Molecular Characterization and Defense Functions of the Nile Tilapia (Oreochromis niloticus) DnaJ B9b and DnaJ C3a Genes in Response to Pathogenic Bacteria under High-Temperature Stress Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Cloning and Characterization of Nile Tilapia cDNA Encoding the On-DnaJ B9b and On-DnaJ C3a Genes
2.3. Gene Organization Structural Analysis of the Nile Tilapia DnaJ B9b and DnaJ C3a Genes
2.4. Evolutionary Tree Construction of the DnaJ B9b and DnaJ C3a Genes
2.5. Normal Tissue Distribution of On-DnaJ B9b and On-DnaJ C3a Gene Expression in Healthy Nile Ti-Lapia
2.6. Quantitative Real-Time RT-PCR (qRT-PCR) Analyses of the On-DnaJ B9b and On-DnaJ C3a Genes in Response to Systemic Pathogenic Infection
2.6.1. Preparation of Bacterial Suspension
2.6.2. Experimental Animal Design
2.6.3. qRT-PCR Analysis
2.7. Silencing Analysis and Effects of On-DnaJ B9b and On-DnaJ C3a Gene Knockdown under Normal Conditions
2.7.1. Experimental Design and Double-Stranded RNA (dsRNA) Preparation
2.7.2. dsRNA Delivery and qRT-PCR Analysis for Gene Knockdown of the On-DnaJ B9b and On-DnaJ C3a Genes
2.8. Silencing Analysis and Effects of On-DnaJ B9b and On-DnaJ C3a Gene Knockdown at High Water Temperature
2.9. Effects of On-DnaJ B9b and On-DnaJ C3a Gene Silencing under High-Temperature Stress with S. agalactiae Infection
2.10. Statistical Analysis
3. Results
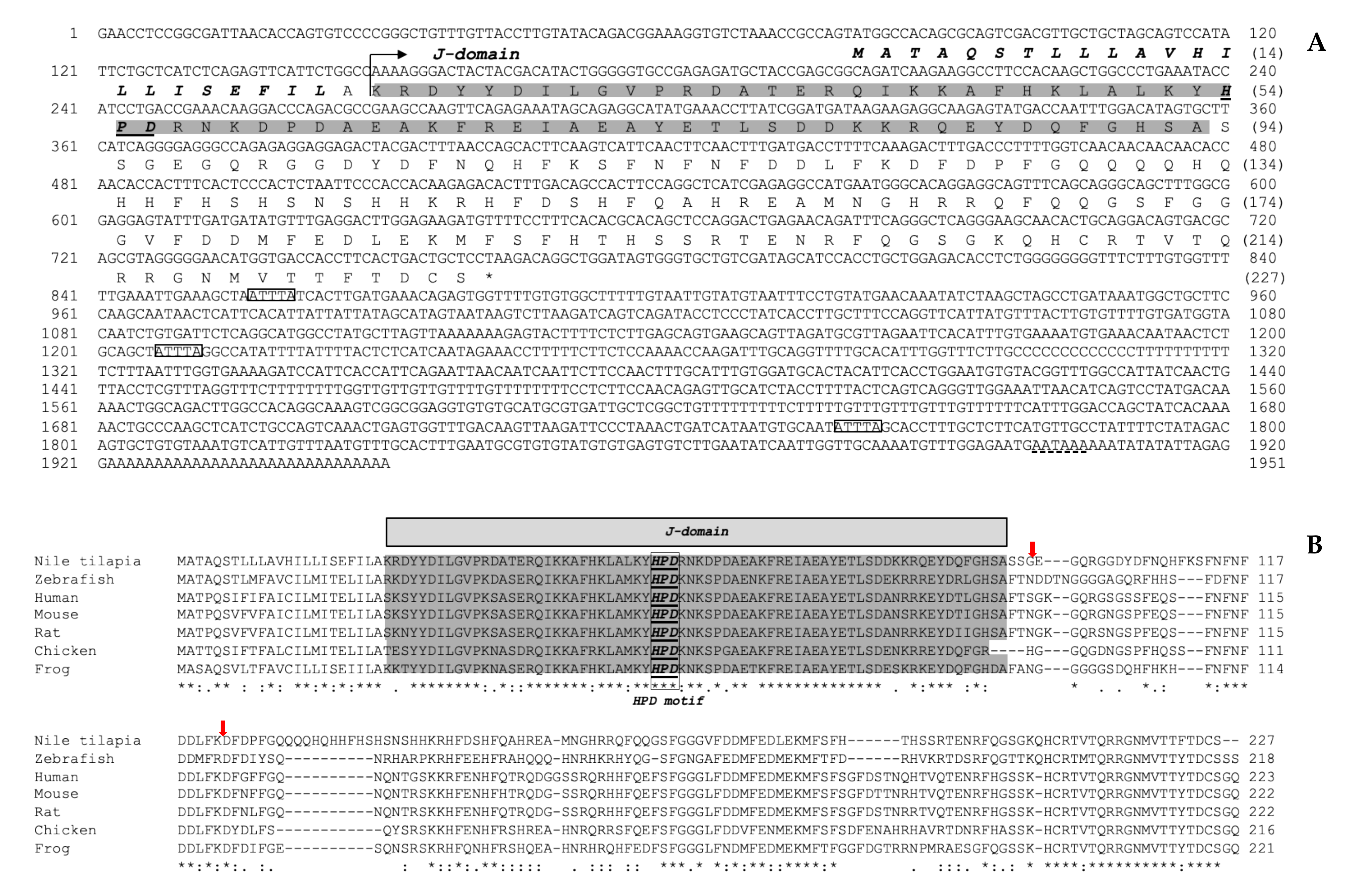
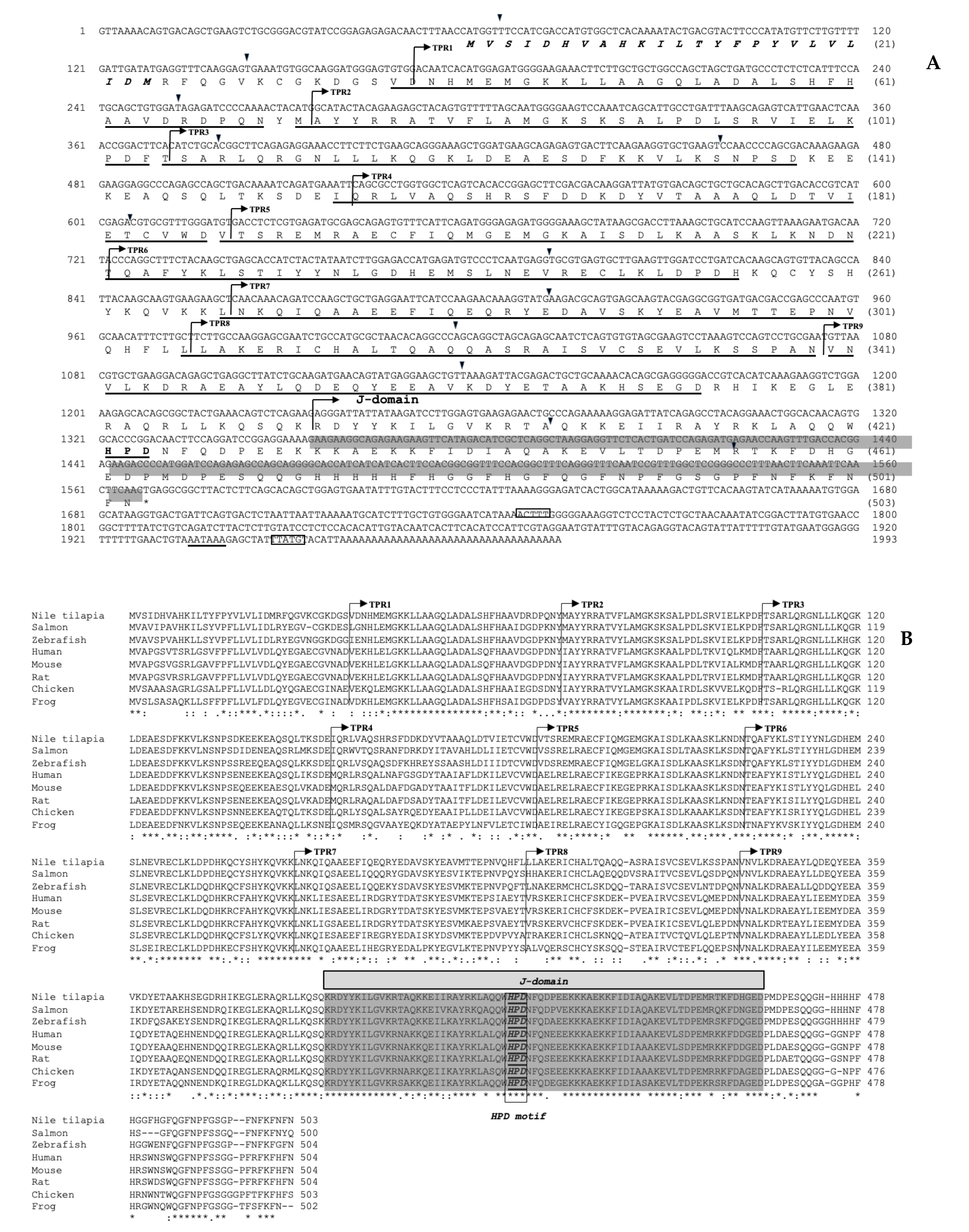
3.1. Structural Characterization of the Nile Tilapia cDNAs Encoding the On-DnaJ B9b and On-DnaJ C3a Genes
3.2. Organization of the On-DnaJ B9b and On-DnaJ C3a Genes
3.3. Evolutionary Analysis of the On-DnaJ B9b and On-DnaJ C3a Genes
3.4. On-DnaJ B9b and On-DnaJ C3a Gene Tissue Distribution in Healthy Nile Tilapia
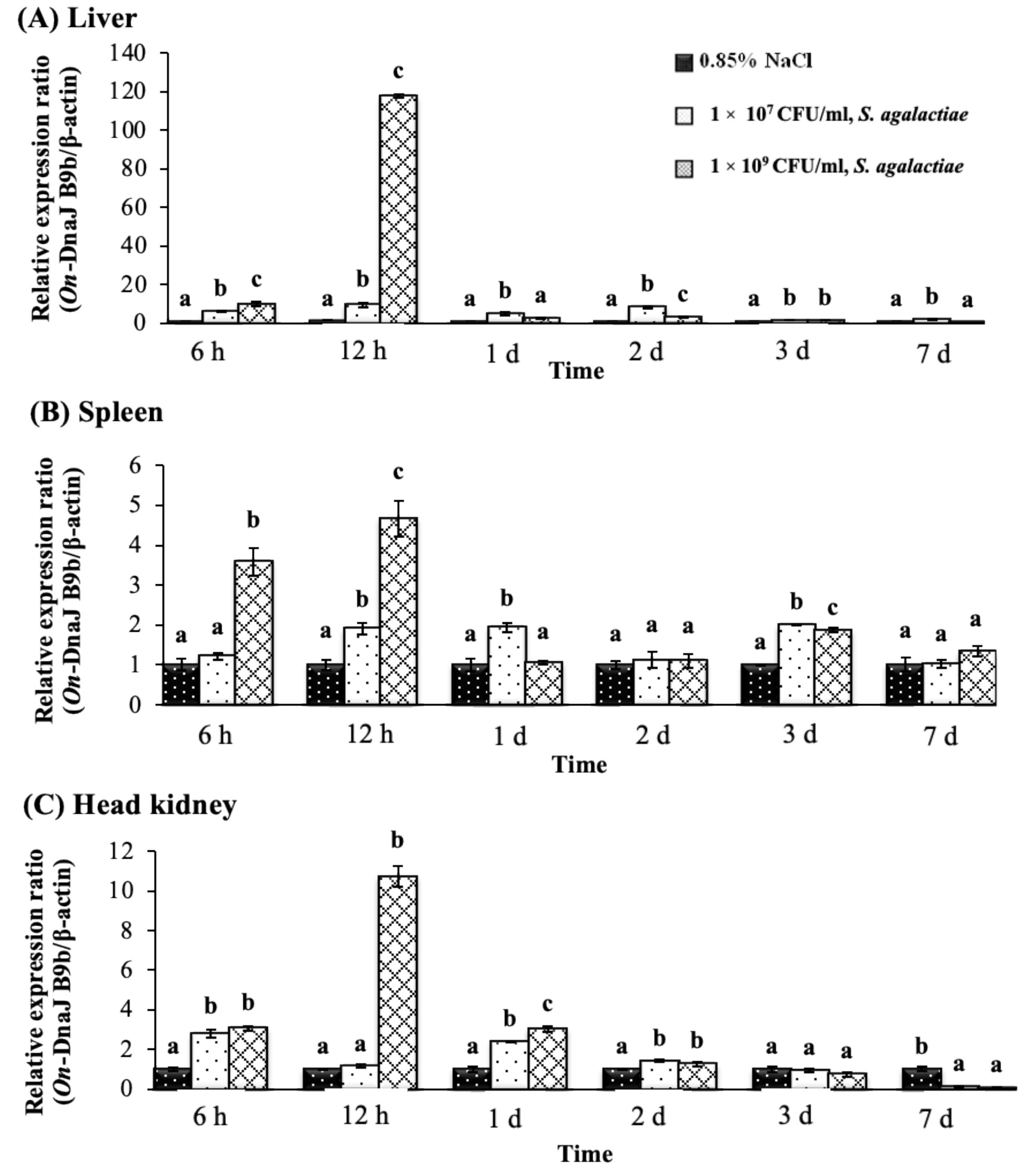
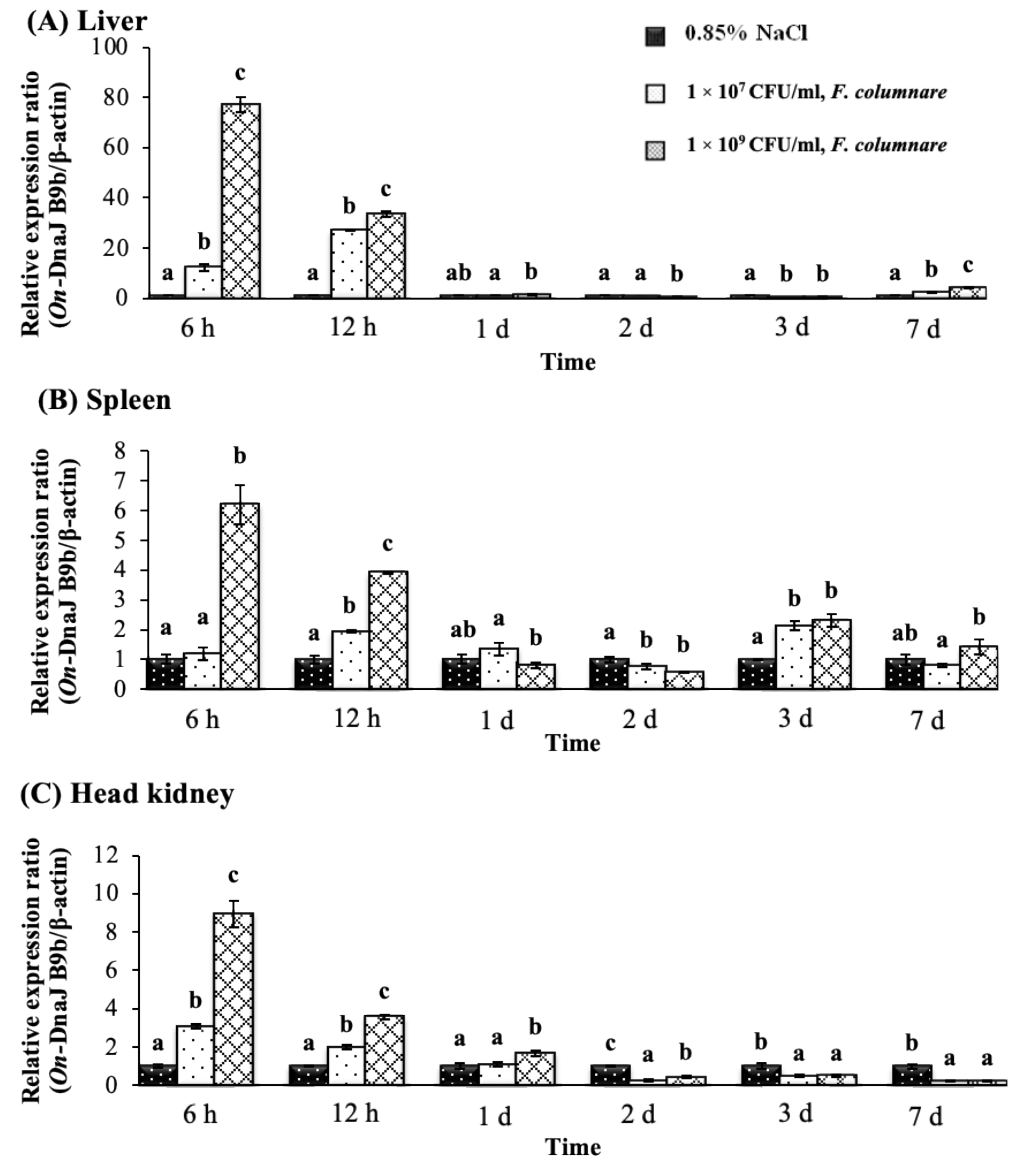
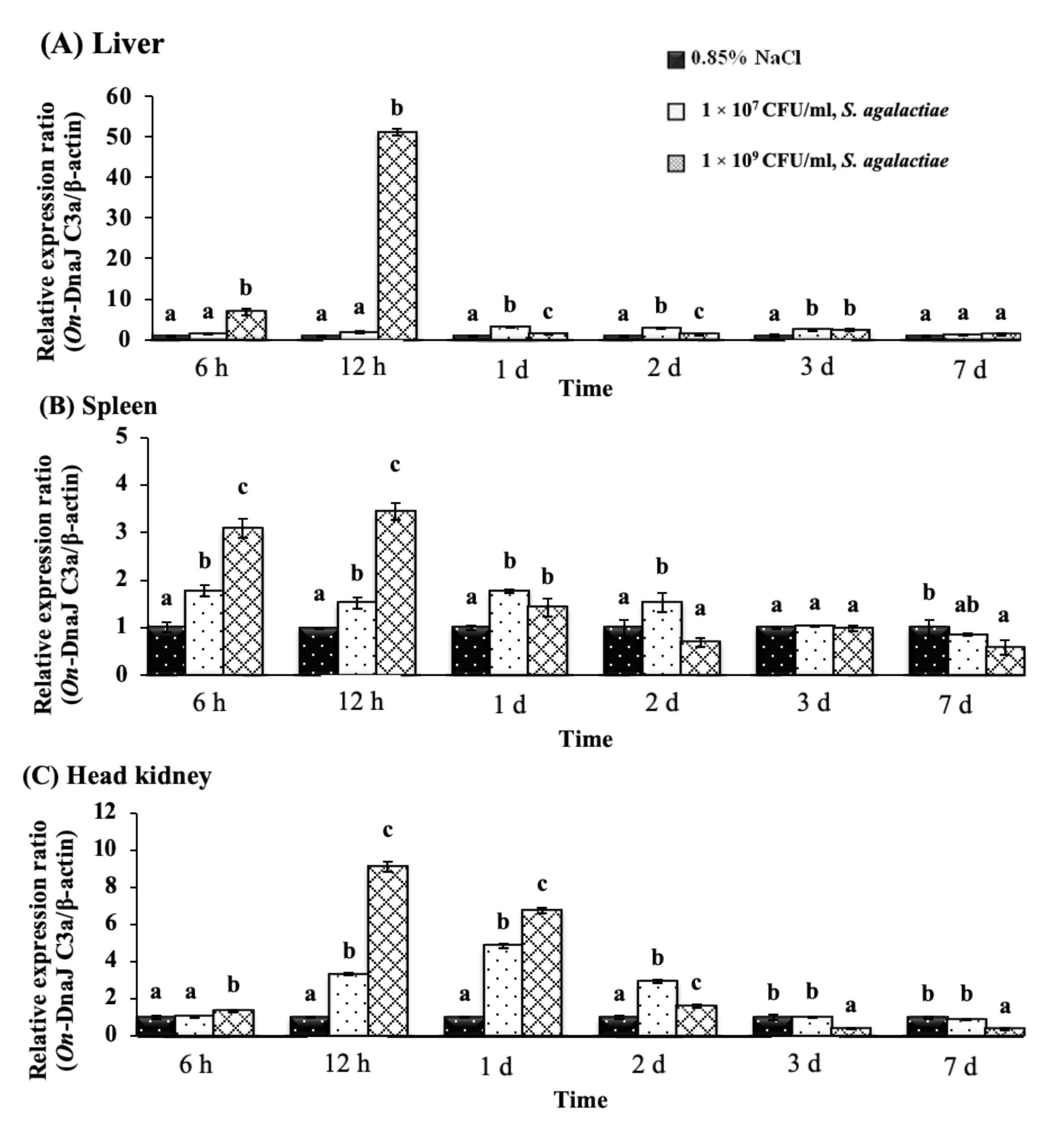
