Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology
Abstract
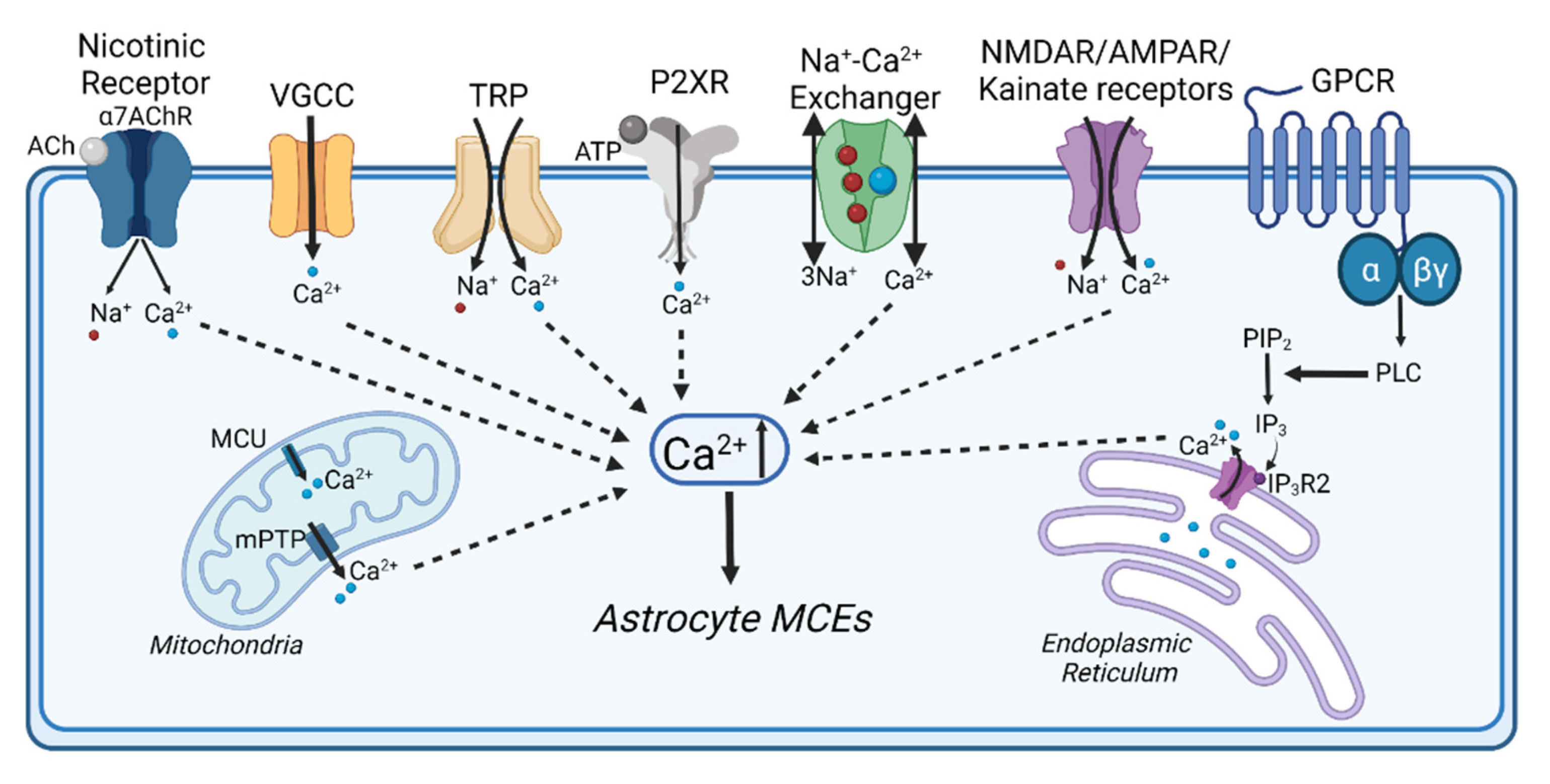
:1. Introduction
2. Functional Roles of Astrocyte Microdomain Ca2+ Events
3. Pathways Underlying Fast Astrocyte MCEs
3.1. Ionotropic Glutamate Receptors (NMDA, AMPA, and Kainate Receptors)
3.1.1. Astrocyte iGluR Expression
3.1.2. Functional Roles of Astrocyte iGluRs
3.2. P2X Receptors
3.2.1. Astrocyte P2X Receptor Expression
3.2.2. Functional Roles of Astrocyte P2XRs
3.3. Nicotinic Receptors
3.3.1. Astrocyte Nicotinic Receptor Expression
3.3.2. Functional Roles of Astrocyte Nicotinic Receptors
3.4. Na+-Ca2+ Exchanger
3.4.1. Astrocyte Na+-Ca2+ Exchanger Expression
3.4.2. Functional Roles of Astrocyte NCX Reversal
3.5. Voltage-Gated Calcium Channels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Wallraff, A.; Köhling, R.; Heinemann, U.; Theis, M.; Willecke, K.; Steinhäuser, C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 2006, 26, 5438–5447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bröer, S.; Bröer, A.; Hansen, J.T.; Bubb, W.A.; Balcar, V.J.; Nasrallah, F.A.; Garner, B.; Rae, C. Alanine metabolism, transport, and cycling in the brain. J. Neurochem. 2007, 102, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Barros, L.F. The astrocyte: Powerhouse and recycling center. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mächler, P.; Wyss, M.T.; Elsayed, M.; Stobart, J.; Gutierrez, R.; Von Faber-Castell, A.; Kaelin, V.; Zuend, M.; San Martín, A.; Romero-Gómez, I.; et al. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 2016, 23, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertz, L.; Gibbs, M.E.; Dienel, G.A. Fluxes of lactate into, from, and among gap junction-coupled astrocytes and their interaction with noradrenaline. Front. Neurosci. 2014, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef] [Green Version]
- Stobart, J.; Anderson, C.M. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front. Cell. Neurosci. 2013, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.R.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef] [Green Version]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.J.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Rosenegger, D.G.; Tran, C.H.T.; Wamsteeker Cusulin, J.I.; Gordon, G.R. Tonic local brain blood flow control by astrocytes independent of phasic neurovascular coupling. J. Neurosci. 2015, 35, 13463–13474. [Google Scholar] [CrossRef]
- Jackson, J.G.; Robinson, M.B. Reciprocal regulation of mitochondrial dynamics and calcium signaling in astrocyte processes. J. Neurosci. 2015, 35, 15199–15213. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Wu, P.H.; Hughes, E.G.; Fukaya, M.; Tischfield, M.A.; Langseth, A.J.; Wirtz, D.; Bergles, D.E. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 2017, 93, 587–605.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Nagai, J.; Khakh, B.S. Improved tools to study astrocytes. Nat. Rev. Neurosci. 2020, 21, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Stobart, J.L.; Ferrari, K.D.; Barrett, M.J.P.; Glück, C.; Stobart, M.J.; Zuend, M.; Weber, B. Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 2018, 98, 726–735.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stobart, J.L.; Ferrari, K.D.; Barrett, M.J.P.P.; Stobart, M.J.; Looser, Z.J.; Saab, A.S.; Weber, B. Long-term in vivo calcium imaging of astrocytes reveals distinct cellular compartment responses to sensory stimulation. Cereb. Cortex 2018, 28, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Bindocci, E.; Savtchouk, I.; Liaudet, N.; Becker, D.; Carriero, G.; Volterra, A. Three-dimensional calcium imaging advances understanding of astrocyte biology. Science 2017, 356, eaai8185. [Google Scholar] [CrossRef] [Green Version]
- Shigetomi, E.; Tong, X.; Kwan, K.Y.; Corey, D.P.; Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 2011, 15, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Arizono, M.; Inavalli, V.V.G.K.; Panatier, A.; Pfeiffer, T.; Angibaud, J.; Levet, F.; Ter Veer, M.J.T.; Stobart, J.; Bellocchio, L.; Mikoshiba, K.; et al. Structural basis of astrocytic Ca2+ signals at tripartite synapses. Nat. Commun. 2020, 11, 1906. [Google Scholar] [CrossRef] [Green Version]
- Shigetomi, E.; Kracun, S.; Sofroniew, M.V.; Khakh, B.S. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat. Neurosci. 2010, 13, 759–766. [Google Scholar] [CrossRef] [Green Version]
- Shigetomi, E.; Kracun, S.; Khakh, B.S. Monitoring astrocyte calcium levels with improved membrane targeted GCaMP reporters. Neuron Glia Biol. 2010, 6, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.; Huang, B.S.; Venugopal, S.; Johnston, A.D.; Chai, H.; Zeng, H.; Golshani, P.; Khakh, B.S. Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat. Neurosci. 2015, 18, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Shigetomi, E.; Jackson-Weaver, O.; Huckstepp, R.T.; O’Dell, T.J.; Khakh, B.S. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 2013, 33, 10143–10153. [Google Scholar] [CrossRef]
