Phytochemical Profile, ?-Glucosidase, and ?-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Sample Preparation
2.4. Phytochemical Investigation of C. aurantium Peel Extracts
2.4.1. Qualitative Screening
2.4.2. Quantitative Screening
2.5. Gaz Chromatography Coupled with Mass Spectroscopy (GC-MS) Analysis
2.6. High-Performance Liquid Chromatography Coupled with Diode Array Detector (HPLC-DAD) Analysis
2.7. Inhibition Assay (In Vitro) for Intestinal α-Glucosidase Activity
2.8. Inhibition Assay (In Vitro) for Pancreatic α-Amylase Activity
2.9. Molecular Docking Analysis
2.10. Acute Oral Toxicity of C. aurantium Samples
2.11. Statistical Analysis
3. Results
3.1. Yield of Extractions
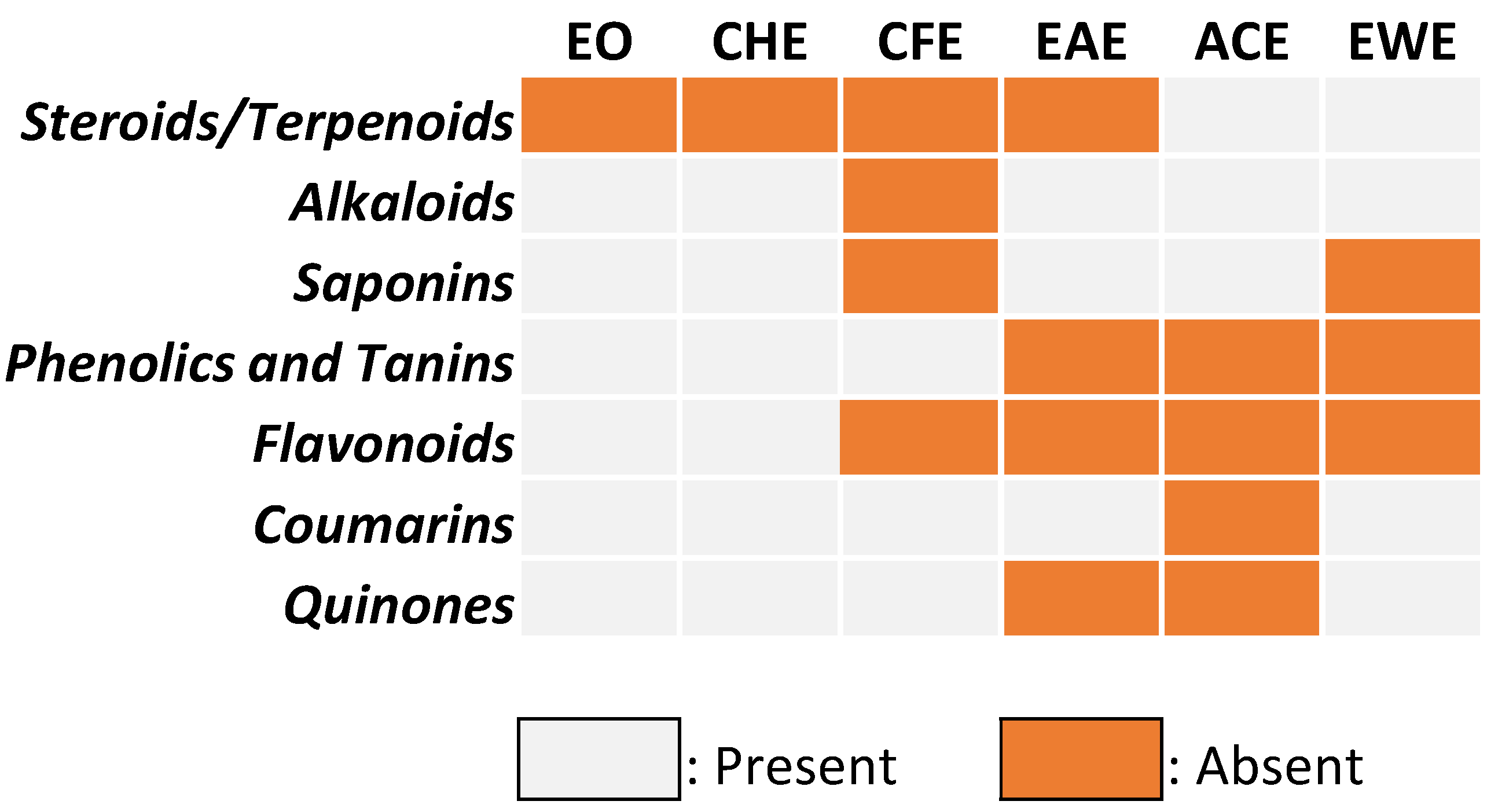
3.2. Qualitative Phytochemical Screening
3.3. Quantitative Phytochemical Screening
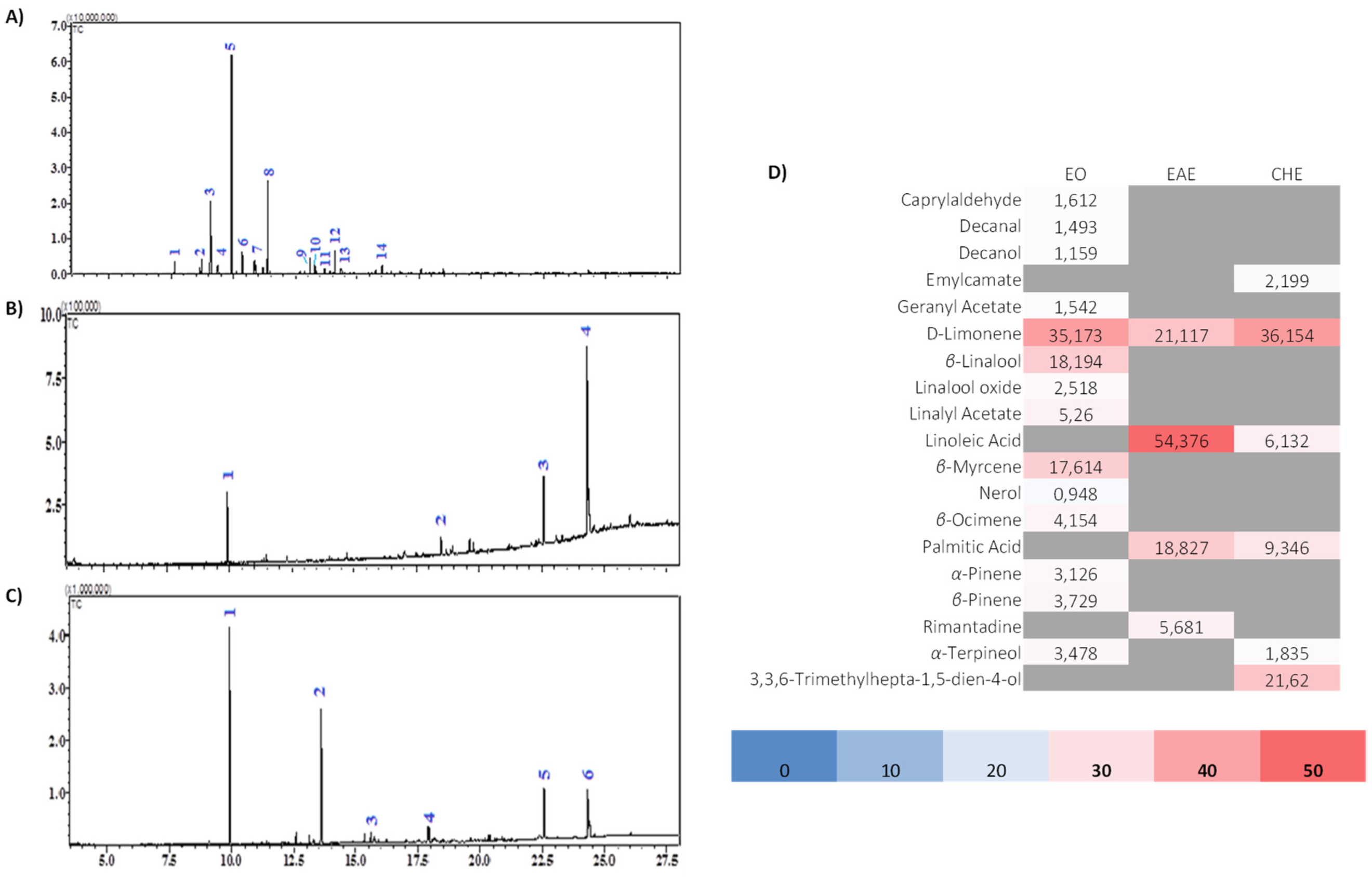
3.4. GC-MS Analysis
3.5. HPLC-DAD Analysis
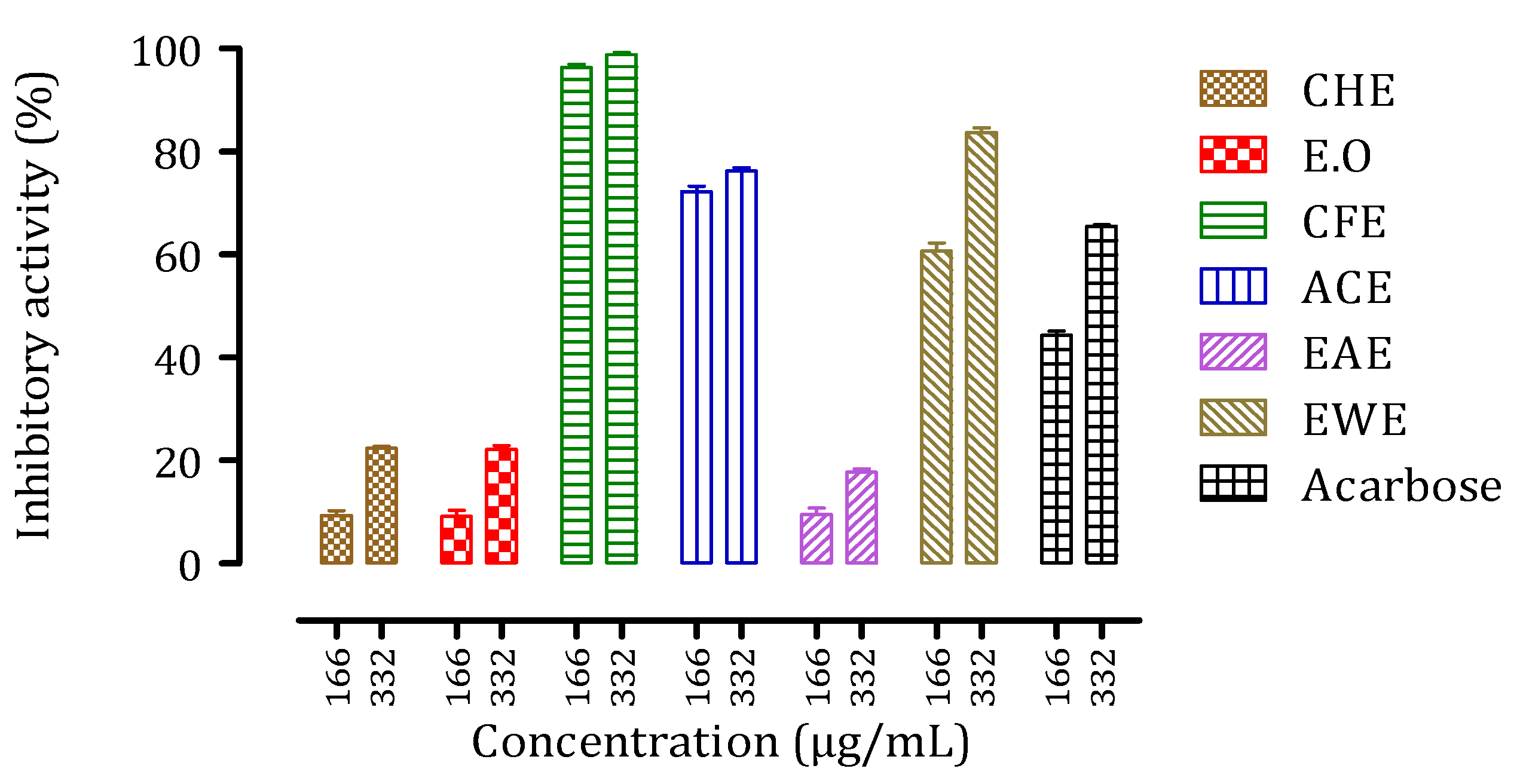
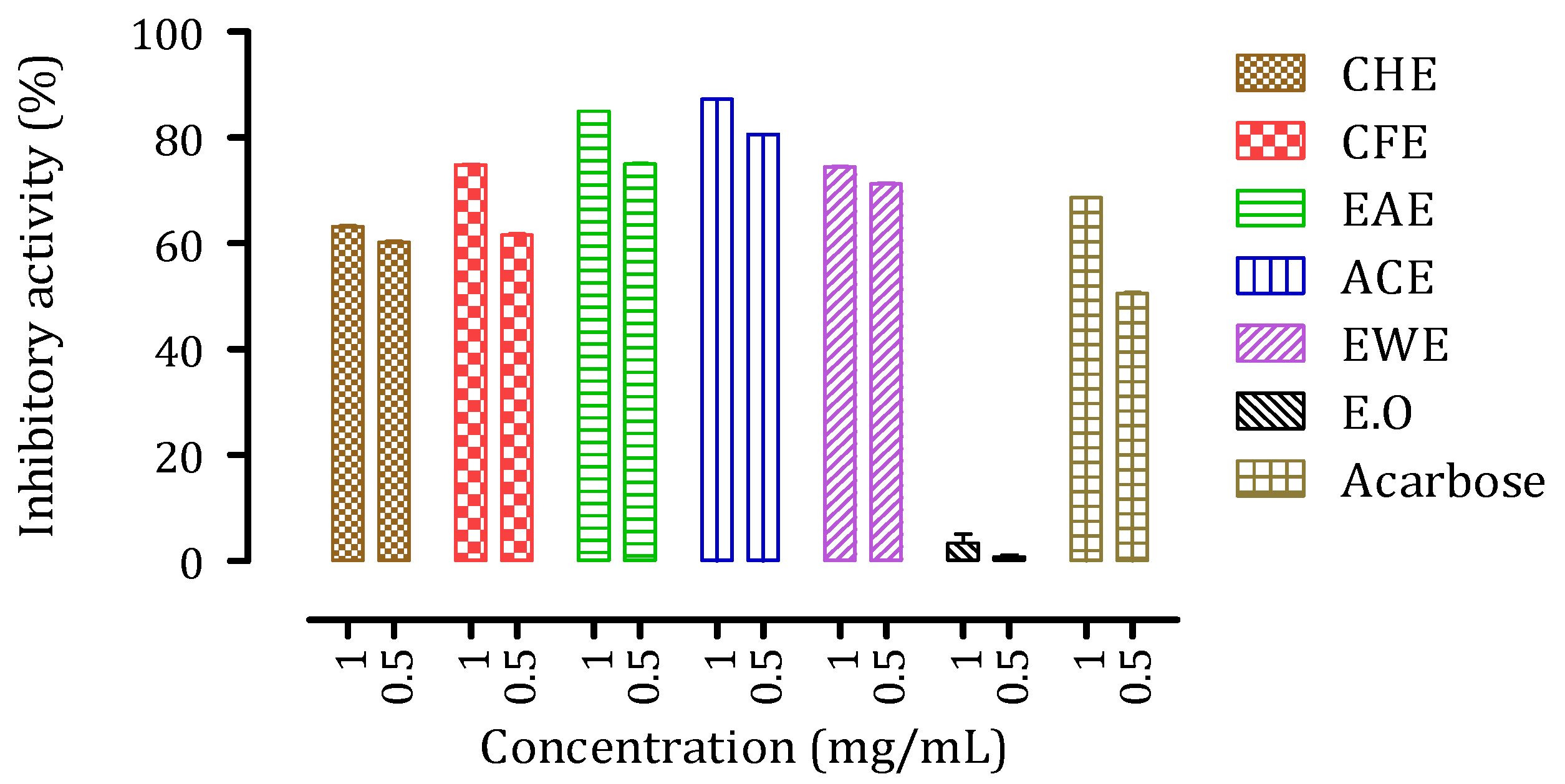
3.6. Inhibitory Activities of Citrus aurantium Peel Extracts against Intestinal α-Glucosidase and Pancreatic α-Amylase
3.7. Acute Toxicity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reuther, W.; Batchelor, L.D.; Webber, H.J. The Citrus Industry. Vol. I.; University of California Press: Berkeley, CA, USA, 1967; ISBN1 9789812707208. ISBN2 9789812569820. [Google Scholar]
- Cerdagne, I. L’oranger amer: Citrus aurantium var.amara link. Diss Thése Dr. Pharm. 2004, 218. [Google Scholar]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An overview on Citrus aurantium L.: Its functions as food ingredient and therapeutic agent. Oxidative Med. Cell. Longev. 