Use of Alternative Gelling Agents Reveals the Role of Rhamnolipids in Pseudomonas aeruginosa Surface Motility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture
2.2. Swarming Motility Assay
2.3. Construction of ΔfliC Mutant
2.4. Construction of rhlC- Mutant
3. Results and Discussion
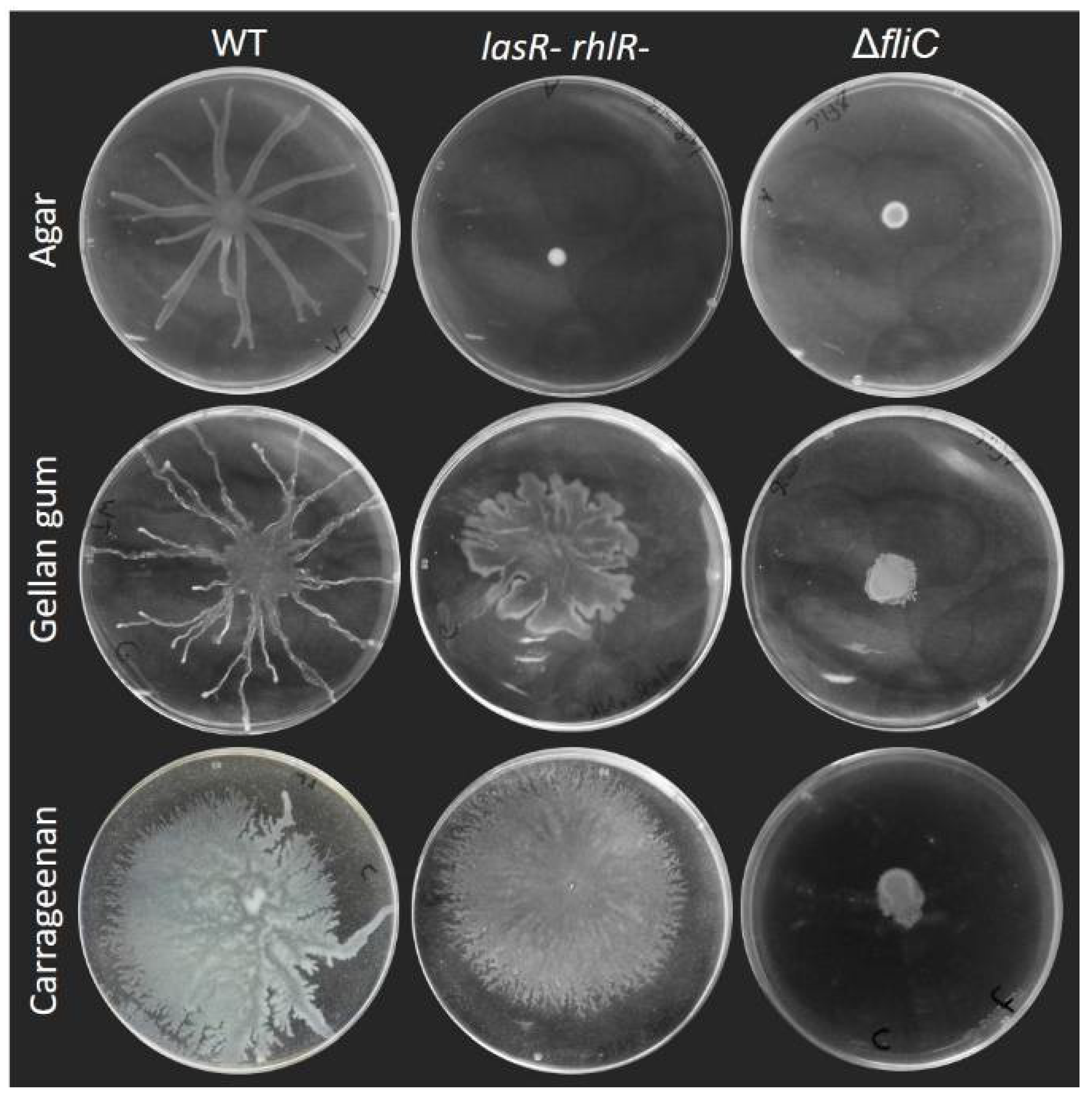
3.1. Surface Motility Pattern Is Affected by the Type of Gelling Agent Used
3.2. Rhamnolipids Are Not Required for Motility on Alternative Gelling Agents but Still Contribute to the Motility Pattern
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valentini, M.; Gonzalez, D.; Mavridou, D.A.; Filloux, A. Lifestyle transitions and adaptive pathogenesis of Pseudomonas aeruginosa. Curr. Opin. Microbiol. 2018, 41, 15–20. [Google Scholar] [CrossRef]
- Henrichsen, J. Bacterial surface translocation: A survey and a classification. Bacteriol. Rev. 1972, 36, 478–503. [Google Scholar] [CrossRef]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and Urinary Tract Infections. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julkowska, D.; Obuchowski, M.; Holland, I.B.; Seror, S.J. Branched swarming patterns on a synthetic medium formed by wild-type Bacillus subtilis strain 3610: Detection of different cellular morphologies and constellations of cells as the complex architecture develops. Microbiology 2004, 150, 1839–1849. [Google Scholar] [CrossRef] [Green Version]
- Kearns, D.B.; Chu, F.; Rudner, R.; Losick, R. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol. Microbiol. 2004, 52, 357–369. [Google Scholar] [CrossRef]
- Matsuyama, T.; Kaneda, K.; Ishizuka, I.; Toida, T.; Yano, I. Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea. J. Bacteriol. 1990, 172, 3015–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Déziel, E.; Lépine, F.; Milot, S.; Villemur, R. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology 2003, 149, 2005–2013. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, J.; Richardson, A.P.; Lépine, F.; Déziel, E. Self-produced extracellular stimuli modulate the Pseudomonas aeruginosa swarming motility behaviour. Environ. Microbiol. 2007, 9, 2622–2630. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, N.C.; Shanks, R.M.; O’Toole, G.A. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J. Bacteriol. 2005, 187, 7351–7361. [Google Scholar] [CrossRef] [Green Version]
- Ochsner, U.A.; Reiser, J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1995, 92, 6424–6428. [Google Scholar] [CrossRef] [Green Version]
- Overhage, J.; Bains, M.; Brazas, M.D.; Hancock, R.E. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 2008, 190, 2671–2679. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.; Tremblay, J.; Déziel, E. Swarming motility: A multicellular behaviour conferring antimicrobial resistance. Environ. Microbiol. 2009, 11, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Burrows, L.L. Pseudomonas aeruginosa twitching motility: Type IV pili in action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef] [Green Version]
- Murray, T.S.; Kazmierczak, B.I. Pseudomonas aeruginosa exhibits sliding motility in the absence of type IV pili and flagella. J. Bacteriol. 2008, 190, 2700–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, A.T.; Parayno, A.; Hancock, R.E. Mucin promotes rapid surface motility in Pseudomonas aeruginosa. mBio 2012, 3, e00073-12. [Google Scholar] [CrossRef] [Green Version]
