Effect of Methyl Jasmonate Doped Nanoparticles on Nitrogen Composition of Monastrell Grapes and Wines
Abstract
1. Introduction
2. Materials and Methods
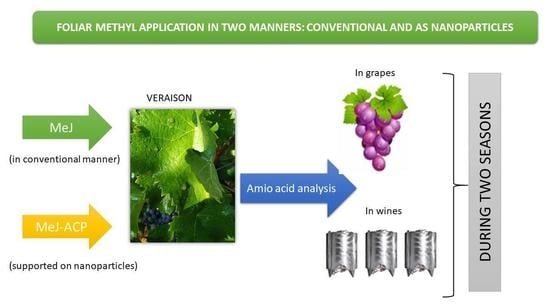
2.1. Experimental Design in Field and Treatments
2.2. Synthesis of MeJ-ACP
2.3. Climatological Conditions
2.4. Physicochemical Parameters of Grapes at Harvest
2.5. Vinifications
2.6. Pre-Sample Preparation for Analysing Nitrogen Composition in Grapes and Wines
2.7. Determination of Nitrogen Composition by HPLC
2.8. Statistical Analysis
3. Results and Discussion
3.1. Must Enological Parameters
3.2. Must Amino Acids and Ammonium Content in Grapes
3.3. Proline/Arginine Relationship
3.4. Amino Acids and Ammonium Content in Wines
3.5. Discriminant Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Martínez-Gil, A.M.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Salinas, M.R. Implications of nitrogen compounds during alcoholic fermentation from some grape varieties at different maturation stages and cultivation systems. Food Chem. 2011, 124, 106–116. [Google Scholar] [CrossRef]
- Bisson, L.F.; Butzke, C.E. Diagnosis and rectification of stuck and sluggish fermentations. Am. J. Enol. Vitic. 2000, 51, 168–177. [Google Scholar]
- Smit, I.; Pfliehinger, M.; Binner, A.; Großmann, M.; Horst, W.J.; Löhnertz, O. Nitrogen fertilisation increases biogenic amines and amino acid concentrations in Vitis vinifera var. Riesling musts and wines. J. Sci. Food Agric. 2014, 94, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- ter Schure, E.G.; van Riel, N.A.; Verrips, C.T. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2000, 24, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Magasanik, B.; Kaiser, A.C. Nitrogen regulation in Saccharomyces cerevisiae. Gene 2002, 290, 1–18. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in enological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Gump, B.H.; Zoecklein, B.W.; Fugelsang, K.C.; Whiton, R.S. Comparison of analytical methods for prediction of prefermentation nutritional status of grape juice. Am. J. Enol. Vitic. 2002, 53, 325–329. [Google Scholar]
- Huang, Z.; Ough, C.S. Effect of vineyard locations, varieties, and rootstocks on the juice amino acid composition of several cultivars. Am. J. Enol. Vitic. 1989, 40, 135–139. [Google Scholar]
- Gutiérrez-Gamboa, G.; Mateluna-Cuadra, R.; Díaz-Gálvez, I.; Mejía, N.; Verdugo-Vásquez, N. Methyl Jasmonate Applications in Viticulture: A Tool to Increase the Content of Flavonoids and Stilbenes in Grapes and Wines. Horticulturae 2021, 7, 133. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Grimes, H. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc. Natl. Acad. Sci. USA 1991, 88, 6745–6749. [Google Scholar] [CrossRef]
- Saniewski, M.; Miyamoto, K.; Ueda, J. Methyl Jasmonate Induces Gums and Stimulates Anthocyanin Accumulation in Peach Shoots. J. Plant Growth Regul. 1998, 17, 121–124. [Google Scholar] [CrossRef]