2018, 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Güven, C.; Taşkin, E.; Kaya, S.T.; Sevgiler, Y. The potential anti-diabetic effects of some plant species. Nat. Eng. Sci. 2018, 3, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Nabavi, S.M.; Sureda, A.; Mehterov, N.; Gulei, D.; Berindan-Neagoe, I.; Taniguchi, H.; Atanasov, A.G. Therapeutic potential of songorine, a diterpenoid alkaloid of the genus Aconitum. Eur. J. Med. Chem. 2018, 153, 29–33. [Google Scholar] [CrossRef]
- Bendaha, H.; Bouchal, B.; El Mounsi, I.; Salhi, A.; Berrabeh, M.; El Bellaoui, M.; Mimouni, M. Chemical composition, antioxidant, antibacterial and antifungal activities of peel essential oils of citrus aurantium grown in Eastern Morocco. Der Pharm. Lett. 2016, 8, 239–245. [Google Scholar]
- Bendaha, H.; Mimouni, M.; Karrouchi, K.; El Mounsi, I.; Bouchal, B. Byproducts evaluation: Phytochemical investigation and byproducts evaluation: Phytochemical investigation and antioxidant activity of extracts of Eastern Moroccan (Oujda) Citrus. Rev. Microbiol. Ind. Sanit. Et Environn. 2016, 10, 107–127. [Google Scholar]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from Citrus wastes. Foods 2019, 523, 523. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, J.A.S. An overview of Citrus aurantium used in treatment of various diseases. Afr. J. Plant. Sci. 2011, 5, 390–395. [Google Scholar]
- Saunders, A.; Messer, L.H.; Forlenza, G.P. MiniMed 670G hybrid closed loop artificial pancreas system for the treatment of type 1 diabetes mellitus: Overview of its safety and efficacy. Expert Rev. Med. Devices 2019, 16, 845–853. [Google Scholar] [CrossRef]