- King, C.M.; Bohmbach, K.; Minge, D.; Delekate, A.; Zheng, K.; Reynolds, J.; Rakers, C.; Zeug, A.; Petzold, G.C.; Rusakov, D.A.; et al. Local resting Ca2+ controls the scale of astroglial Ca2+ signals. Cell Rep. 2020, 30, 3466–3477.e4. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Bard, L.; Reynolds, J.P.; King, C.; Jensen, T.P.; Gourine, A.V.; Rusakov, D.A. Time-resolved imaging reveals heterogeneous landscapes of nanomolar Ca2+ in neurons and astroglia. Neuron 2015, 88, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Otsu, Y.; Couchman, K.; Lyons, D.G.; Collot, M.; Agarwal, A.; Mallet, J.M.; Pfrieger, F.W.; Bergles, D.E.; Charpak, S. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat. Neurosci. 2015, 18, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Asada, A.; Ujita, S.; Nakayama, R.; Oba, S.; Ishii, S.; Matsuki, N.; Ikegaya, Y. Subtle modulation of ongoing calcium dynamics in astrocytic microdomains by sensory inputs. Physiol. Rep. 2015, 3, e12454. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.L.; Jessen, S.B.; Lønstrup, M.; Joséphine, C.; Bonvento, G.; Lauritzen, M. Fast Ca2+ responses in astrocyte end-feet and neurovascular coupling in mice. Glia 2017, 66, 348–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, B.L.; Brazhe, A.R.; Jessen, S.B.; Tan, F.C.C.; Lauritzen, M.J. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, E4678–E4687. [Google Scholar] [CrossRef] [Green Version]
- Nizar, K.; Uhlirova, H.; Tian, P.; Saisan, P.a.; Cheng, Q.; Reznichenko, L.; Weldy, K.L.; Steed, T.C.; Sridhar, V.B.; MacDonald, C.L.; et al. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J. Neurosci. 2013, 33, 8411–8422. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; O’Donnell, J.; Thrane, A.S.; Zeppenfeld, D.; Kang, H.; Xie, L.; Wang, F.; Nedergaard, M. α1-adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 2013, 54, 387–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Lou, N.; Xu, Q.; Tian, G.-F.; Peng, W.G.; Han, X.; Kang, J.; Takano, T.; Nedergaard, M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 2006, 9, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Winship, I.R.; Plaa, N.; Murphy, T.H. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J. Neurosci. 2007, 27, 6268–6272. [Google Scholar] [CrossRef] [Green Version]
- Fellin, T.; Pascual, O.; Gobbo, S.; Pozzan, T.; Haydon, P.G.; Carmignoto, G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 2004, 43, 729–743. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Yoon, B.; Berglund, K.; Oh, S.; Park, H.; Shin, H.; Augustine, G.J.; Lee, C.J. Channel-mediated tonic GABA release from glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef]
- Kozlov, A.S.; Angulo, M.C.; Audinat, E.; Charpak, S. Target cell-specific modulation of neuronal activity by astrocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 10058–10063. [Google Scholar] [CrossRef] [Green Version]
- Panatier, A.; Vallée, J.; Haber, M.; Murai, K.K.; Lacaille, J.C.; Robitaille, R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011, 146, 785–798. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.; Bosson, A.; Riebe, I.; Reynell, C.; Vallée, J.; Laplante, I.; Panatier, A.; Robitaille, R.; Lacaille, C. Astrocytes detect and upregulate transmission at inhibitory synapses of somatostatin interneurons onto pyramidal cells. Nat. Commun. 2018, 9, 4254. [Google Scholar] [CrossRef]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D. a Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Papouin, T.; Henneberger, C.; Rusakov, D.A.; Oliet, S.H.R. Astroglial versus neuronal D-Serine: Fact checking. Trends Neurosci. 2017, 40, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Wolosker, H.; Balu, D.T.; Coyle, J.T. Astroglial versus neuronal D-Serine: Check your controls! Trends Neurosci. 2017, 40, 520–522. [Google Scholar] [CrossRef]
- Wolosker, H.; Balu, D.T.; Coyle, J.T. The rise and fall of the D-Serine-mediated gliotransmission hypothesis. Trends Neurosci. 2016, 39, 712–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Castro, M.A.; Chuquet, J.; Liaudet, N.; Bhaukaurally, K.; Santello, M.; Bouvier, D.; Tiret, P.; Volterra, A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 2011, 14, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Boddum, K.; Jensen, T.P.; Magloire, V.; Kristiansen, U.; Rusakov, D.A.; Pavlov, I.; Walker, M.C. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat. Commun. 2016, 7, 13572. [Google Scholar] [CrossRef] [PubMed]
- De Pittà, M.; Brunel, N.; Volterra, A. Astrocytes: Orchestrating synaptic plasticity? Neuroscience 2016, 323, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Kastanenka, K.V.; Moreno-Bote, R.; De Pittà, M.; Perea, G.; Eraso-Pichot, A.; Masgrau, R.; Poskanzer, K.E.; Galea, E. A roadmap to integrate astrocytes into Systems Neuroscience. Glia 2020, 68, 5–26. [Google Scholar] [CrossRef] [Green Version]