- Kondo, S.; Tsukada, N.; Niimi, Y.; Seto, H. Interactions between Jasmonates and Abscisic Acid in Apple Fruit, and Stimulative Effect of Jasmonates on Anthocyanin Accumulation. J. Jpn. Soc. Hortic. Sci. 2001, 70, 546–552. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Shang, H.; Meng, X. Effect of methyl jasmonate on the anthocyanin content and antioxidant activity of blueberries during cold storage. J. Sci. Food Agric. 2015, 95, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Blanch, G.P.; del Castillo, M.L.R. Postharvest treatment with (−) and (+)-methyl jasmonate stimulates anthocyanin accumulation in grapes. LWT Food Technol. 2015, 62, 807–812. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Olías, R.; Ríos, J.J.; Olías, J.M. Effect of modified atmosphere packaging on strawberry quality during shelf-life. In Proceedings of the Fruits Other than Apples and Pears, Davis, CA, USA, 13–18 July 1997; Kader, A.A., Ed.; University of California Davis: Davis, CA, USA, 1997; Volume 3, pp. 153–158. [Google Scholar]
- Larrondo, F.; Gaudillère, J.P.; Krisa, S.; Decendi, A.; Deffieux, G.; Mérillon, J.M. Airborne methyl jasmonate induces stilbene accumulation in leaves and berries of grapevine plants. Am. J. Enol. Vitic. 2003, 54, 63–66. [Google Scholar]
- Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Effect of methyl jasmonate application to grapevine leaves on grape amino acid content. Food Chem. 2016, 203, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Gonzalo-Diago, A.; Moreno-Simunovic, Y.; Martínez-Gil, A.M. Effect of different foliar nitrogen applications on the must amino acids and glutathione composition in Cabernet Sauvignon vineyard. LWT Food Sci. Technol. 2017, 75, 147–154. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Martínez-Cutillas, A.; Gómez-Plaza, E. Increasing bioactive phenolic compounds in grapes: Response of six monastrell grape clones to benzothiadiazole and methyl jasmonate treatments. Am. J. Enol. Vitic. 2013, 64, 459–465. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Improving phenolic and chromatic characteristics of Monastrell, Merlot and Syrah wines by using methyl jasmonate and benzothiadiazole. J. Int. Sci. Vigne. Vin. 2017, 51, 17–27. [Google Scholar]
- Portu, J.; López-Alfaro, I.; Gómez-Alonso, S.; López, R.; Garde-Cerdán, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Amaratunga, G.A. Urea-hidroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and Ionic Zn Promote Nutrient Loading of Sorghum Grain under Low NPK Fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef]
- White, J.C.; Gardea-Torresdey, J. Achieving food security through the very small. Nat. Nanotechnol. 2018, 13, 627–629. [Google Scholar] [CrossRef]
- Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Gil-Muñoz, R.; Delgado-López, J.M. Nanoelicitors with prolonged retention and sustained release to produce beneficial compounds in wines. Environ. Sci. Nano 2021. [Google Scholar] [CrossRef]
- Pérez-Alvarez, E.P.; Ramirez-Rodriguez, G.; Martinez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainale viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2021, 101, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis; OIV: Paris, France, 2018. [Google Scholar]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC analysis of biogenic amines, amino acids and ammonium ion as aminoenone derivative in wine and beer samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Paladines-Quezada, D.; Moreno-Olivares, J.; Fernández-Fernández, J.; Bautista-Ortín, A.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef]