- Hano, C.; Renouard, S.; Molinié, R.; Corbin, C.; Barakzoy, E.; Doussot, J.; Lamblin, F.; Lainé, E. Flaxseed (Linum usitatissimum L.) extract as well as (+)-secoisolariciresinol diglucoside and its mammalian derivatives are potent inhibitors of α-amylase activity. Bioorganic. Med. Chem. Lett. 2013, 23, 3007–3012. [Google Scholar] [CrossRef]
- Ullah, M.A.; Tungmunnithum, D.; Garros, L.; Drouet, S.; Hano, C.; Abbasi, B.H. Effect of ultraviolet-C radiation and melatonin stress on biosynthesis of antioxidant and antidiabetic metabolites produced in in vitro callus cultures of lepidium sativum L. Int. J. Mol. Sci. 2019, 20, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, M.A.; Tungmunnithum, D.; Garros, L.; Hano, C.; Abbasi, B.H. Monochromatic lights-induced trends in antioxidant and antidiabetic polyphenol accumulation in in vitro callus cultures of Lepidium sativum L. J. Photochem. Photobiol. B Biol. 2019, 196, 111505. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, R.; Ullah, M.A.; Tungmunnithum, D.; Drouet, S.; Shah, M.; Zaeem, A.; Hameed, S.; Hano, C.; Abbasi, B.H. Lights triggered differential accumulation of antioxidant and antidiabetic secondary metabolites in callus culture of Eclipta alba L. PLoS ONE 2020, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tiji, S.; Bouhrim, M.; Addi, M.; Drouet, S.; Lorenzo, J.M.; Hano, C.; Bnouham, M.; Mimouni, M. Linking the phytochemicals and the α-glucosidase and α-amylase enzyme inhibitory effects of nigella sativa seed extracts salima. Foods 2021, 10, 1818. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Abdel Azeem, M.N. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 197–205. [Google Scholar] [CrossRef]