- Letellier, M.; Park, Y.K.; Chater, T.E.; Chipman, P.H.; Gautam, S.G.; Oshima-Takago, T.; Goda, Y. Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc. Natl. Acad. Sci. USA 2016, 113, E2685–E2694. [Google Scholar] [CrossRef] [Green Version]
- Navarrete, M.; Perea, G.; de Sevilla, D.F.; Gómez-Gonzalo, M.; Núñez, A.; Martín, E.D.; Araque, A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012, 10, e1001259. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Bennett, D.V.; Rubinov, M.; Narayan, S.; Yang, C.-T.; Tanimoto, M.; Mensh, B.D.; Looger, L.L.; Ahrens, M.B. Glia accumulate evidence that actions are futile and suppress unsuccessful behavior. Cell 2019, 178, 27–43.e19. [Google Scholar] [CrossRef] [Green Version]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Taylor, A.M.W.; Nagai, J.; Golshani, P.; Evans, C.J.; Coppola, G.; Khakh, B.S. Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 2018, 99, 1170–1187. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Li, L.P.; Wang, Q.; Wu, Q.; Hu, H.H.; Zhang, M.; Fang, Y.Y.; Zhang, J.; Li, S.J.; Xiong, W.C.; et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 2013, 19, 773–777. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, Y.; Wu, D.; Liu, J.-H.; Jie, W.; You, Q.; Huang, L.; Hu, J.; Chu, H.; Gao, F.; et al. Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nat. Commun. 2021, 12, 3321. [Google Scholar] [CrossRef]
- Bonansco, C.; Couve, A.; Perea, G.; Ferradas, C.Á.; Roncagliolo, M.; Fuenzalida, M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur. J. Neurosci. 2011, 33, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gonzalo, M.; Navarrete, M.; Perea, G.; Covelo, A.; Martín-Fernández, M.; Shigemoto, R.; Luján, R.; Araque, A. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb. Cortex 2015, 25, 3699–3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perea, G.; Araque, A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 2007, 317, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; Casper, K.B.; Kubera, C.; Zhang, J.; Revilla-Sanchez, R.; Sul, J.-Y.Y.; Takano, H.; Moss, S.J.; McCarthy, K.; Haydon, P.G. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005, 310, 113–116. [Google Scholar] [CrossRef]
- Min, R.; Nevian, T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat. Neurosci. 2012, 15, 746–753. [Google Scholar] [CrossRef]
- Fossat, P.; Turpin, F.R.; Sacchi, S.; Dulong, J.; Shi, T.; Rivet, J.M.; Sweedler, J.V.; Pollegioni, L.; Millan, M.J.; Oliet, S.H.R.; et al. Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb. Cortex 2012, 22, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Sugihara, H.; Sharma, J.; Perea, G.; Petravicz, J.; Le, C.; Sur, M. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc. Natl. Acad. Sci. USA 2012, 109, E2832–E2841. [Google Scholar] [CrossRef] [Green Version]
- Takata, N.; Mishima, T.; Hisatsune, C.; Nagai, T.; Ebisui, E.; Mikoshiba, K.; Hirase, H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 2011, 31, 18155–18165. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, Y.; Randall, J.; Janett, E.; Nikonenko, I.; König, S.; Jones, E.V.; Flores, C.E.; Murai, K.K.; Bochet, C.G.; Holtmaat, A.; et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 2014, 24, 1679–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molotkov, D.; Zobova, S.; Arcas, J.M.; Khiroug, L. Calcium-induced outgrowth of astrocytic peripheral processes requires actin binding by Profilin-1. Cell Calcium 2013, 53, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Shih, P.-Y.; Gomi, H.; Yoshida, T.; Nakai, J.; Ando, R.; Furuichi, T.; Mikoshiba, K.; Semyanov, A.; Itohara, S. Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol. Brain 2013, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poskanzer, K.E.; Yuste, R. Astrocytic regulation of cortical UP states. Proc. Natl. Acad. Sci. USA 2011, 108, 18453–18458. [Google Scholar] [CrossRef] [Green Version]
- Poskanzer, K.E.; Yuste, R. Astrocytes regulate cortical state switching in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E2675–E2684. [Google Scholar] [CrossRef] [Green Version]
- Paukert, M.; Agarwal, A.; Cha, J.; Doze, V.A.; Kang, J.U.; Bergles, D.E. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 2014, 82, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.H.T.; Peringod, G.; Gordon, G.R. Astrocytes integrate behavioral state and vascular signals during functional hyperemia. Neuron 2018, 100, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidey, J.N.; Peringod, G.; Institoris, A.; Gorzo, K.A.; Nicola, W.; Vandal, M.; Ito, K.; Liu, S.; Fielding, C.; Visser, F.; et al. Astrocytes regulate ultra-slow arteriole oscillations via stretch-mediated TRPV4-COX-1 feedback. Cell Rep. 2021, 36, 109405. [Google Scholar] [CrossRef] [PubMed]