- Oliva, J.; Garde-Cerdán, T.; Martínez-Gil, A.M.; Salinas, M.R.; Barba, A. Fungicide effects on ammonium and amino acids of Monastrell grapes. Food Chem. 2011, 129, 1676–1680. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; Portu, J.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Impact of phenylalanine and urea applications to Tempranillo and Monastrell vineyards on grape amino acid content during two consecutive vintages. Food Res. Int. 2017, 102, 451–457. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Foliar application of methyl jasmonate to Graciano and Tempranillo vines: Effects on grape amino acid content during two consecutive vintages. Oeno One 2019, 53, 1–19. [Google Scholar]
- Bouzas-Cid, Y.; Díaz-Losada, E.; Trigo-Córdoba, E.; Falqué, E.; Orriols, I.; Garde-Cerdán, T.; Mirás-Avalos, J.M. Effects of irrigation over three years on the amino acidcomposition of Albariño (Vitis vinifera L.) musts and wines in two different terroirs. Sci. Hortic. 2018, 227, 313–325. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Rubio-Bretón, P.; Pérez-Alvarez, E.P. Study of must and wine amino acids composition after seaweed applications to Tempranillo blanco grapevines. Food Chem. 2020, 308, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A Tool for Improving Fruit Phenolic Content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Carrasco-Quiroz, M.; Martínez-Gil, A.M.; Pérez-Álvarez, E.P.; Garde-Cerdán, T.; Moreno-Simunovic, Y. Grape and wine amino a composition from Carignan noir grapevines growing under rainfed conditions in the Maule Valley. Chile: Effects of location and rootstock. Food Res. Int. 2018, 105, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Orte, P.; Ibarz, M.; Cacho, J.; Ferreira, V. Addition of amino acids to grape juice of the Merlot variety: Effect on amino acid uptake and aroma generation during alcoholic fermentation. Food Chem. 2006, 98, 300–310. [Google Scholar] [CrossRef]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 77, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Portu, J.; Santamaría, P.; López, R.; Garde-Cerdán, T. Effects on grape amino acid concentration through foliar application of three different elicitors. Food Res. Int. 2017, 99, 688–692. [Google Scholar] [CrossRef]
- Martínez-Gil, A.M.; Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Salinas, M.R. Volatile Compounds Formation in Alcoholic Fermentation from Grapes Collected at 2 Maturation Stages: Influence of Nitrogen Compounds and Grape Variety. J. Food Sci. 2011, 77, C71–C79. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Garde-Cerdán, T.; Zalacain, A.; Garcia, R.; Cabrita, M.; Salinas, M.; Sánchez-Gómez, R.; Garde-Cerdán, T.; Zalacain, A.; Garcia, R.; et al. Vine-shoot waste aqueous extract applied as foliar fertilizer to grapevines: Effect on amino acids and fermentative volatile content. Food Chem. 2016, 197, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Ancín-Azpilicueta, C.; Salinas, M.R. Study of the Evolution of Nitrogen Compounds during Grape Ripening. Application to Differentiate Grape Varieties and Cultivated Systems. J. Agric. Food Chem. 2009, 57, 2410–2419. [Google Scholar] [CrossRef]
