, W. Decorin reduces hypertrophic scarring through inhibition of the tgf-beta1/smad signaling pathway in a rat osteomyelitis model. Exp. Ther. Med. 2016, 12, 2102–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjerpe, E.; Egyhazi Brage, S.; Carlson, J.; Frostvik Stolt, M.; Schedvins, K.; Johansson, H.; Shoshan, M.; Avall-Lundqvist, E. Metabolic markers gapdh, pkm2, atp5b and bec-index in advanced serous ovarian cancer. BMC Clin. Pathol. 2013, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.A.; Nair, S.P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 2010, 300, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Monecke, S.; Slickers, P.; Gawlik, D.; Muller, E.; Reissig, A.; Ruppelt-Lorz, A.; Akpaka, P.E.; Bandt, D.; Bes, M.; Boswihi, S.S.; et al. Molecular typing of st239-mrsa-iii from diverse geographic locations and the evolution of the sccmec iii element during its intercontinental spread. Front. Microbiol. 2018, 9, 1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botelho, A.M.N.; Cerqueira, E.C.M.O.; Moustafa, A.M.; Beltrame, C.O.; Ferreira, F.A.; Cortes, M.F.; Costa, B.S.S.; Silva, D.N.S.; Bandeira, P.T.; Lima, N.C.B.; et al. Local diversification of methicillin- resistant Staphylococcus aureus st239 in south america after its rapid worldwide dissemination. Front. Microbiol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, N.J.; Boelens, H.A.; de Vogel, C.P.; Tavakol, M.; Bode, L.G.; Verbrugh, H.A.; van Belkum, A.; van Wamel, W.J. Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh-Moghaddam, H.; van Wamel, W.; van Belkum, A.; Hamat, R.A.; Tavakol, M.; Neela, V.K. Humoral immune consequences of Staphylococcus aureus st239-associated bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N.; Schwaber, M.J.; Karchmer, A.W.; Carmeli, Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin. Infect. Dis. 2003, 36, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Gastmeier, P.; Sohr, D.; Geffers, C.; Behnke, M.; Daschner, F.; Ruden, H. Mortality risk factors with nosocomial Staphylococcus aureus infections in intensive care units: Results from the german nosocomial infection surveillance system (kiss). Infection 2005, 33, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Engemann, J.J.; Carmeli, Y.; Cosgrove, S.E.; Fowler, V.G.; Bronstein, M.Z.; Trivette, S.L.; Briggs, J.P.; Sexton, D.J.; Kaye, K.S. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 2003, 36, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.D.; Friedman, J.Y.; Engemann, J.J.; Griffiths, R.I.; Anstrom, K.J.; Kaye, K.S.; Stryjewski, M.E.; Szczech, L.A.; Reller, L.B.; Corey, G.R.; et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect. Control. Hosp. Epidemiol. 2005, 26, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gulbins, E.; Edwards, M.J.; Caldwell, C.C.; Fraunholz, M.; Becker, K.A. Staphylococcus aureus alpha-toxin induces inflammatory cytokines via lysosomal acid sphingomyelinase and ceramides. Cell Physiol. Biochem. 2017, 43, 2170–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicholas, S.; Talento, A.F.; O’Gorman, J.; Hannan, M.M.; Lynch, M.; Greene, C.M.; Humphreys, H.; Fitzgerald-Hughes, D. Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect. Dis. 2014, 14, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, C.A.; Jellis, J.; Hughes, S.P.; Remick, D.G.; Friedland, J.S. Tumor necrosis factor-alpha, interleukin-6, and interleukin-8 secretion and the acute-phase response in patients with bacterial and tuberculous osteomyelitis. J. Infect. Dis. 1998, 177, 1582–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marriott, I.; Gray, D.L.; Tranguch, S.L.; Fowler, V.G., Jr.; Stryjewski, M.; Scott Levin, L.; Hudson, M.C.; Bost, K.L. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am. J. Pathol. 2004, 164, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.F.; Moraes, C.; Silveira, A.M.; Correa-Oliveira, R.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Moreno, E.C.; do Carmo, L.S.; Fraga, L.A.; Malaquias, L.C. Distinct cytokine profiles of circulating mononuclear cells stimulated with Staphylococcus aureus enterotoxin a in vitro during early and late episodes of chronic osteomyelitis. Memórias do Inst. Oswaldo Cruz 2012, 107, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, T.; Magara, S.; Miyai, D.; Nishimura, H.; Kuroki, E.; Furudoi, S.; Komori, T.; Ohbayashi, C. Local levels of interleukin-1beta, -4, -6 and tumor necrosis factor alpha in an experimental model of murine osteomyelitis due to Staphylococcus aureus. Cytokine 2002, 19, 59–65. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of atopic dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef]