- Sun, E.; Liu, S.; Hancock, R.E.W. Surfing Motility: A Conserved yet Diverse Adaptation among Motile Bacteria. J. Bacteriol. 2018, 200, e00394-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, W.J.; Chang, Y.K.; Hong, S.K. Agar degradation by microorganisms and agar-degrading enzymes. Appl. Microbiol. Biotechnol. 2012, 94, 917–930. [Google Scholar] [CrossRef]
- Das, N.; Tripathi, N.; Basu, S.; Bose, C.; Maitra, S.; Khurana, S. Progress in the development of gelling agents for improved culturability of microorganisms. Front. Microbiol. 2015, 6, 698. [Google Scholar] [CrossRef]
- Van de Velde, F.; Lourenco, N.D.; Pinheiro, H.M.; Bakker, M. Carrageenan: A food-grade and biocompatible support for immobilisation techniques. Adv. Synth. Catal. 2002, 344, 815–835. [Google Scholar] [CrossRef]
- Datta, S.; Mody, K.; Gopalsamy, G.; Jha, B. Novel application of κ-carrageenan: As a gelling agent in microbiological media to study biodiversity of extreme alkaliphiles. Carbohydr. Polym. 2011, 85, 465–468. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Childers, S.E.; Vargas, M.; Noll, K.M. Improved Methods for Cultivation of the Extremely Thermophilic Bacterium Thermotoga neapolitana. Appl. Environ. Microbiol. 1992, 58, 3949–3953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Soto, N.; Anyan, M.E.; Mattingly, A.E.; Madukoma, C.S.; Harvey, C.W.; Alber, M.; Déziel, E.; Kearns, D.B.; Shrout, J.D. Preparation, imaging, and quantification of bacterial surface motility assays. J. Vis. Exp. 2015, 98, 52338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, J.; Déziel, E. Improving the reproducibility of Pseudomonas aeruginosa swarming motility assays. J. Basic Microbiol. 2008, 48, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Tang, W.S.; Si, T.; Tang, J.X. Influence of physical effects on the swarming motility of Pseudomonas aeruginosa. Biophys. J. 2017, 112, 1462–1471. [Google Scholar] [CrossRef] [Green Version]
- Hara, S.; Isoda, R.; Tahvanainen, T.; Hashidoko, Y. Trace amounts of furan-2-carboxylic acids determine the quality of solid agar plates for bacterial culture. PLoS ONE 2012, 7, e41142. [Google Scholar] [CrossRef] [Green Version]
- Harshey, R.M.; Matsuyama, T. Dimorphic transition in Escherichia coli and Salmonella typhimurium: Surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. USA 1994, 91, 8631–8635. [Google Scholar] [CrossRef] [Green Version]
- Leech, A.J.; Mattick, J.S. Effect of site-specific mutations in different phosphotransfer domains of the chemosensory protein ChpA on Pseudomonas aeruginosa motility. J. Bacteriol. 2006, 188, 8479–8486. [Google Scholar] [CrossRef] [Green Version]
- Rahme, L.G.; Stevens, E.J.; Wolfort, S.F.; Shao, J.; Tompkins, R.G.; Ausubel, F.M. Common virulence factors for bacterial pathogenicity in plants and animals. Science 1995, 268, 1899–1902. [Google Scholar] [CrossRef] [Green Version]