- Stines, A.; Grubb, J.; Gockowiak, H.; Henschke, P.; Høj, P.; van Heeswijck, R. Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: Influence of vine cultivar, berry maturity and tissue type. Aust. J. Grape Wine Res. 2000, 6, 150–158. [Google Scholar] [CrossRef]
- Orte, M.P.H.; Ibarz, M.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 89, 163–174. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Undesirable Compounds and Spoilage Microorganisms in Wine. In Wine Safety, Consumer Preference, and Human Health; Moreno-Arribas, M.V., Bartolomé Suáldea, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3–26. [Google Scholar]
- Lorenzo, C.; Bordiga, M.; Pérez-Álvarez, E.; Travaglia, F.; Arlorio, M.; Salinas, M.R.; Coïsson, J.D.; Garde-Cerdán, T. Impacts of temperature, alcoholic degree and amino acids content on biogenic amines and their precursor amino acids content in red wine. Food Res. Int. 2017, 99, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, E.P.; Garde-Cerdán, T.; Cabrita, M.J.; García-Escudero, E.; Peregrina, F. Influence on wine biogenic amine composition of modifications to soil N availability and grapevine N by cover crops. J. Sci. Food Agric. 2017, 97, 4800–4806. [Google Scholar] [CrossRef]
- Díez, L.; Solopova, A.; Fernández-Pérez, R.; González, M.; Tenorio, C.; Kuipers, O.P.; Ruiz-Larrea, F. Transcriptome analysis shows activation of the argininedeiminase pathway in Lactococcus lactis as a response to ethanol stress. Int. J. Food Microbiol. 2017, 257, 41–48. [Google Scholar] [CrossRef]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma Precursors in Grapes and Wine: Flavor Release during Wine Production and Consumption. J. Agric. Food Chem. 2017, 66, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, G.G.; Garde-Cerdán, T.; Portu, J.; Moreno-Simunovic, Y.; Martínez-Gil, A.M. Foliar nitrogen application in Cabernet Sauvignon vines: Effects on wine flavonoid and amino acid content. Food Res. Int. 2017, 96, 46–53. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; López, R.; Portu, J.; González-Arenzana, L.; López-Alfaro, I.; Santamaría, P. Study of the effects of proline, phenylalanine, and urea foliar application to Tempranillo vineyards on grape amino acid content. Comparison with commercial nitrogen fertilisers. Food Chem. 2014, 163, 136–141. [Google Scholar] [CrossRef]
- Gamboa, G.G.; Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Elicitor and nitrogen applications to Garnacha, Graciano and Tempranillo vines: Effect on grape amino acid composition. J. Sci. Food Agric. 2017, 98, 2341–2349. [Google Scholar] [CrossRef]
- Ougha, C.S.; Kriel, A. Ammonia Concentrations of Musts of Different Grape Cultivars and Vineyards in the Stellenbosch Area. S. Afr. J. Enol. Vitic. 1985, 6, 7–11. [Google Scholar] [CrossRef][Green Version]
- Alcaide-Hidalgo, J.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Polo, M.C. Influence of malolactic fermentation, postfermentative treatments and ageing with lees on nitrogen compounds of red wines. Food Chem. 2007, 103, 572–581. [Google Scholar] [CrossRef]

| 2019 | 2020 | |||||||
|---|---|---|---|---|---|---|---|---|
| July | August | September | October | July | August | September | October | |
| Rmean (w/m2) | 363 | 352 | 376 | 375 | 365 | 356 | 295 | 271 |
