A Scoping Review of the Role of Metalloproteinases in the Pathogenesis of Autoimmune Pemphigus and Pemphigoid
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Selection, Collection and Synthesis
3. Results
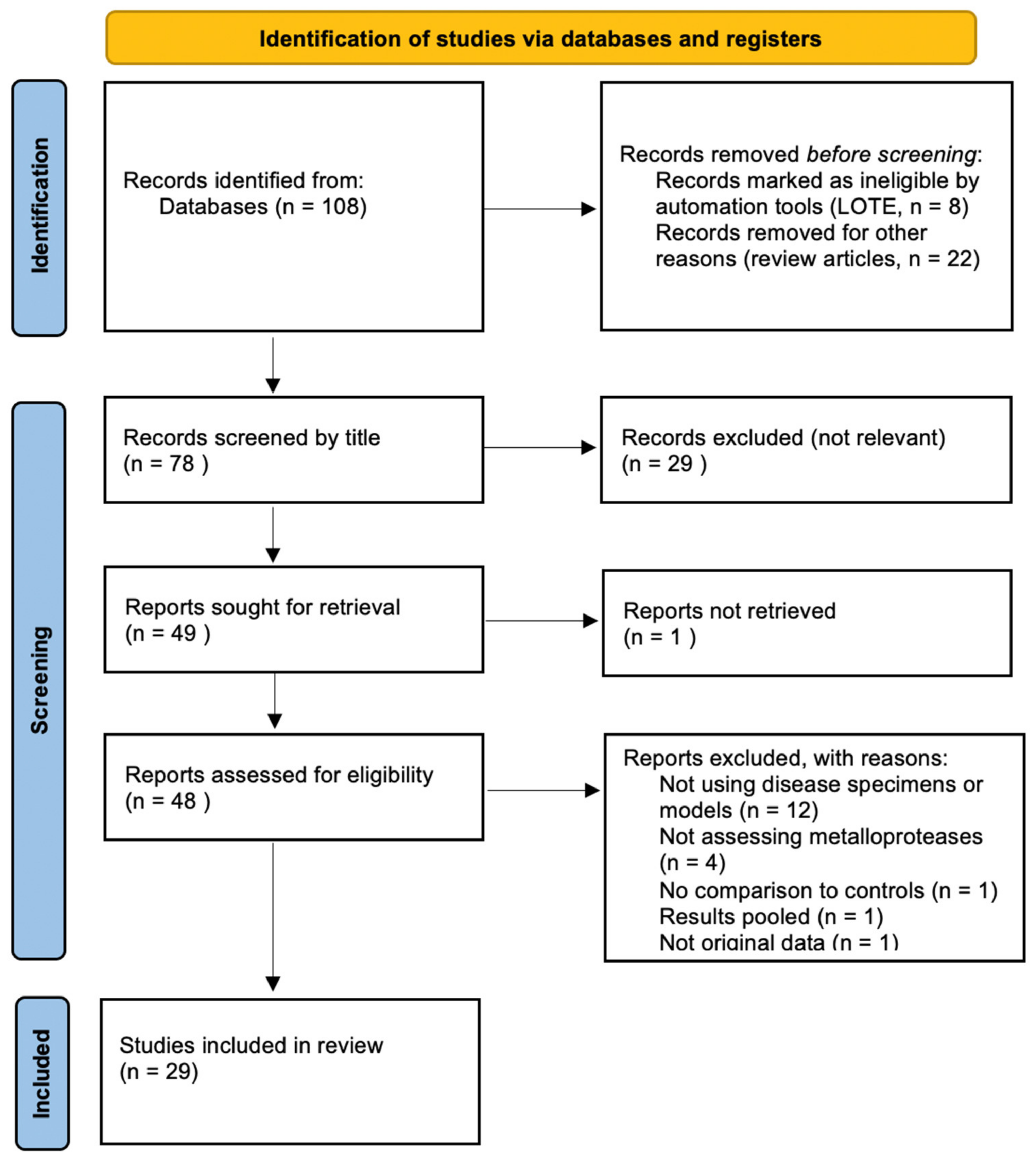
3.1. Overview of the Search Process
3.2. Summary of Findings
| Ref. | First Author, Year | Type | Disease | Target |
|---|---|---|---|---|
| [18] | Woodward et al., 2020 | Human | OCP | MMP9 |
| [19] | Le Jan et al., 2019 | In vitro | BP | MMP9 |
| [20] | Riani et al., 2019 | In vitro, human | BP | MMP9 |
| [21] | Ivars et al., 2020 | In vivo | PV | ADAM10 |
| [22] | de Graauw et al., 2018 | In vitro | BP | MMP2/9 |
| [23] | Shen et al., 2018 | Human | BP | ADAM10 |
| [24] | Riani et al., 2017 | In vitro | BP | MMP9 |
| [25] | Fujimura et al., 2017 | Human | PV, BP | MMP12 |
| [26] | Zebrowska et al., 2014 | Human | BP, DH | MMP9 |
| [27] | Massie et al., 2015 | In vitro | OCP | MMP2/9 |
| [28] | Le Jan et al., 2014 | In vitro, human | BP | MMP9 |
| [29] | Arafat et al., 2014 | Human | BP | MMP8, MMP9, TIMP1 |
| [30] | Zebrowska et al., 2012 | Human | BP, DH | ADAM17 |
| [31] | Oswald et al., 2012 | In vitro | BP | MMP9 |
| [32] | Lin et al., 2011 | In vitro, in vivo | BP | MMP9 |
| [33] | Chan et al., 2011 | Human | OCP | MMP9 |
| [34] | Saw et al., 2011 | In vitro | OCP | MMP3/8/13 |
| [35] | Zebrowska et al., 2009 | Human | BP, DH | ADAM8/15/17 |
| [36] | Cirillo et al., 2007 | In vitro, in vivo | PV | TIMP3, ADAM5, MMP9 |
| [37] | Niimi et al., 2006 | Human | BP | MMP2/9/13 |
| [38] | Liu et al., 2005 | In vitro, in vivo | BP | MMP3/9 |
| [39] | Shimanovich et al., 2004 | In vitro | BP, EBA | MMP9 |
| [40] | Verraes et al., 2001 | In vitro, human | BP | MMP2/9, TIMP1 |
| [41] | Liu et al., 2000 | In vitro, in vivo | BP | MMP9 |
| [42] | Liu et al., 1998 | In vivo | BP | MMP9 |
| [43] | Saarialho-Kere et al., 1995 | Human | EB, PV, BP | MMP1 |
| [44] | Ståhle-Bäckdahl et al., 1994 | In vitro, human | BP | MMP9 |
| [45] | Oikarinen et al., 1993 | Human | BP | MMP2/9 |
| [46] | Oikarinen et al., 1983 | Human | BP, DH, PV | |
4. Discussion
4.1. MMP-9 and Pemphigoid
4.2. Metalloproteinases in Pemphigus
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lever, W.F. Pemphigus. Medicine 1953, 32, 1–123. [Google Scholar]
- Schmidt, E.; Kasperkiewicz, M.; Joly, P. Pemphigus. Lancet 2019, 394, 882–894. [Google Scholar] [CrossRef]
- Bystryn, J.C.; Rudolph, J.L. Pemphigus. Lancet 2005, 366, 61–73. [Google Scholar] [CrossRef]
- Schmidt, E.; Zillikens, D. Pemphigoid diseases. Lancet 2013, 381, 320–332. [Google Scholar] [CrossRef]