3.5. On-DnaJ B9b and On-DnaJ C3a Gene Expression Levels in Nile Tilapia Infected with Two Pathogenic Bacteria
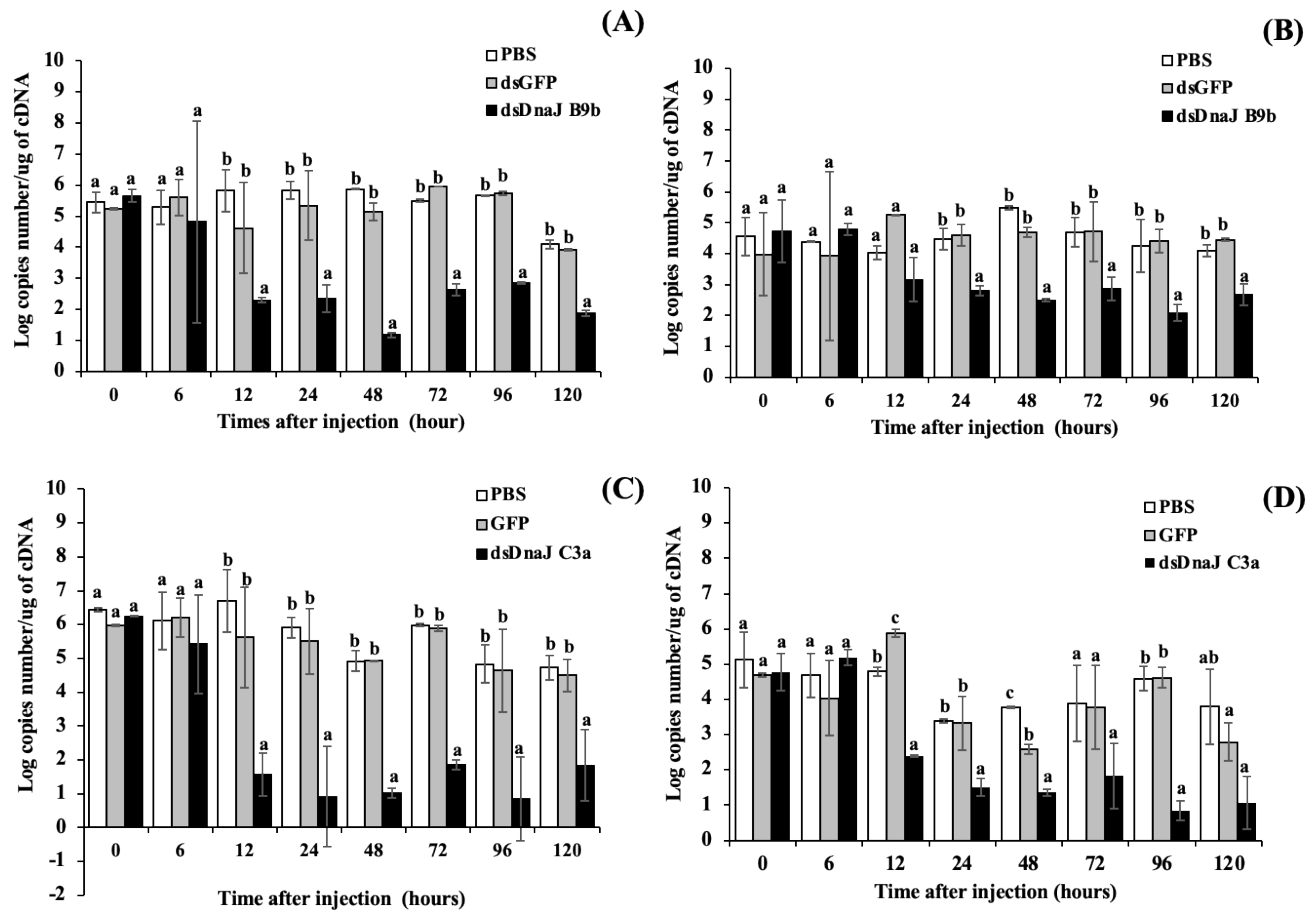
3.6. Gene Silencing Analysis
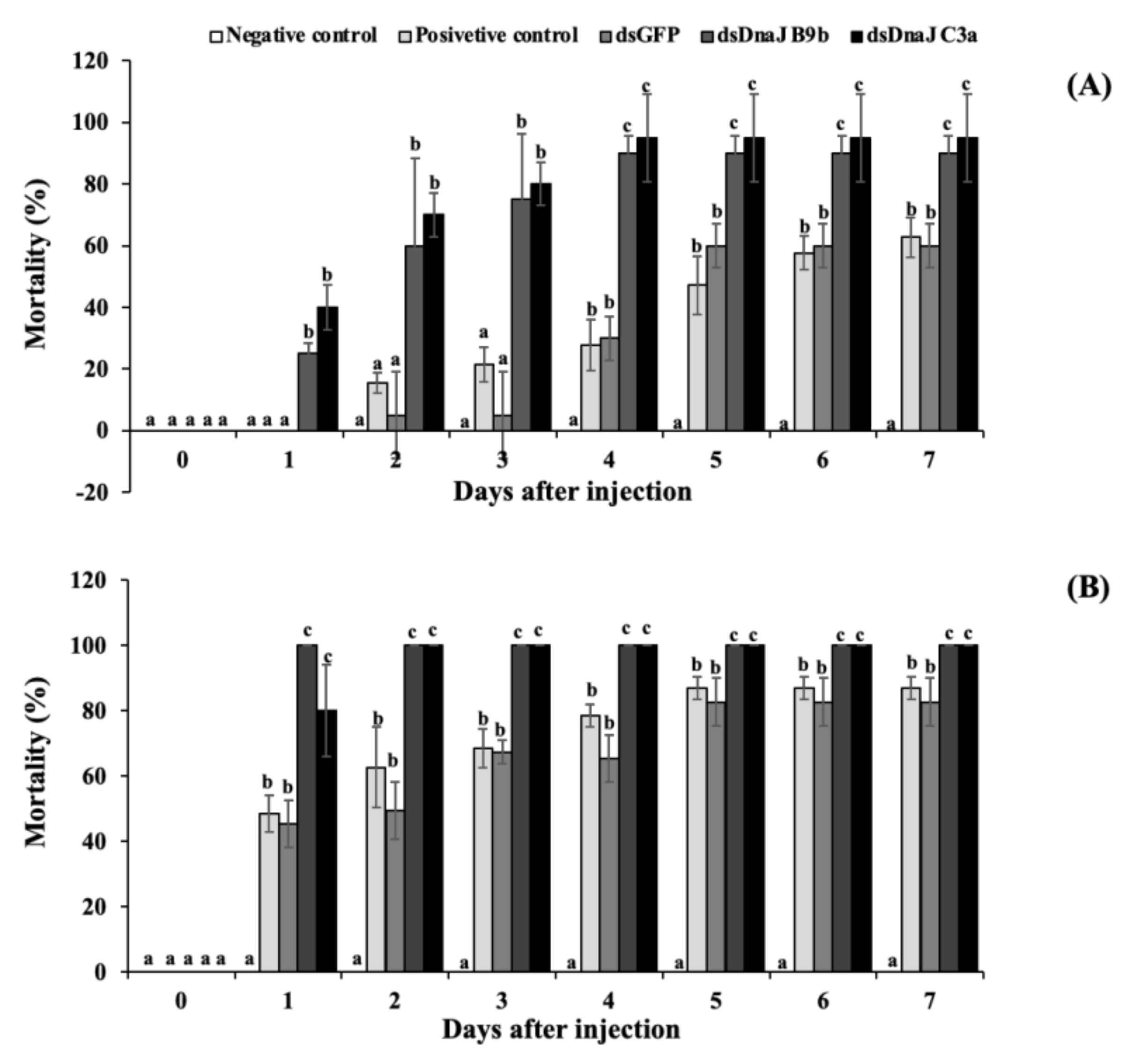
3.7. Effects of Gene Knockdown under High-Temperature Stress and Coinjection with S. agalactiae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Valentin, F.N.; Nascimento, N.F.; Silva, R.C.; Tsuji, E.A.; Paes, M.C.F.; Koberstein, T.C.R.D.; Nakaghi, L.S.O. Maternal age influ-ences on reproductive rates in Nile tilapia (Oreochromis niloticus). R. Bras. Zootec. 2015, 44, 161–163. [Google Scholar] [CrossRef] [Green Version]
- Pretto-Giordano, L.G.; Müller, E.E.; De Freitas, J.C.; Da Silva, V.G. Evaluation on the Pathogenesis of Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus). Braz. Arch. Biol. Technol. 2010, 53, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Amal, M.N.A.; Zamri-Saad, M. Streptococcosis in tilapia (Oreochromis niloticus): A review. Pertanika J. Trop. Agric. Sci. 2011, 34, 195–206. [Google Scholar]
- Mian, G.; Godoy, D.; Leal, C.; Yuhara, T.; Costa, G.; Figueiredo, H. Aspects of the natural history and virulence of S. agalactiae infection in Nile tilapia. Vet. Microbiol. 2009, 136, 180–183. [Google Scholar] [CrossRef]
- Rodkhum, C.; Kayansamruaj, P.; Pirarat, N. Effect of water temperature on susceptibility to Streptococcus agalactiae sero-type Ia infection in Nile tilapia (Oreochromis niloticus). Thai J. Vet. Med. 2011, 41, 309–314. [Google Scholar]
- Durborow, R.M.; Thune, R.L.; Hawke, J.P.; Camus, A.C. Columnaris disease a bacterial infection caused by Flavobacterium columnare. SRAC 1998, 479, 1–4. [Google Scholar]