| Rainfall (mm) | 1.4 | 22.7 | 147.7 | 39.2 | 4.7 | 0.4 | 5.3 | 8.7 |
| Tmin (°C) | 22.8 | 21.4 | 15.8 | 9.6 | 21.5 | 19.4 | 16.2 | 9.5 |
| Tmax (°C) | 31.6 | 28.4 | 24.3 | 19.8 | 28.7 | 29.1 | 23.1 | 20.0 |
| Tmean (°C) | 25.5 | 24.9 | 20.7 | 15.7 | 25.3 | 25.1 | 20.3 | 14.0 |
| 2019 | 2020 | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | MeJ | MeJ-ACP | p-Value | Control | MeJ | MeJ-ACP | p-Value | |
| °Brix | 23.4 | 23.2 | 23.5 | ns | 26.3 | 25.6 | 24.9 | ns |
| pH | 3.8 | 3.9 | 3.8 | ns | 4.1 | 4.1 | 4.1 | ns |
| Total acidity (g/L) a | 2.8 | 3.2 | 2.9 | ns | 2.3b | 2.7a | 2.4b | * |
| Tartaric acid (g/L) | 3.9 | 3.8 | 3.9 | ns | 4.5 | 4.4 | 4.2 | ns |
| Malic acid (g/L) | 1.4 | 1.7 | 1.4 | ns | 1.4b | 1.9a | 1.4b | *** |
| 2019 | 2020 | Multifactorial Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | MeJ | MeJ-ACP | Control | MeJ | MeJ-ACP | T | S | TxS | |
| Asp | 27.6c | 38.7a | 33.3b | 10.2 | 11.3 | 10.7 | *** | *** | *** |
| Glu | 33.3b | 43.4a | 45.4a | 13.1c | 21.8a | 16.6b | *** | *** | *** |
| Asn + Ser | 42.3c | 106.7a | 77.5b | 91.0b | 114.1a | 114.1a | *** | *** | *** |
| Gln | 53.6c | 148.3a | 127.7b | 198.5b | 280.9a | 213.0b | *** | *** | ** |
| His | 13.1c | 46.3a | 32.0b | 69.7b | 95.1a | 75.3b | *** | *** | ns |
| Gly | 6.0c | 10.3a | 7.5b | 12.3 | 13.8 | 13.8 | *** | *** | ** |
| Thr | 14.9c | 42.4a | 27.7b | 43.1 | 47.9 | 44.4 | *** | *** | *** |
| β-Ala | 10.5c | 30.3a | 19.7b | 28.9b | 39.6a | 37.7a | *** | *** | * |
| Arg + GABA | 42.5c | 275.4a | 188.4b | 213.8c | 354.3a | 301.5b | *** | *** | ** |
| α-Ala | 21.5b | 27.6a | 20.3b | 23.8c | 30.7b | 130.3a | *** | *** | *** |
| Pro | 199.3c | 564.1a | 335.0b | 345.9b | 449.6a | 436.2a | *** | *** | *** |
| NH4+ | 14.9c | 33.0a | 23.9b | 21.6b | 27.3a | 25.1a | *** | ns | *** |
| Tyr | 7.0b | 11.9a | 9.2b | 26.1b | 37.8a | 37.0a | *** | *** | ** |
| Val | 8.9c | 20.4a | 14.6b | 33.6 | 34.4 | 37.1 | *** | *** | *** |
| Met | 2.8b | 4.1a | 3.4b | 4.8b | 5.5ab | 6.3a | ** | *** | * |
| Cys | 10.4a | 7.7ab | 6.8b | 13.2a | 9.9b | 9.0b | *** | ** | ns |
| Iso | 6.5c | 11.0a | 8.4b | 15.1b | 15.0b | 17.5a | *** | *** | *** |
| Leu | 8.1c | 16.6a | 12.1b | 24.8 | 25.1 | 27.2 | *** | *** | *** |
| Trp | 20.3c | 47.8a | 35.2b | 59.3b | 80.1a | 54.4b | *** | *** | ** |
| Phe | 5.3c | 13.2a | 9.8b | 17.3 | 17.2 | 18.9 | *** | *** | ** |
| Orn | 2.1c | 2.9a | 2.6b | 2.6b | 3.3a | 2.1c | *** | ** | *** |
| Lys | 2.6c | 4.4a | 3.2b | 3.4b | 4.2a | 2.8c | *** | ns | *** |
| Pro/Arg | 4.7a | 2.1b | 1.8b | 1.6a | 1.3b | 1.5ab | *** | *** | *** |
| Totals | 553.6c | 1506.7a | 1043.6b | 1271.9b | 1718.8a | 1631.3a | *** | *** | *** |
| Totals-Pro | 354.3c | 942.5a | 708.6b | 926.0b | 1269.2a | 1195.1a | *** | *** | ** |
| 2019 | 2020 | Multifactorial Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | MeJ | MeJ-ACP | Control | MeJ | MeJ-ACP | T | S | TxS | |
| Asp | 2.1b | 4.0a | 3.6a | 3.3c | 5.2a | 4.1b | *** | *** | ns |
| Glu | 4.1b | 9.8a | 8.3a | 9.4b | 18.8a | 12.4b | *** | *** | ns |
| Asn + Ser | 1.3c | 5.9a | 3.8b | 6.4b | 10.7a | 8.7ab | *** | *** | ns |
| Gln | 2.2b | 7.1a | 5.8a | 5.7b | 8.4a | 7.3a | *** | *** | ns |
| His | 3.4b | 4.8a | 4.6a | 5.6 | 7.9 | 5.9 | ** | *** | ns |
| Gly | 4.6b | 6.6a | 6.1a | 7.7b | 11.1a | 9.3ab | *** | *** | ns |