- Genovese, G.; Di Zenzo, G.; Cozzani, E.; Berti, E.; Cugno, M.; Marzano, A.V. New Insights Into the Pathogenesis of Bullous Pemphigoid: 2019 Update. Front Immunol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Walter, E.; Vielmuth, F.; Rotkopf, L.; Sárdy, M.; Horváth, O.N.; Goebeler, M.; Schmidt, E.; Eming, R.; Hertl, M.; Spindler, V.; et al. Different signaling patterns contribute to loss of keratinocyte cohesion dependent on autoantibody profile in pemphigus. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Chernyavsky, A.; Patel, K.G.; Grando, S.A. Mechanisms of synergy of autoantibodies to M3 muscarinic acetylcholine receptor and secretory pathway Ca2+/Mn2+-ATPase isoform 1 in patients with non-desmoglein pemphigus vulgaris. Int. Immunopharmacol. 2020, 80, 106149. [Google Scholar] [CrossRef]
- Oktem, A.; Hayran, Y.; Uysal, P.İ.; Atılan, A.U.; Yalçın, B. Evaluation of the Importance of Immunological Profile for Pemphigus Vulgaris in the Light of Necessity to Modify Compensation Theory. Acta Dermatovenerol. Croat. 2018, 26, 100–104. [Google Scholar]
- Gualtieri, B.; Marzano, A.V.; Grando, S. Atypical pemphigus: Autoimmunity against desmocollins and other non-desmoglein autoantigens. Ital. J. Dermatol. Venereol. 2020, 156, 134–141. [Google Scholar]
- Kaur, B.; Kerbrat, J.; Kho, J.; Kaler, M.; Kanatsios, S.; Cirillo, N. Mechanism-based therapeutic targets of pemphigus vulgaris: A scoping review of pathogenic intracellular pathways. Exp. Dermatol. 2021, in press. [Google Scholar] [CrossRef]
- Gornowicz-Porowska, J.; Bowszyc-Dmochowska, M.; Dmochowski, M. Autoimmunity-driven enzymatic remodeling of the dermal–epidermal junction in bullous pemphigoid and dermatitis herpetiformis. Autoimmunity 2011, 45, 71–80. [Google Scholar] [CrossRef]
- Cirillo, N.; Dell’Ermo, A.; Gombos, F.; Lanza, A. The specific proteolysis hypothesis of pemphigus: Does the song remain the same? Med. Hypotheses 2008, 70, 333–337. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. [13] Evolutionary families of metallopeptidases. Meth. Enzymol. 1995, 248, 183–228. [Google Scholar] [CrossRef]
- Amar, S.; Minond, D.; Fields, G.B. Clinical Implications of Compounds Designed to Inhibit ECM-Modifying Metalloproteinases. Proteomics 2017, 17, 1600389. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Riihilä, P.; Nissinen, L.; Kähäri, V. Matrix metalloproteinases in keratinocyte carcinomas. Exp. Dermatol. 2020, 30, 50–61. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Woodward, A.M.; Di Zazzo, A.; Bonini, S.; Argüeso, P. Endoplasmic reticulum stress promotes inflammation-mediated proteolytic activity at the ocular surface. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Le Jan, S.; Muller, C.; Plée, J.; Durlach, A.; Bernard, P.; Antonicelli, F. IL-23/IL-17 Axis Activates IL-1β-Associated Inflammasome in Macrophages and Generates an Auto-Inflammatory Response in a Subgroup of Patients With Bullous Pemphigoid. Front Immunol. 2019, 10, 1972. [Google Scholar] [CrossRef]