- LeMaistre Stobart, J.L.; Lu, L.; Anderson, H.D.I.; Mori, H.; Anderson, C.M. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2013, 110, 3149–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, G.G.; Choi, H.B.; Rungta, R.L.; Ellis-Davies, G.C.R.; MacVicar, B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008, 456, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, S.J.; MacVicar, B.A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004, 431, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Zonta, M.; Angulo, M.C.; Gobbo, S.; Rosengarten, B.; Hossmann, K.-A.A.; Pozzan, T.; Carmignoto, G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003, 6, 43–50. [Google Scholar] [CrossRef]
- Sharma, K.; Gordon, G.R.J.; Tran, C.H.T. Heterogeneity of sensory-induced astrocytic Ca2+ dynamics during functional hyperemia. Front. Physiol. 2020, 11, 611884. [Google Scholar] [CrossRef]
- Gu, X.; Chen, W.; Volkow, N.D.; Koretsky, A.P.; Du, C.; Pan, Y. Synchronized astrocytic Ca2+ responses in neurovascular coupling during somatosensory stimulation and for the resting state. Cell Rep. 2018, 23, 3878–3890. [Google Scholar] [CrossRef]
- Tran, C.H.T.; George, A.G.; Teskey, G.C.; Gordon, G.R. Seizures elevate gliovascular unit Ca2+ and cause sustained vasoconstriction. JCI Insight 2020, 5, e136469. [Google Scholar] [CrossRef]
- Volnova, A.; Tsytsarev, V.; Ptukha, M.; Inyushin, M. In vitro and in vivo study of the short-term vasomotor response during epileptic seizures. Brain Sci. 2020, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Grutzendler, J.; Nedergaard, M. Cellular control of brain capillary blood flow: In vivo imaging veritas. Trends Neurosci. 2019, 42, 528–536. [Google Scholar] [CrossRef]
- Dunn, K.M.; Hill-Eubanks, D.C.; Liedtke, W.B.; Nelson, M.T. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc. Natl. Acad. Sci. USA 2013, 110, 6157–6162. [Google Scholar] [CrossRef] [Green Version]
- Malarkey, E.B.; Ni, Y.; Parpura, V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 2008, 56, 821–835. [Google Scholar] [CrossRef]
- Butenko, O.; Dzamba, D.; Benesova, J.; Honsa, P.; Benfenati, V.; Rusnakova, V.; Ferroni, S.; Anderova, M. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral Hypoxia/Ischemia. PLoS ONE 2012, 7, e39959. [Google Scholar] [CrossRef] [Green Version]
- Benfenati, V.; Amiry-Moghaddam, M.; Caprini, M.; Mylonakou, M.N.; Rapisarda, C.; Ottersen, O.P.; Ferroni, S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 2007, 148, 876–892. [Google Scholar] [CrossRef]
- Pizzo, P.; Burgo, A.; Pozzan, T.; Fasolato, C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J. Neurochem. 2001, 79, 98–109. [Google Scholar] [CrossRef]
- Grimaldi, M.; Maratos, M.; Verma, A. Transient receptor potential channel activation causes a novel form of [Ca2+]i oscillations and is not involved in capacitative Ca2+ entry in glial cells. J. Neurosci. 2003, 23, 4737–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agulhon, C.; Fiacco, T.A.; McCarthy, K.D. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 2010, 327, 1250–1254. [Google Scholar] [CrossRef] [Green Version]
- Takata, N.; Nagai, T.; Ozawa, K.; Oe, Y.; Mikoshiba, K.; Hirase, H. Cerebral blood flow modulation by basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS ONE 2013, 8, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petravicz, J.; Fiacco, T.a.; McCarthy, K.D. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 2008, 28, 4967–4973. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.T.; McCarthy, K.D. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 1996, 16, 5073–5081. [Google Scholar] [CrossRef] [Green Version]
- Pasti, L.; Volterra, A.; Pozzan, T.; Carmignoto, G. Intracellular calcium oscillations in astrocytes: A highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997, 17, 7817–7830. [Google Scholar] [CrossRef] [Green Version]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; McConnell, E.; Pare, J.-F.F.; Xu, Q.; Chen, M.; Peng, W.; Lovatt, D.; Han, X.; Smith, Y.; Nedergaard, M.; et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 2013, 339, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Lavialle, M.; Aumann, G.; Anlauf, E.; Pröls, F.; Arpin, M.; Derouiche, A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 12915–12919. [Google Scholar] [CrossRef] [Green Version]
- Collingridge, G.L.; Olsen, R.W.; Peters, J.; Spedding, M. A nomenclature for ligand-gated ion channels. Neuropharmacology 2009, 56, 2–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Ma, Y.-Y. Calcium permeable-AMPA receptors and excitotoxicity in neurological disorders. Front. Neural Circuits 2021, 15, 711564. [Google Scholar] [CrossRef]
- Frandsen, A.; Schousboe, A. AMPA receptor-mediated neurotoxicity: Role of Ca2+ and desensitization. Neurochem. Res. 2003, 28, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, L.A.; Bernardinelli, Y.; Muller, D. GluN3A: An NMDA receptor subunit with exquisite properties and functions. Neural Plast. 2013, 2013, 145387. [Google Scholar] [CrossRef] [Green Version]