- Alu’Datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Ereifej, K.; Al-Karaki, G.; Al-Duais, M.; Andrade, J.E.; Tranchant, C.C.; Kubow, S.; et al. Profiles of free and bound phenolics extracted from: Citrus fruits and their roles in biological systems: Content, and antioxidant, anti-diabetic and anti-hypertensive properties. Food Funct. 2017, 8, 3187–3197. [Google Scholar] [CrossRef]
- Jaradat, N.; Hussen, F.; Ali, A.A. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of Ephedra alata decne. J. Mater. Environ. Sci. 2015, 6, 1771–1778. [Google Scholar]
- Alilou, H.; Bencharki, B.; Mina, L.; Hassani, I. Screening phytochimique et identification spectroscopique des flavonoïdes d’ Asteriscusgraveolenssubsp. odorus. Afr. Sci. Rev. Int. Des. Sci. Et Technol. 2014, 10, 316–328. [Google Scholar]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Tiji, S.; Benayad, O.; Berrabah, M.; El Mounsi, I.; Mimouni, M. Phytochemical profile and antioxidant activity of Nigella sativa L growing in Morocco. Sci. World J. 2021, 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.D.G.; Markham, K.R. Structure Information from HPLC and On-Line Measured Absorption Spectra: Flavones, Flavonols and Phenolic Acids; Coimbra University Press: Portugal, 2007. [Google Scholar]
- Abid, S.; Lekchiri, A.; Mekhfi, H.; Ziyyat, A.; Legssyer, A.; Aziz, M.; Bnouham, M. Inhibition of α-glucosidase and glucose intestinal absorption by Thymelaea hirsuta fractions. J. Diabetes 2014, 6, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Juvekar, A.R.; Khatri, D.K. α-Glucosidase and α-amylase inhibitory activity of Indigofera cordifolia seeds and leaves extract. Int. J. Pharm. Pharm. Sci. 2014, 6, 152–155. [Google Scholar]