- Riani, M.; Muller, C.; Bour, C.; Bernard, P.; Antonicelli, F.; Le Jan, S. Blister Fluid Induces MMP-9-Associated M2-Type Macrophages in Bullous Pemphigoid. Front. Immunol. 2019, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Ivars, M.; España, A.; Alzuguren, P.; Pelacho, B.; Lasarte, J.; López-Zabalza, M. The involvement of ADAM 10 in acantholysis in mucocutaneous pemphigus vulgaris depends on the autoantibody profile of each patient. Br. J. Dermatol. 2019, 182, 1194–1204. [Google Scholar] [CrossRef]
- De Graauw, E.; Sitaru, C.; Horn, M.P.; Borradori, L.; Yousefi, S.; Simon, D.; Simon, H.U. Monocytes enhance neutrophil-induced blister formation in an ex vivo model of bullous pemphigoid. Allergy 2018, 73, 1119–1130. [Google Scholar] [CrossRef]
- Shen, S.; Ke, Y.; Dang, E.; Fang, H.; Chang, Y.; Zhang, J.; Zhu, Z.; Shao, S.; Qiao, P.; Zhang, T.; et al. Semaphorin 4D from CD15+ Granulocytes via ADAM10-Induced Cleavage Contributes to Antibody Production in Bullous Pemphigoid. J. Investig. Dermatol. 2018, 138, 588–597. [Google Scholar] [CrossRef]
- Riani, M.; Le Jan, S.; Plée, J.; Durlach, A.; Le Naour, R.; Haegeman, G.; Bernard, P.; Antonicelli, F. Bullous pemphigoid outcome is associated with CXCL10-induced matrix metalloproteinase 9 secretion from monocytes and neutrophils but not lymphocytes. J. Allergy Clin. Immunol. 2017, 139, 863–872. [Google Scholar] [CrossRef]
- Fujimura, T.; Kakizaki, A.; Furudate, S.; Aiba, S. A possible interaction between periostin and CD163(+) skin-resident macro-phages in pemphigus vulgaris and bullous pemphigoid. Exp. Dermatol. 2017, 26, 1193–1198. [Google Scholar] [CrossRef]
- Żebrowska, A.; Wagrowska-Danilewicz, M.; Danilewicz, M.; Stasikowska-Kanicka, O.; Kulczycka-Siennicka, L.; Woźniacka, A.; Waszczykowska, E. Mediators of Mast Cells in Bullous Pemphigoid and Dermatitis Herpetiformis. Mediat. Inflamm. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Massie, I.; Dale, S.B.; Daniels, J.T. Limbal Fibroblasts Maintain Normal Phenotype in 3D RAFT Tissue Equivalents Suggesting Po-tential for Safe Clinical Use in Treatment of Ocular Surface Failure. Tissue Eng. Part C Methods 2015, 21, 576–584. [Google Scholar] [CrossRef]
- Le Jan, S.; Plée, J.; Vallerand, D.; Dupont, A.; Delanez, E.; Durlach, A.; Jackson, P.L.; Blalock, J.E.; Bernard, P.; Antonicelli, F. Innate immune cell-produced IL-17 sustains inflammation in bullous pemphigoid. J. Investig. Dermatol. 2014, 134, 2908–2917. [Google Scholar] [CrossRef]