- Grand, T.; Abi Gerges, S.; David, M.; Diana, M.A.; Paoletti, P. Unmasking GluN1/GluN3A excitatory glycine NMDA receptors. Nat. Commun. 2018, 9, 4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkhratsky, A.; Steinhäuser, C. Ion channels in glial cells. Brain Res. Rev. 2000, 32, 380–412. [Google Scholar] [CrossRef]
- Conti, F.; Minelli, A.; Brecha, N. Cellular localization and laminar distribution of AMPA glutamate receptor subunits mRNAs and proteins in the rat cerebral cortex. J. Comp. Neurol. 1994, 350, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Steinhäuser, C. Glial cells in the mouse hippocampus express AMPA receptors with an intermediate Ca2+ permeability. Eur. J. Neurosci. 1995, 7, 1872–1881. [Google Scholar] [CrossRef]
- Fan, D.; Grooms, S.Y.; Araneda, R.C.; Johnson, A.B.; Dobrenis, K.; Kessler, J.A.; Zukin, R.S. AMPA receptor protein expression and function in astrocytes cultured from hippocampus. J. Neurosci. Res. 1999, 57, 557–571. [Google Scholar] [CrossRef]
- Seifert, G.; Zhou, M.; Steinhäuser, C. Analysis of AMPA receptor properties during postnatal development of mouse hippocampal astrocytes. J. Neurophysiol. 1997, 78, 2916–2923. [Google Scholar] [CrossRef]
- Palygin, O.; Lalo, U.; Pankratov, Y. Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br. J. Pharmacol. 2011, 163, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Palygin, O.; Lalo, U.; Verkhratsky, A.; Pankratov, Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+signalling in cortical astrocytes. Cell Calcium 2010, 48, 225–231. [Google Scholar] [CrossRef]
- Dzamba, D.; Honsa, P.; Valny, M.; Kriska, J.; Valihrach, L.; Novosadova, V.; Kubista, M.; Anderova, M. Quantitative analysis of glutamate receptors in glial cells from the cortex of GFAP/EGFP mice following ischemic injury: Focus on NMDA receptors. Cell. Mol. Neurobiol. 2015, 35, 1187–1202. [Google Scholar] [CrossRef]
- García-Barcina, J.; Matute, C. Expression of kainate-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Eur. J. Neurosci. 1996, 8, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Brand-Schieber, E.; Lowery, S.; Werner, P. Select ionotropic glutamate AMPA/kainate receptors are expressed at the astrocyte-vessel interface. Brain Res. 2004, 1007, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.; Albrecht, J.; Morrow, C.; Murphy, S. Astrocyte glutamate receptor activation promotes inositol phospholipid turnover and calcium flux. Neurosci. Lett. 1986, 72, 335–340. [Google Scholar] [CrossRef]
- Cornell-Bell, A.H.; Finkbeiner, S.M.; Cooper, M.S.; Smith, S.J. Glutamate induces calcium waves in cultured astrocytes: Long-range glial signaling. Science 1990, 247, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.M.; Chiu, S.Y. Fluorescence measurement of changes in intracellular calcium induced by excitatory amino acids in cultured cortical astrocytes. J. Neurosci. 1990, 10, 1165–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnashev, N.; Khodorova, A.; Jonas, P.; Helm, P.; Wisden, W.; Monyer, H.; Seeburg, P.; Sakmann, B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science 1992, 256, 1566–1570. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y.; Parpura, V.; Verkhratsky, A. Ionotropic receptors in neuronal-astroglial signalling: What is the role of “excitable” molecules in non-excitable cells. Biochim. Biophys. Acta 2011, 1813, 992–1002. [Google Scholar] [CrossRef]
- Cai, Z.; Kimelberg, H.K. Glutamate receptor-mediated calcium responses in acutely isolated hippocampal astrocytes. Glia 1997, 21, 380–389. [Google Scholar] [CrossRef]
- Papadia, S.; Soriano, F.X.; Léveillé, F.; Martel, M.-A.; Dakin, K.A.; Hansen, H.H.; Kaindl, A.; Sifringer, M.; Fowler, J.; Stefovska, V.; et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 2008 114 2008, 11, 476–487. [Google Scholar] [CrossRef]
- Wroge, C.M.; Hogins, J.; Eisenman, L.; Mennerick, S. Synaptic NMDA receptors mediate hypoxic excitotoxic death. J. Neurosci. 2012, 32, 6732–6742. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Blasco, D.; Santofimia-Castanõ, P.; Gonzalez, A.; Almeida, A.; Bolanõs, J.P. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015, 22, 1877–1889. [Google Scholar] [CrossRef] [Green Version]
- Glaum, S.R.; Holzwarth, J.A.; Miller, R.J. Glutamate receptors activate Ca2+ mobilization and Ca2+ influx into astrocytes. Proc. Natl. Acad. Sci. USA 1990, 87, 3454–3458. [Google Scholar] [CrossRef] [Green Version]
- Kou, Z.W.; Mo, J.L.; Wu, K.W.; Qiu, M.H.; Huang, Y.L.; Tao, F.; Lei, Y.; Lv, L.L.; Sun, F.Y. Vascular endothelial growth factor increases the function of calcium-impermeable AMPA receptor GluA2 subunit in astrocytes via activation of protein kinase C signaling pathway. Glia 2019, 67, 1344–1358. [Google Scholar] [CrossRef]