- Decostere, A.; Haesebrouck, F.; A Devriese, L. Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. J. Clin. Microbiol. 1997, 35, 322–324. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Yang, G.; Zhang, Y.; Yuan, J.; An, L. Generation of Biotechnology-Derived Flavobacterium columnare Ghosts by PhiX174 GeneE-Mediated Inactivation and the Potential as Vaccine Candidates against Infection in Grass Carp. J. Biomed. Biotechnol. 2012, 2012, 760730. [Google Scholar] [CrossRef] [Green Version]
- Pilarski, F.; Rossini, A.J.; Ceccarelli, P.S. Isolation and characterization of Flavobacterium columnare (Bernardet et al. 2002) from four tropical fish species in Brazil. Braz. J. Biol. 2008, 68, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Robert, J. Evolution of heat shock protein and immunity. Dev. Comp. Immunol. 2003, 27, 449–464. [Google Scholar] [CrossRef]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef]
- Ryckaert, J.; Pasmans, F.; Tobback, E.; Duchateau, L.; Decostere, A.; Haesebrouck, F.; Sorgeloos, P.; Bossier, P. Heat shock proteins protect platyfish (Xiphophorus maculatus) from Yersinia ruckeri induced mortality. Fish Shellfish. Immunol. 2010, 28, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Cha, I.S.; Kwon, J.; Bin Park, S.; Bin Jang, H.; Nho, S.W.; Kim, Y.K.; Hikima, J.-I.; Aoki, T.; Jung, T.S. Heat shock protein profiles on the protein and gene expression levels in olive flounder kidney infected with Streptococcus parauberis. Fish Shellfish. Immunol. 2013, 34, 1455–1462. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, J.; Su, L.; Zhou, F.; Zhao, X.; Deng, W.; Zhang, J.; Liu, S.; Wang, W.; Liu, H. Expression of heat shock protein 90 genes during early devel-opment and infection in Megalobrama amblycephala and evidence for adaptive evolution in teleost. Dev. Comp. Immunol. 2013, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and eco-logical physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [Green Version]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat shock protein expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Udono, H. Heat shock protein magic in antigen trafficking within dendritic cells: Implications in antigen cross-presentation in immunity. Acta Med. Okayama 2012, 66, 1–6. [Google Scholar]