- Arafat, S.N.; Suelves, A.M.; Spurr-Michaud, S.; Chodosh, J.; Foster, C.S.; Dohlman, C.H.; Gipson, I.K. Neutrophil Collagenase, Gelatinase, and Myeloperoxidase in Tears of Patients with Stevens-Johnson Syndrome and Ocular Cicatricial Pemphigoid. Ophthalmology 2013, 121, 79–87. [Google Scholar] [CrossRef]
- Zebrowska, A.; Wagrowska-Danilewicz, M.; Danilewicz, M.; Sokolowska, M.; Stasikowska-Kawecka, O.; Erkiert-Polguj, A.; Cynkier, A.; Pawliczak, R.; Sysa-Jedrzejowska, A.; Waszczykowska, E. Does Adam17 cause the destruction of anchoring fibers via shedding tumor necrosis factor α in bullous pemphigoid and dermatitis herpetiformis? J. Cutan. Med. Surg. 2012, 16, 149–150. [Google Scholar] [CrossRef]
- Oswald, E.; Sesarman, A.; Franzke, C.W.; Wölfle, U.; Bruckner-Tuderman, L.; Jakob, T.; Martin, S.F.; Sitaru, C. The flavonoid luteolin inhibits Fcγ-dependent respiratory burst in granulocytes, but not skin blistering in a new model of pemphigoid in adult mice. PLoS ONE 2012, 7, e31066. [Google Scholar] [CrossRef]
- Lin, L.; Bankaitis, E.; Heimbach, L.; Li, N.; Abrink, M.; Pejler, G.; An, L.; Diaz, L.; Werb, Z.; Liu, Z. Dual Targets for Mouse Mast Cell Protease-4 in Mediating Tissue Damage in Experimental Bullous Pemphigoid. J. Biol. Chem. 2011, 286, 37358–37367. [Google Scholar] [CrossRef]
- Chan, M.F.; Sack, R.; Quigley, D.A.; Sathe, S.; Vijmasi, T.; Li, S.; Holsclaw, D.; Strauss, E.C.; McNamara, N.A. Membrane Array Analysis of Tear Proteins in Ocular Cicatricial Pemphigoid. Optom. Vis. Sci. 2011, 88, 1005–1009. [Google Scholar] [CrossRef]
- Saw, V.P.; Schmidt, E.; Offiah, I.; Galatowicz, G.; Zillikens, D.; Dart, J.K.; Calder, V.L.; Daniels, J.T. Profibrotic Phenotype of Conjunctival Fibroblasts from Mucous Membrane Pemphigoid. Am. J. Pathol. 2011, 178, 187–197. [Google Scholar] [CrossRef]
- Żebrowska, A.; Wagrowska-Danilewicz, M.; Danilewicz, M.; Wodz, K.; Sokolowska, M.; Erkiert-Polguj, A.; Sysa-Jedrzejowska, A.; Waszczykowska, E.; Pawliczak, R. Expression of selected ADAMs in bullous pemphigoid and dermatitis herpetiformis. J. Dermatol. Sci. 2009, 56, 58–61. [Google Scholar] [CrossRef]
- Cirillo, N.; Lanza, M.; Rossiello, L.; Gombos, F.; Lanza, A. Defining the involvement of proteinases in pemphigus vulgaris: Evi-dence of matrix metalloproteinase-9 overexpression in experimental models of disease. J. Cell. Physiol. 2007, 212, 36–41. [Google Scholar] [CrossRef]
- Niimi, Y.; Pawankar, R.; Kawana, S. Increased Expression of Matrix Metalloproteinase-2, Matrix Metalloproteinase-9 and Matrix Metalloproteinase-13 in Lesional Skin of Bullous Pemphigoid. Int. Arch. Allergy Immunol. 2006, 139, 104–113. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Diaz, L.A.; Shipley, M.; Senior, R.M.; Werb, Z. Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J. Clin. Investig. 2005, 115, 879–887. [Google Scholar] [CrossRef]