- Hamilton, N.; Vayro, S.; Kirchhoff, F.; Verkhratsky, A.; Robbins, J.; Gorecki, D.C.; Butt, A.M. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia 2008, 56, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Ziemens, D.; Oschmann, F.; Gerkau, N.J.; Rose, C.R. Heterogeneity of activity-induced sodium transients between astrocytes of the mouse hippocampus and neocortex: Mechanisms and consequences. J. Neurosci. 2019, 39, 2620–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, S.C.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A.; Norenberg, M.D. Primary cultures of astrocytes: Their value in understanding astrocytes in health and disease. Neurochem. Res. 2012, 37, 2569–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelton, M.K.; McCarthy, K. Mature hippocampal astrocytes exhibit functional metabotropic and ionotropic glutamate receptors in situ. Glia 1999, 26, 1–11. [Google Scholar] [CrossRef]
- Schipke, C.G.; Ohlemeyer, C.; Matyash, M.; Nolte, C.; Kettenmann, H.; Kirchhoff, F. Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. FASEB J. 2001, 15, 1270–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, A.; Robitaille, R.; Lacaille, J.C. Differential NMDA-dependent activation of glial cells in mouse hippocampus. Glia 2008, 56, 1648–1663. [Google Scholar] [CrossRef]
- Lalo, U.; Palygin, O.; Rasooli-Nejad, S.; Andrew, J.; Haydon, P.G.; Pankratov, Y. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 2014, 12, e1001747. [Google Scholar] [CrossRef]
- Mehina, E.M.F.; Murphy-Royal, C.; Gordon, G.R. Steady-state free Ca2+ in astrocytes is decreased by experience and impacts arteriole tone. J. Neurosci. 2017, 37, 8150–8165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalo, U.; Palygin, O.; North, R.A.; Verkhratsky, A.; Pankratov, Y. Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell 2011, 10, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benz, B.; Grima, G.; Do, K.Q. Glutamate-induced homocysteic acid release from astrocytes: Possible implication in glia-neuron signaling. Neuroscience 2004, 124, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Droste, D.; Seifert, G.; Seddar, L.; Jädtke, O.; Steinhaüser, C.; Lohr, C. Ca2+-permeable AMPA receptors in mouse olfactory bulb astrocytes. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Haustein, M.D.; Kracun, S.; Lu, X.H.; Shih, T.; Jackson-Weaver, O.; Tong, X.; Xu, J.; Yang, X.W.; O’Dell, T.J.; Marvin, J.S.; et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 2014, 82, 413–429. [Google Scholar] [CrossRef] [Green Version]
- Saab, A.S.; Neumeyer, A.; Jahn, H.M.; Cupido, A.; Šimek, A.A.M.; Boele, H.J.; Scheller, A.; Le Meur, K.; Götz, M.; Monyer, H.; et al. Bergmann glial AMPA receptors are required for fine motor coordination. Science 2012, 337, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Iino, M.; Goto, K.; Kakegawa, W.; Okado, H.; Sudo, M.; Ishiuchi, S.; Miwa, A.; Takayasu, Y.; Saito, I.; Tsuzuki, K.; et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 2001, 292, 926–929. [Google Scholar] [CrossRef]
- Olney, J.W. Neurotoxicity of NMDA receptor antagonists: An overiew. Psychopharmacol. Bull. 1994, 30, 533–540. [Google Scholar]
- Chipman, P.H.; Fernandez, A.P.; Fung, C.C.A.; Tedoldi, A.; Kawai, A.; Gautam, S.G.; Kurosawa, M.; Abe, M.; Sakimura, K.; Fukai, T.; et al. Astrocyte GluN2C NMDA receptors control basal synaptic strengths of hippocampal CA1 pyramidal neurons in the stratum radiatum. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y.; Wichert, S.P.; Rossner, M.J.; North, R.A.; Kirchhoff, F.; Verkhratsky, A. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J. Neurosci. 2008, 28, 5473–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, W.; Appelt, K.; Grohmann, M.; Franke, H.; Nörenberg, W.; Illes, P. Increase of intracellular Ca2+ by P2X and P2Y receptor-subtypes in cultured cortical astroglia of the rat. Neuroscience 2009, 160, 767–783. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Surprenant, A.; North, R.A. Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 2009, 71, 333–359. [Google Scholar] [CrossRef] [Green Version]
- Franke, H.; Verkhratsky, A.; Burnstock, G.; Illes, P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012, 8, 629–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, H.; Gunther, A.; Grosche, J.; Schmidt, R.; Rossner, S.; Reinhardt, R.; Faber-Zuschratter, H.; Schneider, D.; Illes, P. P2X 7 receptor expression after ischemia in the cerebral cortex of rats. J. Neuropathol. Exp. Neurol. 2004, 63, 686–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Reynolds, J.P.; Chen, Y.; Gourine, A.V.; Rusakov, D.A.; Attwell, D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 2016, 19, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Lalo, U.; Bogdanov, A.; Pankratov, Y. Age- and experience-related plasticity of ATP-mediated signaling in the neocortex. Front. Cell. Neurosci. 