| Thr | 3.4b | 3.8a | 3.8a | 4.2b | 5.2a | 4.4b | *** | *** | * |
| β-Ala | 2.4b | 2.8a | 2.8a | 3.6 | 4.5 | 3.6 | ns | *** | ns |
| Arg | 4.3c | 7.4a | 6.3b | 10.8b | 21.7a | 15.3b | *** | *** | ** |
| GABA | 4.7c | 10.8a | 8.8b | 15.4b | 27.2a | 19.8b | *** | *** | ns |
| α-Ala | 2.2c | 4.3a | 3.4b | 5.5b | 9.9a | 7.2b | *** | *** | * |
| Pro | 102.7b | 466.0a | 358.2a | 564.9b | 1109.8a | 893.1a | *** | *** | ns |
| NH4+ | 2.5 | 2.7 | 2.9 | 4.1b | 8.5a | 5.6b | *** | *** | *** |
| Tyr | 3.2b | 4.7a | 4.8a | 5.4b | 9.3a | 6.0b | *** | *** | *** |
| Val | 2.6 | 2.9 | 2.7 | 2.9b | 3.8a | 3.3b | ** | *** | ns |
| Met | 2.7 | 3.1 | 3.1 | 3.1 | 3.7 | 3.3 | * | * | ns |
| Cys | 3.2 | 3.2 | 3.3 | 4.6 | 4.2 | 4.0 | ns | *** | ns |
| Iso | 3.6b | 4.4a | 4.1ab | 4.7 | 5.5 | 4.7 | * | *** | ns |
| Leu | 3.9b | 4.9a | 4.8a | 5.3b | 7.6a | 5.7b | *** | *** | ** |
| Trp | 4.0b | 4.8a | 4.7a | 5.0c | 6.5a | 5.8b | *** | *** | * |
| Phe | 2.7b | 3.9a | 3.7a | 3.7b | 6.1a | 4.2b | *** | *** | ** |
| Orn | 2.0b | 2.5a | 2.3a | 3.1b | 4.6a | 3.5b | *** | *** | ** |
| Lys | 4.3b | 7.6a | 6.2ab | 8.6b | 16.0a | 9.3b | ** | *** | ns |
| Totals | 171.9b | 578.0a | 458.0a | 693.0b | 1316.2a | 1046.7a | *** | *** | ns |
| Totals-Pro | 69.2c | 112.0a | 99.8b | 128.1b | 206.3a | 153.6b | *** | *** | * |
| Function 1 | Function 2 | |
|---|---|---|
| Asp | −0.52 | 0.72 |
| Glu | −1.89 | −5.44 |
| Asn + Ser | 12.34 | 23.68 |
| Gln | 11.45 | −23.41 |
| His | 1.64 | −13.10 |
| Gly | 1.10 | 1.28 |
| Thr | −10.58 | −8.37 |
| β-Ala | −9.93 | −7.23 |
| Arg + GABA | −4.09 | 15.95 |
| α-Ala | −1.95 | 1.43 |
| Pro | 19.58 | −11.38 |
| NH4+ | 5.29 | −3.83 |
| Tyr | 6.25 | −2.33 |
| Val | 2.33 | 0.13 |
| Met | −1.53 | 1.40 |
| Cys | −0.29 | 2.28 |
| Iso | −15.69 | −1.50 |
| Leu | −3.57 | 10.34 |
| Trp | −2.56 | 22.07 |
| Phe | 6.78 | −11.34 |
| Orn | 3.60 | 2.13 |
| Lys | 5.50 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of Methyl Jasmonate Doped Nanoparticles on Nitrogen Composition of Monastrell Grapes and Wines. Biomolecules 2021, 11, 1631. https://doi.org/10.3390/biom11111631
Gil-Muñoz R, Giménez-Bañón MJ, Moreno-Olivares JD, Paladines-Quezada DF, Bleda-Sánchez JA, Fernández-Fernández JI, Parra-Torrejón B, Ramírez-Rodríguez GB, Delgado-López JM. Effect of Methyl Jasmonate Doped Nanoparticles on Nitrogen Composition of Monastrell Grapes and Wines. Biomolecules. 2021; 11(11):1631. https://doi.org/10.3390/biom11111631
Chicago/Turabian StyleGil-Muñoz, Rocío, María José Giménez-Bañón, Juan Daniel Moreno-Olivares, Diego Fernando Paladines-Quezada, Juan Antonio Bleda-Sánchez, José Ignacio Fernández-Fernández, Belén Parra-Torrejón, Gloria Belén Ramírez-Rodríguez, and José Manuel Delgado-López. 2021. "Effect of Methyl Jasmonate Doped Nanoparticles on Nitrogen Composition of Monastrell Grapes and Wines" Biomolecules 11, no. 11: 1631. https://doi.org/10.3390/biom11111631
APA StyleGil-Muñoz, R., Giménez-Bañón, M. J., Moreno-Olivares, J. D., Paladines-Quezada, D. F., Bleda-Sánchez, J. A., Fernández-Fernández, J. I., Parra-Torrejón, B., Ramírez-Rodríguez, G. B., & Delgado-López, J. M. (2021). Effect of Methyl Jasmonate Doped Nanoparticles on Nitrogen Composition of Monastrell Grapes and Wines. Biomolecules, 11(11), 1631. https://doi.org/10.3390/biom11111631