- Roy, C.R. Exploitation of the endoplasmic reticulum by bacterial pathogens. Trends Microbiol. 2002, 10, 418–424. [Google Scholar] [CrossRef]
- Zhao, L.; Ackerman, S.L. Endoplasmic reticulum stress in health and disease. Curr. Opin. Cell Biol. 2006, 18, 444–452. [Google Scholar] [CrossRef]
- Komura, T.; Sakai, Y.; Honda, M.; Takamura, T.; Wada, T.; Kaneko, S. ER stress induced impaired TLR signaling and mac-rophage differentiation of human monocytes. Cell Immunol. 2013, 282, 44–52. [Google Scholar] [CrossRef]
- Martinon, F. The endoplasmic reticulum: A sensor of cellular stress that modulates immune responses. Microbes Infect. 2012, 14, 1293–1300. [Google Scholar] [CrossRef]
- Prols, F.; Mayer, M.P.; Renner, O.; Czarnecki, P.G.; Ast, M.; Gassler, C.; Wilting, J.; Kurz, H.; Christ, B. Upregulation of the cochaperone Mdg1 in en-dothelial cells is induced by stress and during in vitro angiogenesis. Exp. Cell Res. 2001, 269, 42–53. [Google Scholar] [CrossRef]
- Shen, Y.; Meunier, L.; Hendershot, L.M. Identification and Characterization of a Novel Endoplasmic Reticulum (ER) DnaJ Homologue, Which Stimulates ATPase Activity of BiP in Vitro and Is Induced by ER Stress. J. Biol. Chem. 2002, 277, 15947–15956. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef]
- Otero, J.H.; Lizák, B.; Hendershot, L.M. Life and death of a BiP substrate. Semin. Cell Dev. Biol. 2010, 21, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, R.J.; Back, S.H.; Song, B.; Han, J.; Hassler, J. The unfolded protein response is required to maintain the integrity of the ER, prevent oxidative stress, and preserve differentiation in β-cells. Diabetes Obes. Metab. 