2019, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourine, A.V.; Kasymov, V.; Marina, N.; Tang, F.; Figueiredo, M.F.; Lane, S.; Teschemacher, A.G.; Spyer, K.M.; Deisseroth, K.; Kasparov, S.; et al. Astrocytes control breathing through pH-dependent release of ATP. Science 2010, 329, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Lalo, U.; Bogdanov, A.; Pankratov, Y. Diversity of astroglial effects on aging- and experience-related cortical metaplasticity. Front. Mol. Neurosci. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Sharma, G.; Vijayaraghavan, S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc. Natl. Acad. Sci. USA 2001, 98, 4148–4153. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Yakel, J.L. Functional α7 nicotinic ACh receptors on Astrocytes in rat hippocampal CA1 slices. J. Mol. Neurosci. 2012, 48, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Corradi, J.; Bouzat, C. Understanding the bases of function and modulation of a7 nicotinic receptors: Implications for drug discovery. Mol. Pharmacol. 2016, 90, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Papouin, T.; Dunphy, J.M.; Tolman, M.; Dineley, K.T.; Haydon, P.G. Septal cholinergic neuromodulation tunes the astrocyte-dependent gating of hippocampal NMDA receptors to wakefulness. Neuron 2017, 94, 840–854.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryal, S.; Fu, X.; Sandin, J.N.; Neupane, K.R.; Lakes, J.E.; Grady, M.E.; Richards, C.I. Nicotine induces morphological and functional changes in astrocytes via nicotinic receptor activity. Glia 2021, 69, 2037–2053. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; McIntire, J.; Ryan, S.; Dunah, A.; Loring, R. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-ΚB pathway and activation of the Nrf2 pathway. J. Neuroinflammation 2017, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kirischuk, S.; Parpura, V.; Verkhratsky, A. Sodium dynamics: Another key to astroglial excitability? Trends Neurosci. 2012, 35, 497–506. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Noda, M.; Parpura, V.; Kirischuk, S. Sodium fluxes and astroglial function. Adv. Exp. Med. Biol. 2013, 961, 295–305. [Google Scholar] [CrossRef]
- Rose, C.R.; Ziemens, D.; Verkhratsky, A. On the special role of NCX in astrocytes: Translating Na+-transients into intracellular Ca2+ signals. Cell Calcium 2020, 86, 102154. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Juhaszova, M.; Golovina, V.A.; Church, P.J.; Stanley, E.F. Na/Ca exchager and PMCA localizaiton in neurons and astrocytes: Functional implications. Ann. N. Y. Acad. Sci. 2002, 976, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Golovina, V.A.; Song, H.; James, P.F.; Lingrel, J.B.; Blaustein, M.P. Na+ pump alpha2 -subunit expression modulates Ca2+ signaling. Am. J. Physiol. - Cell Physiol. 2003, 284, C475–C486. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Song, H.; Nakai, J.; Ohkura, M.; Kotlikoff, M.I.; Kinsey, S.P.; Golovina, V.A.; Blaustein, M.P. Local subplasma membrane Ca2+ signals detected by a tethered Ca2+ sensor. Proc. Natl. Acad. Sci. USA 2006, 103, 13232–13237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takuma, K.; Matsuda, T.; Hashimoto, H.; Kitanaka, J.; Asano, S.; Kishida, Y.; Baba, A. Role of Na(+)-Ca2+ exchanger in agonist-induced Ca2+ signaling in cultured rat astrocytes. J. Neurochem. 1996, 67, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Goldman, W.; Yarowsky, P.; Juhaszova, M.; Krueger, B.; Blaustein, M. Sodium/calcium exchange in rat cortical astrocytes. J. Neurosci. 1994, 14, 5834–5843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, H.; Colina, C.; Ramos, M.; Benaim, G.; Jaffe, E.H.; Caputo, C.; DiPolo, R. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers -induced Ca2+ release in rat cerebellar Type-1 astrocytes. J. Neurochem. 2007, 100, 1188–1202. [Google Scholar] [CrossRef]
- Jackson, J.G.; O’Donnell, J.C.; Takano, H.; Coulter, D.A.; Robinson, M.B. Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J. Neurosci. 2014, 34, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Doengi, M.; Hirnet, D.; Coulon, P.; Pape, H.-C.; Deitmer, J.W.; Lohr, C. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 17570. [Google Scholar] [CrossRef] [Green Version]
- Reyes, R.C.; Verkhratsky, A.; Parpura, V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro 2012, 4, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Paluzzi, S.; Alloisio, S.; Zappettini, S.; Milanese, M.; Raiteri, L.; Nobile, M.; Bonanno, G. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J. Neurochem. 2007, 103, 1196–1207. [Google Scholar] [CrossRef]
- Brazhe, A.R.; Verisokin, A.Y.; Verveyko, D.V.; Postnov, D.E. Sodium–calcium exchanger can account for regenerative Ca2+ entry in thin astrocyte processes. Front. Cell. Neurosci. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- Héja, L.; Kardos, J. NCX activity generates spontaneous Ca2+ oscillations in the astrocytic leaflet microdomain. Cell Calcium 2020, 86, 102137. [Google Scholar] [CrossRef]