- Daoudi, N.E.; Bouhrim, M.; Ouassou, H.; Legssyer, A.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M. Inhibitory effect of roasted/unroasted Argania spinosa seeds oil on α-glucosidase, α-amylase and intestinal glucose absorption activities. South Afr. J. Bot. 2020, 135, 413–420. [Google Scholar] [CrossRef]
- Tchoumtchoua, J.; Mouchili, O.R.; Ateba, S.B.; Zingue, S.; Halabalaki, M.; Mbanya, J.C.; Skaltsounis, A.L.; Njamen, D. Safety assessment of the methanol extract of the stem bark of Amphimas pterocarpoides harms: Acute and subchronic oral toxicity studies in Wistar rats. Toxicol. Rep. 2014, 1, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Terry, P.; Giovannucci, E.; Michels, K.B.; Bergkvist, L.; Hansen, H.; Holmberg, L.; Wolk, A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J. Natl. Cancer Inst. 2001, 93, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R, S.; Gurunathan, J. Metabolites from the citrus extracts inhibit the activity of selected proteins in Indian Cobra (Naja naja) venom. J. Ethnopharmacol. 2020, 252, 112575. [Google Scholar] [CrossRef]
- Gunwantrao, B.B.; Bhausaheb, S.K.; Ramrao, B.S.; Subhash, K.S. Antimicrobial activity and phytochemical analysis of orange (Citrus aurantium L.) and pineapple (Ananas comosus (L.) Merr.) peel extract. Ann. Phytomedicine 2016, 5, 156–160. [Google Scholar] [CrossRef]
- Costa, C.A.R.A.; Cury, T.C.; Cassettari, B.O.; Takahira, R.K.; Flório, J.C.; Costa, M. Citrus aurantium L. essential oil exhibits anxiolytic-like activity mediated by 5-HT1A-receptors and reduces cholesterol after repeated oral treatment. BMC Complementary Altern. Med. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Teneva, D.; Denkova-Kostova, R.; Goranov, B.; Hristova-Ivanova, Y.; Slavchev, A.; Denkova, Z.; Kostov, G. Chemical composition, antioxidant activity and antimicrobial activity of essential oil from Citrus aurantium L zest against some pathogenic microorganisms. Z. Fur Nat.-Sect. C J. Biosci. 2019, 74, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Essadik, F.Z.; Haida, S.; Kribii, A.; Kribii, A.R.; Ounine, K. Antioxidant activity of Citrus aurantium L. var. amara Peel from western of Morocco, identification of volatile compounds of its essential oil by GC-MS and a preliminary study of their antibacterial activity. Int. J. Innov. Sci. Res. 2015, 16, 425–432. [Google Scholar]
- Sanei-Dehkordi, A.; Sedaghat, M.M.; Vatandoost, H.; Abai, M.R. Chemical compositions of the peel essential oil of Citrus aurantium and its natural larvicidal activity against the malaria vector Anopheles stephensi (Diptera: Culicidae) in comparison with Citrus paradisi. J. Arthropod-Borne Dis. 2016, 10, 577–585. [Google Scholar]
- Abderrezak, M.K.; Abaza, I.; Aburjai, T.; Kabouche, A.; Kabouche, Z. Comparative compositions of essential oils of Citrus aurantium growing in different soils. J. Mater. Environ. Sci. 2014, 5, 1913–1918. [Google Scholar]
- Sandoval-Montemayor, N.E.; García, A.; Elizondo-Treviño, E.; Garza-González, E.; Alvarez, L.; Del Rayo Camacho-Corona, M. Chemical composition of hexane extract of Citrus aurantifolia and anti-Mycobacterium tuberculosis activity of some of its constituents. Molecules 2012, 17, 11173–11184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmah, N.; Suniarti, D.; Margono, A.; Mas’ud, Z.; Bachtiar, E. Identification of active compounds in ethyl acetate, chloroform, and N-hexane extracts from peels of Citrus aurantifolia from Maribaya, West Java, Indonesia. J. Adv. Pharm. Technol. Res. 2020, 11, 107–112. [Google Scholar] [CrossRef]