2010, 12, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.W.; Otero, J.H.; Hendershot, L.M.; Snapp, E. ERdj4 protein is a soluble endoplasmic reticulum (ER) DnaJ family pro-tein that interacts with ER-associated degradation machinery. J. Biol. Chem. 2012, 287, 7969–7978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, J.; Petrova, K.; Ron, D.; Sha, B. Crystal Structure of P58(IPK) TPR Fragment Reveals the Mechanism for its Molecular Chaperone Activity in UPR. J. Mol. Biol. 2010, 397, 1307–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, K.; Oyadomari, S.; Hendershot, L.M.; Ron, D. Regulated association of misfolded endoplasmic reticulum lumenal proteins with P58/DnaJ C3a. EMBO J. 2008, 27, 2862–2872. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbour Laboratory Press: Cold Spring Harbour, NY, USA, 2001; Volumes 1–3. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Sha, B. Heat Shock Protein 40: Structural Studies and Their Functional Implications. Protein Pept. Lett. 2009, 16, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.-B.; Shao, Y.-M.; Miao, S.; Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 2006, 63, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Korth, M.J.; Lyons, C.N.; Wambach, M.; Katze, M.G. Cloning, expression, and cellular localization of the oncogenic 58-kDa inhibitor of the RNA-activated human and mouse protein kinase. Gene 1996, 170, 181–188. [Google Scholar] [CrossRef]
- Melville, M.W.; Katze, M.G.; Tan, S.-L. P58IPK, a novel cochaperone containing tetratricopeptide repeats and a J-domain with oncogenic potential. Cell. Mol. Life Sci. 2000, 57, 311–322. [Google Scholar] [CrossRef]
- D’Andrea, L.D.; Regan, L. TPR proteins: The versatile helix. TIBS 2003, 28, 655–662. [Google Scholar] [CrossRef]