- Liberati, N.T.; Urbach, J.M.; Miyata, S.; Lee, D.G.; Drenkard, E.; Wu, G.; Villanueva, J.; Wei, T.; Ausubel, F.M. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. USA 2006, 103, 2833–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekimpe, V.; Déziel, E. Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: The transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 2009, 155, 712–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hmelo, L.R.; Borlee, B.R.; Almblad, H.; Love, M.E.; Randall, T.E.; Tseng, B.S.; Lin, C.; Irie, Y.; Storek, K.M.; Yang, J.J.; et al. Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nat. Protoc. 2015, 10, 1820–1841. [Google Scholar] [CrossRef] [PubMed]
- Jean-Pierre, F.; Tremblay, J.; Déziel, E. Broth versus surface-grown cells: Differential regulation of RsmY/Z Small RNAs in Pseudomonas aeruginosa by the Gac/HptB System. Front. Microbiol. 2016, 7, 2168. [Google Scholar] [CrossRef] [PubMed]
- Giverso, C.; Verani, M.; Ciarletta, P. Emerging morphologies in round bacterial colonies: Comparing volumetric versus chemotactic expansion. Biomech. Model. Mechanobiol. 2016, 15, 643–661. [Google Scholar] [CrossRef] [Green Version]
- Trinschek, S.; John, K.; Thiele, U. Modelling of surfactant-driven front instabilities in spreading bacterial colonies. Soft Matter 2018, 14, 4464–4476. [Google Scholar] [CrossRef] [Green Version]
- Fauvart, M.; Phillips, P.; Bachaspatimayum, D.; Verstraeten, N.; Fransaer, J.; Michiels, J.; Vermant, J. Surface tension gradient control of bacterial swarming in colonies of Pseudomonas aeruginosa. Soft Matter 2012, 8, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Rashid, M.H.; Kornberg, A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2000, 97, 4885–4890. [Google Scholar] [CrossRef] [Green Version]
- Harshey, R.M.; Partridge, J.D. Shelter in a Swarm. J. Mol. Biol. 2015, 427, 3683–3694. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, J.; Déziel, E. Gene expression in Pseudomonas aeruginosa swarming motility. BMC Genom. 2010, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holscher, T.; Kovacs, A.T. Sliding on the surface: Bacterial spreading without an active motor. Environ. Microbiol. 2017, 19, 2537–2545. [Google Scholar] [CrossRef]
- Kollaran, A.M.; Joge, S.; Kotian, H.S.; Badal, D.; Prakash, D.; Mishra, A.; Varma, M.; Singh, V. Context-specific requirement of forty-four two-component loci in Pseudomonas aeruginosa swarming. iScience 2019, 13, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrout, J.D.; Chopp, D.L.; Just, C.L.; Hentzer, M.; Givskov, M.; Parsek, M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006, 62, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Kamatkar, N.G.; Shrout, J.D. Surface hardness impairment of quorum sensing and swarming for Pseudomonas aeruginosa. PLoS ONE 2011, 6, e20888. [Google Scholar] [CrossRef] [Green Version]
- Hara, S.; Hashidoko, Y.; Desyatkin, R.V.; Hatano, R.; Tahara, S. High rate of N2 fixation by East Siberian cryophilic soil bacteria as determined by measuring acetylene reduction in nitrogen-poor medium solidified with gellan gum. Appl. Environ. Microbiol. 2009, 75, 2811–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyonyo, T.; Shinkai, T.; Tajima, A.; Mitsumori, M. Effect of media composition, including gelling agents, on isolation of previously uncultured rumen bacteria. Lett. Appl. Microbiol. 2013, 56, 63–70. [Google Scholar] [CrossRef]