- Chanthaphon, S.; Chanthachum, S.; Hongpattarakere, T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. Against food-related microorganisms. Songklanakarin J. Sci. Technol. 2008, 30, 125–131. [Google Scholar]
- Hernández-Aquino, E.; Muriel, P. Naringenin and the liver. In Liver Pathophysiology: Therapies and Antioxidants; 2017; pp. 633–651. ISBN 9780128043219. [Google Scholar]
- Jabri Karoui, I.; Marzouk, B. Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xu, X.; Jiang, T.; Wu, K.; Ding, C.; Liu, Z.; Zhang, X.; Yu, T.; Song, C. Citrus aurantium naringenin prevents osteosarcoma progression and recurrence in the patients who underwent osteosarcoma surgery by improving antioxidant capability. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.M.; Guarino, M.P.; Barroso, S.; Gil, M.M. Phytopharmacological strategies in the management of type 2 diabetes mellitus. Foods 2020, 9, 271. [Google Scholar] [CrossRef] [Green Version]
- Kamtekar, S.; Keer, V.; Patil, V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J. Appl. Pharm. Sci. 2014, 4, 61–65. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Sahnoun, M.; Trabelsi, S.; Bejar, S. Citrus flavonoids collectively dominate the α-amylase and α-glucosidase inhibitions. Biologia 2017, 72, 764–773. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, Z.; Duan, W.; Mo, M.; Su, Z.; Bi, Y.; Kong, F. Optimized preparation process for naringenin and evaluation of its antioxidant and α-glucosidase inhibitory activities. J. Food Process. Preserv. 2020, 44, 14931. [Google Scholar] [CrossRef]