- Savant-Bhonsale, S.; Cleveland, D. Evidence for instability of mRNAs containing AUUUA motifs mediated through translation-dependent assembly of a > 20S degradation complex. Genes Dev. 1992, 6, 1927–1939. [Google Scholar] [CrossRef] [Green Version]
- Declercq, A.M.; Haesebrouck, F.; Broeck, W.V.D.; Bossier, P.; Decostere, A. Columnaris disease in fish: A review with emphasis on bacterium-host interactions. Vet. Res. 2013, 44, 27. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Nam, G.H.; Gim, J.A.; Lee, H.E.; Jo, A.; Kim, H.S. Current challenges of Streptococcus infection and effective mo-lecular, cellular, and environmental control methods in aquaculture. Mol. Cells 2018, 41, 495–505. [Google Scholar] [PubMed]
- Cao, S.S.; Zimmermann, E.M.; Chuang, B.; Song, B.; Nwokoye, A.; Wilkinson, J.E.; Eaton, K.A.; Kaufman, R.J. The Unfolded Protein Response and Chemical Chaperones Reduce Protein Misfolding and Colitis in Mice. Gastroenterology 2013, 144, 989–1000. [Google Scholar] [CrossRef] [Green Version]
- Tort, L.; Balasch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Inmunología 2003, 22, 277–286. [Google Scholar]
- Bode, J.G.; Albrecht, U.; Häussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins—Regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell. Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef]
- Kovacevic, N.; Hagen, M.O.; Xie, J.; Belosevic, M. The analysis of the acute phase response during the course of Trypanoso-ma carassii infection in the goldfish (Carassius auratus L.). Dev. Comp. Immunol. 2015, 53, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Baruch, M.; Hertzog, B.B.; Ravins, M.; Anand, A.; Youting, C.C.; Biswas, D.; Tirosh, B.; Ehanski, E. Induction of endoplasmic reticulum stress and unfolded protein response constitutes a pathogenic strategy of group A streptococcus. Front. Cell. Infect. Microbiol. 2014, 4, 105. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhang, W.; Lu, C. Comparative genomics analysis of Streptococcus agalactiae reveals that isolates from cultured ti-lapia in China are closely related to the human strain A909. BMC Genom. 2013, 14, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, M.; Oyadomari, S.; Suga, M.; Mori, M.; Gotoh, T. The ER Stress Pathway Involving CHOP Is Activated in the Lungs of LPS-Treated Mice. J. Biochem. 2005, 138, 501–507. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colaco, C.A.; Bailey, C.R.; Walker, K.B.; Keeble, J. Heat Shock Proteins: Stimulators of Innate and Acquired Immunity. BioMed Res. Int. 2013, 2013, 461230. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Zhang, J.; Li, C.; Yao, J.; Jiang, C.; Li, Y.; Liu, S.; Liu, Z. Genome-wide identification of Hsp40 genes in channel cat-fish and their regulated expression after bacterial infection. PLoS ONE 2014, 9, e115752. [Google Scholar] [CrossRef] [Green Version]
- Faust, O.; Abayev-Avraham, M.; Wentink, A.S.; Maurer, M.; Nillegoda, N.; London, N.; Bukau, B.; Rosenzweig, R. HSP40 proteins use class-specific regulation to drive HSP70 functional diversity nature research. Nature 2020, 587, 1–6. [Google Scholar] [CrossRef]
- Benarroch, E.E. Heat shock proteins: Multiple neuroprotective functions and implications for neurologic disease. Neurology 2011, 76, 660–667. [Google Scholar] [CrossRef]




| Gene Name | Oligonucleotide Primer | Accession Number | Sequence (5’ to 3’) | Amplicon Size | Purpose of Use |
|---|---|---|---|---|---|
| DnaJ subfamily B member 9b (DnaJB9b) | On-DnaJ B9b F | KM_081674 | GGAGACTACGACTTTAACCAGCAC | 160 | Real-time PCR, RACE |
| On-DnaJ B9b R | CCTGGAAGTGGCTGTCAAAGTGTC | 160 | Real-time PCR, RACE | ||
| DnaJB9b_F | GCCGAGAGATGCTACCGAGC | 225 | Gene knockdown | ||
| DnaJB9b_R | GCCGAGAGATGCTACCGAGC | ||||
| DnaJB9bT7_F | GGATCCTAATACGACTCACTATAGGGCCGAGAGATGCTACCGAGC | 250 | Gene knockdown | ||