- Bauche, D.; Marie, J.C. Transforming growth factor beta: A master regulator of the gut microbiota and immune cell interactions. Clin. Transl. Immunol. 2017, 6, e136. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Kasagi, S.; Chen, W. Tgf-beta1 on osteoimmunology and the bone component cells. Cell Biosci. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, Y.; Fukumoto, S.; Matsumoto, T. Relationship between actions of transforming growth factor (tgf)-beta and cell surface expression of its receptors in clonal osteoblastic cells. J. Cell Physiol. 1995, 162, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Sriram, S.; Robinson, P.; Pi, L.; Lewin, A.S.; Schultz, G. Triple combination of sirnas targeting tgfbeta1, tgfbetar2, and ctgf enhances reduction of collagen i and smooth muscle actin in corneal fibroblasts. Investig. Ophthalmol Vis. Sci. 2013, 54, 8214–8223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Xi, C. Hepatocyte growth factor reduces hypertrophy of skin scar: In vivo study. Adv. Skin Wound Care 2013, 26, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, F.; Redini, F. Transforming growth factor-beta signaling plays a pivotal role in the interplay between osteosarcoma cells and their microenvironment. Front. Oncol. 2018, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Y.; Ye, C.L.; Zhu, L.L.; Tian, Z.Y.; Yang, Z.B. A homolog of glyceraldehyde-3-phosphate dehydrogenase from riemerella anatipestifer is an extracellular protein and exhibits biological activity. J. Zhejiang Univ. Sci. B 2014, 15, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Nahlik, K.W.; Mleczko, A.K.; Gawlik, M.K.; Rokita, H.B. Modulation of gapdh expression and cellular localization after vaccinia virus infection of human adherent monocytes. Acta Biochim. Pol. 2003, 50, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal response to oxidative stress. Front. Cell Infect. Microbiol. 2012, 2, 33. [Google Scholar] [CrossRef] [Green Version]




| LAB CODE | ST-SCCmec-spa Type | Source | FOX | CN | DA | E | CIP | TE | SXT | K | RD | BPR |
| ATCC-12598 | 30-MSSA-III-t976 | - | - | - | - | - | - | - | - | - | - | - |
| 2SA | 239-III-t037 | wound | R | R | Ri | R | R | R | R | R | 2 | 2 |
| 5SA | 5-II-t2154 | blood | R | R | S | R | R | R | S | R | >32 | 2 |
| 10SA | 228-I-t041 | blood | R | R | R | R | R | S | S | R | >32 | 2 |
| 14SA | 22-IVh-t032 | blood | R | S | Ri | R | R | S | S | S | 0.008 | 1 |
| LAB CODE | ST-SCCmec-spa type | Source | DAL | CPT | LNZ | DPT | TGC | FU | VA | TC | GRD | |
| ATCC-12598 | 30-MSSA-III-t976 | - | - | - | - | - | - | - | - | - | - | |
| 2SA | 239-III-t037 | wound | 0.125 | 2 | 2 | 0.5 | 0.25 | >256 | 1 | 2 | VSSA | |
| 5SA | 5-II-t2154 | blood | 0.012 | 4 | 32 | 0.5 | 0.5 | >256 | 1 | 0.5 | hVISA | |
| 10SA | 228-I-t041 | blood | 0.125 | 1 | 8 | 1 | 0.125 | 0.125 | 1 | 1 | VSSA | |
| 14SA | 22-IVh-t032 | blood | 0.064 | 1 | 1 | 1 | 0.125 | 0.064 | 0.5 | 0.25 | VSSA |
| Official Name # | Official Symbol | Alternative Titles/Symbols | Detected Transcript | Amplicon Length | Cat. No. § |
|---|---|---|---|---|---|
| nitric oxide synthase 2, inducible | NOS2 | NOS; INOS; NOS2A; HEP-NOS | NM_000625 NM_153292 | 92 bp | QT00068740 |
| cytochrome b-245 beta chain | CYBB | CGD; NOX2; IMD34; AMCBX2; GP91-1; GP91PHOX; p91-PHOX; GP91-PHOX | NM_000397 | 124 bp | QT00029533 |
| transforming growth factor beta 1 | TGFB1 | CED; LAP; DPD1; TGFB; IBDIMDE; TGFbeta; TGF-beta1 | NM_000660 | 108 bp | QT00000728 |
| interleukin 6 | IL6 | CDF; HGF; HSF; BSF2; IL-6; BSF-2; IFNB2; IFN-beta-2 | NM_000600 XM_005249745 | 107 bp | QT00083720 |
| tumor necrosis factor | TNF | DIF; TNFA; TNFSF2; TNLG1F; TNF-alpha | NM_000594 | 98 bp | QT00029162 |
| glyceraldehyde-3-phosphate dehydrogenase | GAPDH | G3PD; GAPD; HEL-S-162eP | NM_001256799 NM_002046 NM_001289745 NM_001289746 | 95 bp | QT00079247 |
| nuclear factor, erythroid 2 like 2 | NFE2L2 | NRF2; HEBP1; Nrf-2; IMDDHH | NM_006164 | 153 bp | QT00027384 |
| heme oxygenase 1 | HMOX1 | HO-1; HSP32; HMOX1D; bK286B10 | NM_002133 | 99 bp | QT00092645 |
| actin beta | ACTB | BRWS1; PS1TP5BP1 | NM_001101 | 146 bp | QT00095431 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musso, N.; Caruso, G.; Bongiorno, D.; Grasso, M.; Bivona, D.A.; Campanile, F.; Caraci, F.; Stefani, S. Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells. Biomolecules 2021, 11, 72. https://doi.org/10.3390/biom11010072
Musso N, Caruso G, Bongiorno D, Grasso M, Bivona DA, Campanile F, Caraci F, Stefani S. Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells. Biomolecules. 2021; 11(1):72. https://doi.org/10.3390/biom11010072
Chicago/Turabian StyleMusso, Nicolò, Giuseppe Caruso, Dafne Bongiorno, Margherita Grasso, Dalida A. Bivona, Floriana Campanile, Filippo Caraci, and Stefania Stefani. 2021. "Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells" Biomolecules 11, no. 1: 72. https://doi.org/10.3390/biom11010072
APA StyleMusso, N., Caruso, G., Bongiorno, D., Grasso, M., Bivona, D. A., Campanile, F., Caraci, F., & Stefani, S. (2021). Different Modulatory Effects of Four Methicillin-Resistant Staphylococcus aureus Clones on MG-63 Osteoblast-Like Cells. Biomolecules, 11(1), 72. https://doi.org/10.3390/biom11010072