- Shimanovich, I.; Mihai, S.; Oostingh, G.J.; Ilenchuk, T.T.; Bröcker, E.-B.; Opdenakker, G.; Zillikens, D.; Sitaru, C. Granulocyte-derived elastase and gelatinase B are required for dermal–epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J. Pathol. 2004, 204, 519–527. [Google Scholar] [CrossRef]
- Verraes, S.; Hornebeck, W.; Bernard, P.; Polette, M.; Borradori, L. Respective contribution of neutrophil elastase and matrix met-alloproteinase 9 in the degradation of BP180 (type XVII collagen) in human bullous pemphigoid. J. Investig. Dermatol. 2001, 117, 1091–1096. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Shapiro, S.D.; Shipley, J.; Twining, S.S.; Diaz, L.A.; Senior, R.M.; Werb, Z. The Serpin α1-Proteinase Inhibitor Is a Critical Substrate for Gelatinase B/MMP-9 In Vivo. Cell 2000, 102, 647–655. [Google Scholar] [CrossRef]
- Liu, Z.; Shipley, J.M.; Vu, T.H.; Zhou, X.; Diaz, L.A.; Werb, Z.; Senior, R.M. Gelatinase B–deficient Mice Are Resistant to Experimental Bullous Pemphigoid. J. Exp. Med. 1998, 188, 475–482. [Google Scholar] [CrossRef]
- Saarialho-Kere, U.K.; Vaalamo, M.; Airola, K.; Niemi, K.-M.; Oikarinen, A.I.; Parks, W.C. Interstitial Collagenase Is Expressed by Keratinocytes That Are Actively Involved in Reepithelialization in Blistering Skin Diseases. J. Investig. Dermatol. 1995, 104, 982–988. [Google Scholar] [CrossRef][Green Version]
- Ståhle-Bäckdahl, M.; Inoue, M.; Guidice, G.J.; Parks, W.C. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. J. Clin. Investig. 1994, 93, 2022–2030. [Google Scholar] [CrossRef]
- Oikarinen, A.; Kylmäniemi, M.; Autio-Harmainen, H.; Autio, P.; Salo, T. Demonstration of 72-kDa and 92-kDa Forms of Type IV Collagenase in Human Skin: Variable Expression in Various Blistering Diseases, Induction During Re-Epithelialization, and Decrease by Topical Glucocorticoids. J. Investig. Dermatol. 1993, 101, 205–210. [Google Scholar] [CrossRef][Green Version]
- Olkarinen, A.I.; Zone, J.J.; Ahmed, A.R.; Kiistala, U.; Uitto, J.; Olkarinen, J.J.Z.A.I. Demonstration of Collagenase and Elastase Activities in the Blister Fluids from Bullous Skin Diseases. Comparison Between Dermatitis Herpetiformis and Bullous Pemphigoid. J. Investig. Dermatol. 1983, 81, 261–266. [Google Scholar] [CrossRef]
- Cancemi, P.; Aiello, A.; Accardi, G.; Caldarella, R.; Candore, G.; Caruso, C.; Ciaccio, M.; Cristaldi, L.; Di Gaudio, F.; Siino, V.; et al. The Role of Matrix Metalloproteinases (MMP-2 and MMP-9) in Ageing and Longevity: Focus on Sicilian Long-Living Individuals (LLIs). Mediat. Inflamm. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochem-istry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Lin, L.; Betsuyaku, T.; Heimbach, L.; Li, N.; Rubenstein, D.; Shapiro, S.D.; Liu, Z. Neutrophil elastase cleaves the murine hemidesmosomal protein BP180/type XVII collagen and generates degradation products that modulate experimental bullous pemphigoid. Matrix Biol. 2012, 31, 38–44. [Google Scholar] [CrossRef]