- Stott, M.B.; Crowe, M.A.; Mountain, B.W.; Smirnova, A.V.; Hou, S.; Alam, M.; Dunfield, P.F. Isolation of novel bacteria, including a candidate division, from geothermal soils in New Zealand. Environ. Microbiol. 2008, 10, 2030–2041. [Google Scholar] [CrossRef]
- Tamaki, H.; Hanada, S.; Sekiguchi, Y.; Tanaka, Y.; Kamagata, Y. Effect of gelling agent on colony formation in solid cultivation of microbial community in lake sediment. Environ. Microbiol. 2009, 11, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Sekiguchi, Y.; Hanada, S.; Nakamura, K.; Nomura, N.; Matsumura, M.; Kamagata, Y. Comparative analysis of bacterial diversity in freshwater sediment of a shallow eutrophic lake by molecular and improved cultivation-based techniques. Appl. Environ. Microbiol. 2005, 71, 2162–2169. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Feng, J.Q.; Zhou, L.; Mbadinga, S.M.; Gu, J.D.; Mu, B.Z. Characterization of bacterial composition and diversity in a long-term petroleum contaminated soil and isolation of high-efficiency alkane-degrading strains using an improved medium. World J. Microbiol. Biotechnol. 2018, 34, 34. [Google Scholar] [CrossRef]
- Nishizuka, H.; Hashidoko, Y. Comparison of Nostocean hormogonium induction and its motility on solid plates between agar and gellan gum at varying gel matrix concentrations. Biosci. Biotechnol. Biochem. 2018, 82, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottcher, T.; Elliott, H.L.; Clardy, J. Dynamics of snake-like swarming behavior of Vibrio alginolyticus. Biophys. J. 2016, 110, 981–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Strain | Stock # | Genotype | Reference |
|---|---|---|---|
| Pseudomonas aeruginosa | |||
| PA14 WT | ED14 | UCBPP-PA14, parental wild-type strain | [30] |
| PA14 rhlA- | ED1 | PA14 rhlA::Mar2xT7 | [31] |
| PA14 ΔfliC | ED3956 | PA14 ΔfliC | This study |
| PA14 rhlB- | ED286 | PA14 rhlB::Mar2xT7 | [31] |
| PA14 rhlC- | ED777 | PA14 rhlC::tetR | This study |
| PA14 lasR- rhlR- | ED266 | PA14 lasR::GmR rhlR::tetR | [32] |
| Escherichia coli | |||
| S17.1 (pEX18Gm-ΔfliC) | ED3955 | E. coli strain with suicide vector pEX18Gm-ΔfliC for allelic exchange deletion of fliC | [15] |
| SM10 (pNTPS138-rhlC::tetR) | ED1271 | E coli strain with suicide vector to insert a tetracycline resistance cassette into rhlC by allelic exchange. | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, C.D.; Déziel, E. Use of Alternative Gelling Agents Reveals the Role of Rhamnolipids in Pseudomonas aeruginosa Surface Motility. Biomolecules 2021, 11, 1468. https://doi.org/10.3390/biom11101468
Morin CD, Déziel E. Use of Alternative Gelling Agents Reveals the Role of Rhamnolipids in Pseudomonas aeruginosa Surface Motility. Biomolecules. 2021; 11(10):1468. https://doi.org/10.3390/biom11101468
Chicago/Turabian StyleMorin, Charles D., and Eric Déziel. 2021. "Use of Alternative Gelling Agents Reveals the Role of Rhamnolipids in Pseudomonas aeruginosa Surface Motility" Biomolecules 11, no. 10: 1468. https://doi.org/10.3390/biom11101468
APA StyleMorin, C. D., & Déziel, E. (2021). Use of Alternative Gelling Agents Reveals the Role of Rhamnolipids in Pseudomonas aeruginosa Surface Motility. Biomolecules, 11(10), 1468. https://doi.org/10.3390/biom11101468