| DnaJB9bT7_R | GGATCCTAATACGACTCACTATAGGGCCCTCCCCTGATGAAGCAC | ||||
| DnaJ subfamily C member 3 (DnaJC3a) | On-DnaJ C3a F | KM_081675 | CAAGGATTATGTGACAGCTGCTGC | 150 | Real-time PCR, RACE |
| On-DnaJ C3a R | TGGTGCTCAGCTTGTAGAAAGCCT | 150 | Real-time PCR, RACE | ||
| DnaJC3a_F | CAGTGGCACCCGGACAACTT | 225 | Gene knockdown | ||
| DnaJC3a_R | CAGTGGCACCCGGACAACTT | ||||
| DnaJC3aT7_F | GGATCCTAATACGACTCACTATAGGCAGTGGCACCCGGACAACTT | 250 | Gene knockdown | ||
| DnaJC3aT7_R | GGATCCTAATACGACTCACTATAGGCCCGGAGCCAAACGGATTGA | ||||
| Green fluorescence gene | GFP_F | TAATACGACTCACTAAGGGAGACACATGAAGCAGCACGACCT | Gene knockdown | ||
| (GFP) | GFP_R | TAATACGACTCACTATAGGGAGAAGTTCACCTTGATGCCGTTC | |||
| Beta-actin | On-Beta-actin F | XM_003443127 | ACAGGATGCAGAAGGAGATCACAG | 155 | Real-time PCR |
| On-Beta-actin R | GTACTCCTGCTTGCTGATCCACAT | 155 | Real-time PCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisapoome, P.; Thummabancha, K.; Wongpanya, R. Molecular Characterization and Defense Functions of the Nile Tilapia (Oreochromis niloticus) DnaJ B9b and DnaJ C3a Genes in Response to Pathogenic Bacteria under High-Temperature Stress Conditions. Biomolecules 2021, 11, 1509. https://doi.org/10.3390/biom11101509
Srisapoome P, Thummabancha K, Wongpanya R. Molecular Characterization and Defense Functions of the Nile Tilapia (Oreochromis niloticus) DnaJ B9b and DnaJ C3a Genes in Response to Pathogenic Bacteria under High-Temperature Stress Conditions. Biomolecules. 2021; 11(10):1509. https://doi.org/10.3390/biom11101509
Chicago/Turabian StyleSrisapoome, Prapansak, Kubpaphas Thummabancha, and Ratree Wongpanya. 2021. "Molecular Characterization and Defense Functions of the Nile Tilapia (Oreochromis niloticus) DnaJ B9b and DnaJ C3a Genes in Response to Pathogenic Bacteria under High-Temperature Stress Conditions" Biomolecules 11, no. 10: 1509. https://doi.org/10.3390/biom11101509