- Sharma, M.; Fernandes, J.; Ahirwar, D.; Jain, R. Hypoglycemic and hypolipidimic activity of alcoholic extract of citrus aurantium in normal and alloxan-induced diabetic rats. Pharmacologyonline 2008, 3, 161–171. [Google Scholar]


| Solvent Extract | Yield (%) | Total Phenol Content * | Total Flavonoid Content ** |
|---|---|---|---|
| Cyclohexane (CHE) | 3.56 | – | – |
| Chloroform (CFE) | 3.85 | – | 18.53 ± 0.67 |
| Ethyl acetate (EAE) | 2.40 | 126.46 ± 1.41 | 9.72 ± 0.21 |
| Acetone (ACE) | 4.45 | 125.54 ± 1.92 | 42.96 ± 1.86 |
| Ethanol-water (EWE) | 32.10 | 421.95 ± 5.24 | 188.04 ± 1.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical Profile, ?-Glucosidase, and ?-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules 2021, 11, 1555. https://doi.org/10.3390/biom11111555
Benayad O, Bouhrim M, Tiji S, Kharchoufa L, Addi M, Drouet S, Hano C, Lorenzo JM, Bendaha H, Bnouham M, et al. Phytochemical Profile, ?-Glucosidase, and ?-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules. 2021; 11(11):1555. https://doi.org/10.3390/biom11111555
Chicago/Turabian StyleBenayad, Ouijdane, Mohamed Bouhrim, Salima Tiji, Loubna Kharchoufa, Mohamed Addi, Samantha Drouet, Christophe Hano, Jose Manuel Lorenzo, Hasnae Bendaha, Mohamed Bnouham, and et al. 2021. "Phytochemical Profile, ?-Glucosidase, and ?-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco" Biomolecules 11, no. 11: 1555. https://doi.org/10.3390/biom11111555
APA StyleBenayad, O., Bouhrim, M., Tiji, S., Kharchoufa, L., Addi, M., Drouet, S., Hano, C., Lorenzo, J. M., Bendaha, H., Bnouham, M., & Mimouni, M. (2021). Phytochemical Profile, ?-Glucosidase, and ?-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules, 11(11), 1555. https://doi.org/10.3390/biom11111555