- Wade, J.J.; Breslin, K.; Wong-Lin, K.F.; Harkin, J.; Flanagan, B.; Van Zalinge, H.; Hall, S.; Dallas, M.; Bithell, A.; Verkhratsky, A.; et al. Calcium microdomain formation at the perisynaptic cradle due to NCX Reversal: A computational study. Front. Cell. Neurosci. 2019, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- MacVicar, B.A. Voltage-dependent calcium channels in glial cells. Science 1984, 226, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Latour, I.; Hamid, J.; Beedle, A.M.; Zamponi, G.W.; MacVicar, B.A. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia 2003, 41, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, T.; Nishizaki, T. Extracellular high K+ stimulates vesicular glutamate release from astrocytes by activating voltage-dependent calcium channels. J. Cell. Physiol. 2010, 225, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Rungta, R.L.; Bernier, L.; Dissing-olesen, L.; Groten, C.J.; Ledue, J.M.; Ko, R.; Drissler, S.; Macvicar, B.A. Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia 2016, 64, 2093–2103. [Google Scholar] [CrossRef]


| Culture Preparation | Pharmacology | Receptor Functionality | Reference |
|---|---|---|---|
| Rat cortical astrocytes 14–21 days in culture | Agonist: Glu, KA NMDA (100 µM) | ✓Kainate/AMPA receptors ✕ NMDARs | Pearce et al., 1986. [113] |
| Rat hippocampal astrocytes 1–3 weeks in culture | Agonist: Glu, QA, KA, Gly, NMDA (100 µM) Blocker: Ca2+-free saline aCSF (EGTA) | ✓Kainate/AMPA receptors ✕ NMDARs | Cornell-Bell et al., 1990. [114] |
| Rat cortical astrocytes 4–9 weeks in culture | Agonist: Glu, KA, QA NMDA (100 µM) Blocker: kynurenic acid, Ca2+-free saline (EGTA) | ✓Kainate/AMPA receptors ✕ NMDARs | Jensen et al., 1990. [115] |
| Rat hippocampal astrocytes 2–4 weeks in culture | Agonist: KA, AMPA, Gly, NMDA (100 µM) | ✕ iGluRs (no Ca2+ permeable forms) | Cai et al., 1997. [118] |
| Rat cerebellar, hippocampal, and cortical astrocytes 10–20-days in culture | Agonist: QA, AMPA Antagonist: CNQX | ✓ AMPARs | Glaum et al., 1990. [122] |
| Rat cortical astrocytes 12–14-days in culture | Agonist: Glu, NMDA (20 µM) Antagonist: MK801, CNQX | ✕ Kainate/AMPA receptors ✓ NMDARs | Jimenez-Blasco et al., 2015. [121] |
| Rat cerebellar astrocytes 4 weeks in culture | Agonist: Glu/Hypoxia Antagonist: CNQX | ✓ AMPARs | Kou et al., 2019. [123] |
| Astrocyte Preparation | iGluR Pharmacology | Receptor Functionality | Reference |
|---|---|---|---|
| Hippocampal slices from 10–13-days-old rats | Bath-applied | ✓ iGluRs (type not specified) | Porter et al., 1996. [92] |
| Hippocampal slices from 8-day-old rats | Bath-applied | ✓ NMDARs | Pasti et al., 1997. [93] |
| Hippocampal slices of 31–38-days-old rats | Bath-applied | ✓AMPARs ✕ NMDARs | Shelton et al., 1999. [127] |
| Cortical slice from 1–4-week-old GFAP-EGFP mice | Patch-applied | ✓ NMDARs | Schipke et al., 2001. [128] |
| Hippocampal slice from 10–18-month-old GFAP-EGFP mice | Patch-applied | ✓ NMDARs | Serrano et al., 2008. [129] |
| Optic nerve isolated from 15–30-day-old- GFAP-EGFP mice | Bath-applied | ✓ AMPARs ✓ NMDARs | Hamilton et al., 2008. [124] |
| Brain slices and acutely isolated cortical astrocytes from 3-month-old GFAP-EGFP mice | Patch-applied | ✓NMDARs | Palygin et al., 2010. [109] |
| Neocortical slice from 1–21-months-old GFAP-EGFP mice | Patch-applied | ✓AMPAR ✓NMDAR | Lalo et al., 2011. [132] |
| Cortical astrocytes isolated from adult GFAP-EGFP mice | Patch-applied | ✓ NMDAR | Palygin et al., 2011. [108] |
| Cortical astrocytes isolated from adult mice | Bath-applied | ✓ NMDAR | Lalo et al., 2014. [130] |
| Brain slices and acutely isolated cortical astrocytes from 35–59-day-old GFAP-EGFP mice | Bath-applied | ✓ NMDARs | Dzamba et al., 2015. [110] |
| Olfactory bulb slice from 14–21-day-old Aldh1l1-eGFP mice | Bath-applied | ✓ AMPARs ✓ NMDARs | Otsu et al., 2015. [28] |
| Somatosensory neocortex slice from 21–30-day-old-rats | Patch-applied | ✓ NMDARs | Mehina et al., 2017. [131] |
| Olfactory bulb slice from 8–12-day-old GFAP-EGFP and GLAST-CreERT2-GCaMP6sfl/fl mice | Bath-applied | ✓ AMPARs | Droste et al., 2017. [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadpour, N.; Kantroo, M.; Stobart, J.L. Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology. Biomolecules 2021, 11, 1467. https://doi.org/10.3390/biom11101467
Ahmadpour N, Kantroo M, Stobart JL. Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology. Biomolecules. 2021; 11(10):1467. https://doi.org/10.3390/biom11101467
Chicago/Turabian StyleAhmadpour, Noushin, Meher Kantroo, and Jillian L. Stobart. 2021. "Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology" Biomolecules 11, no. 10: 1467. https://doi.org/10.3390/biom11101467
APA StyleAhmadpour, N., Kantroo, M., & Stobart, J. L. (2021). Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology. Biomolecules, 11(10), 1467. https://doi.org/10.3390/biom11101467