- Pal-Ghosh, S.; Blanco, T.; Tadvalkar, G.; Pajoohesh-Ganji, A.; Parthasarathy, A.; Zieske, J.; Stepp, M.A. MMP9 cleavage of the β4 integrin ectodomain leads to recurrent epithelial erosions in mice. J. Cell Sci. 2011, 124, 2666–2675. [Google Scholar] [CrossRef]
- Klessner, J.L.; Desai, B.V.; Amargo, E.V.; Getsios, S.; Green, K.J. EGFR and ADAMs Cooperate to Regulate Shedding and Endocytic Trafficking of the Desmosomal Cadherin Desmoglein 2. Mol. Biol. Cell 2009, 20, 328–337. [Google Scholar] [CrossRef]
- Cirillo, N.; Femiano, F.; Gombos, F.; Lanza, A. Metalloproteinase 9 is the outer executioner of desmoglein 3 in apoptotic keratinocytes. Oral Dis. 2006, 13, 341–345. [Google Scholar] [CrossRef]
- Weiske, J.; Schöneberg, T.; Schröder, W.; Hatzfeld, M.; Tauber, R.; Huber, O. The Fate of Desmosomal Proteins in Apoptotic Cells. J. Biol. Chem. 2001, 276, 41175–41181. [Google Scholar] [CrossRef]
- Bech-Serra, J.J.; Santiago-Josefat, B.; Esselens, C.; Saftig, P.; Baselga, J.; Arribas, J.; Canals, F. Proteomic Identification of Desmoglein 2 and Activated Leukocyte Cell Adhesion Molecule as Substrates of ADAM17 and ADAM10 by Difference Gel Electrophoresis. Mol. Cell. Biol. 2006, 26, 5086–5095. [Google Scholar] [CrossRef]
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next generation matrix metalloproteinase inhibitors—Novel strategies bring new pro-spects. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 1927–1939. [Google Scholar] [CrossRef]
- Lenci, E.; Cosottini, L.; Trabocchi, A. Novel matrix metalloproteinase inhibitors: An updated patent review (2014–2020). Expert Opin. Ther. Patents 2021, 31, 509–523. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, N.; Prime, S.S. A Scoping Review of the Role of Metalloproteinases in the Pathogenesis of Autoimmune Pemphigus and Pemphigoid. Biomolecules 2021, 11, 1506. https://doi.org/10.3390/biom11101506
Cirillo N, Prime SS. A Scoping Review of the Role of Metalloproteinases in the Pathogenesis of Autoimmune Pemphigus and Pemphigoid. Biomolecules. 2021; 11(10):1506. https://doi.org/10.3390/biom11101506
Chicago/Turabian StyleCirillo, Nicola, and Stephen S. Prime. 2021. "A Scoping Review of the Role of Metalloproteinases in the Pathogenesis of Autoimmune Pemphigus and Pemphigoid" Biomolecules 11, no. 10: 1506. https://doi.org/10.3390/biom11101506
APA StyleCirillo, N., & Prime, S. S. (2021). A Scoping Review of the Role of Metalloproteinases in the Pathogenesis of Autoimmune Pemphigus and Pemphigoid. Biomolecules, 11(10), 1506. https://doi.org/10.3390/biom11101506







