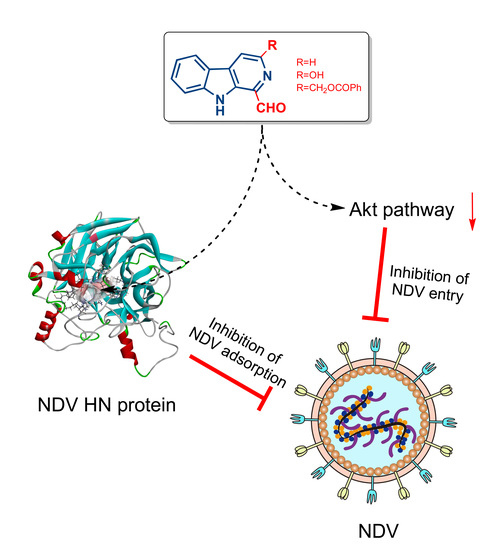
1-Formyl-β-carboline Derivatives Block Newcastle Disease Virus Proliferation through Suppressing Viral Adsorption and Entry Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. β-Carboline Derivative Samples, Reagents, Cell Lines, and Virus
2.2. Cytotoxicity Assay
2.3. Plaque Assay
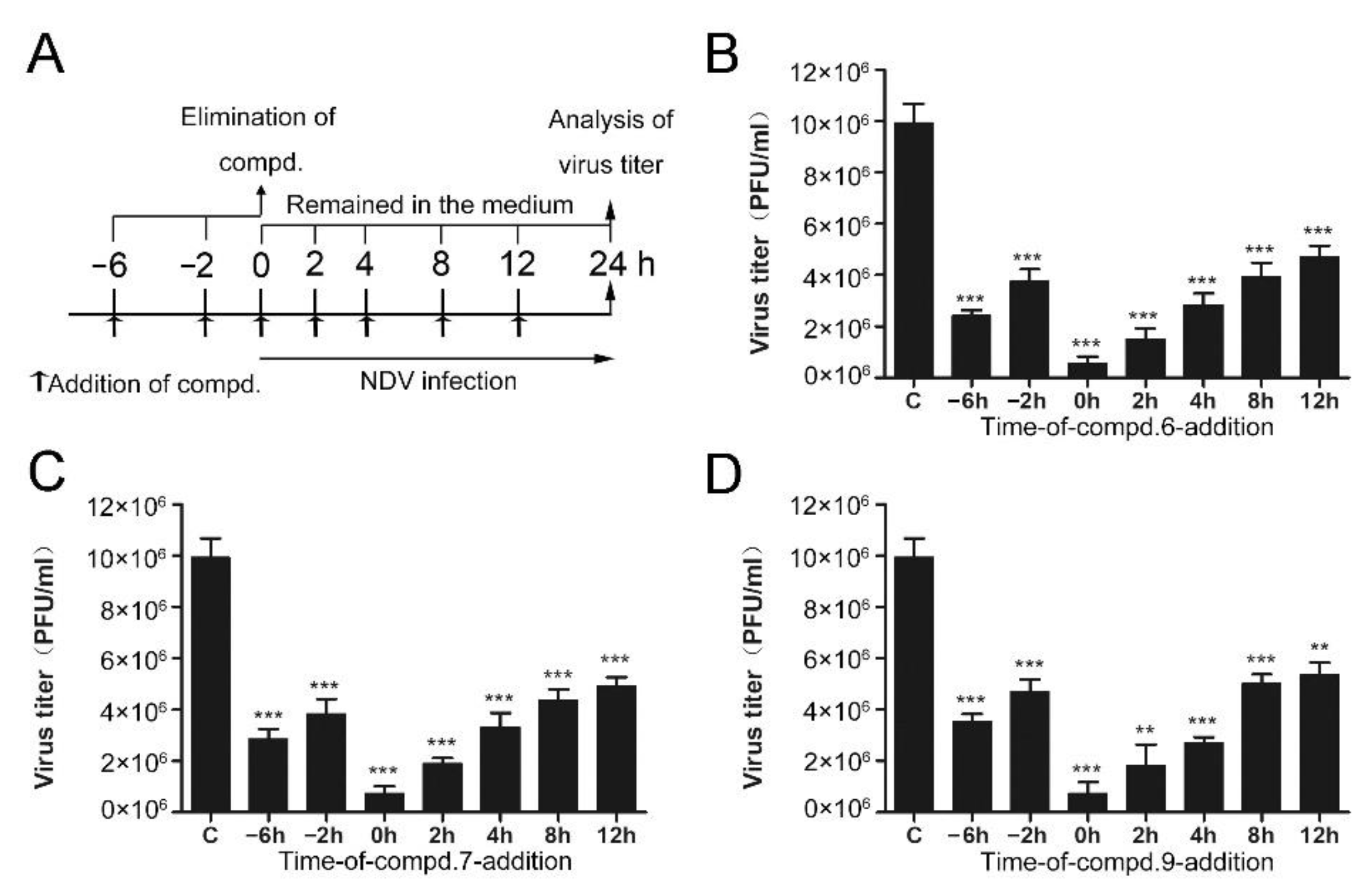
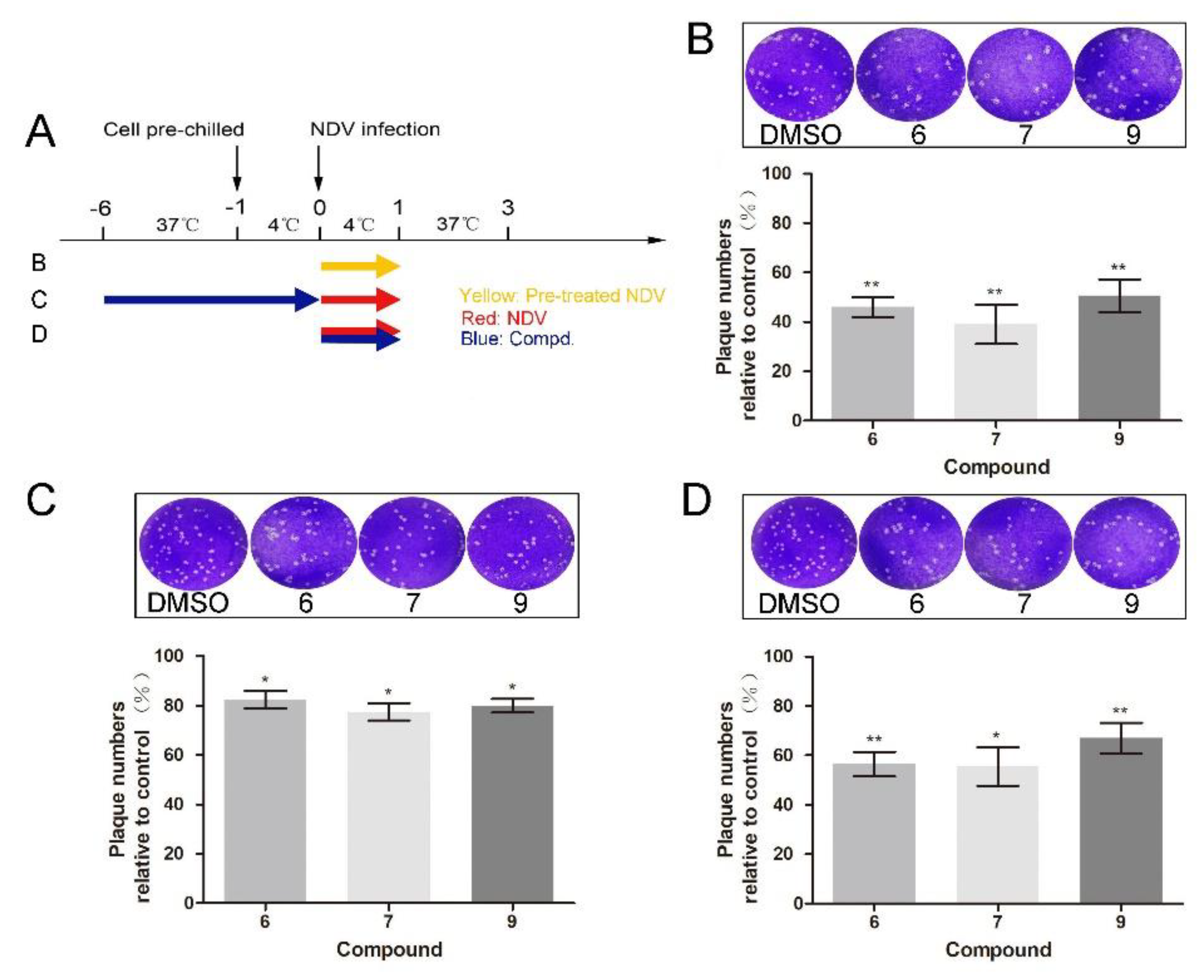
2.4. “Time of Drug-Addition” Assay
2.5. Adsorption Assay
2.6. Entry Assay
2.7. Homology Modeling and Molecular Docking
2.8. Drug Affinity Responsive Target Stability (DARTS)
2.9. Cellular Thermal Shift Assay (CETSA)
2.10. Plasmids, Transfections, and RBC Binding Assay
2.11. RNA Extraction and Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
2.12. Western Blot Analysis
2.13. Indirect Immunofluorescence
2.14. Statistical Analysis
3. Results
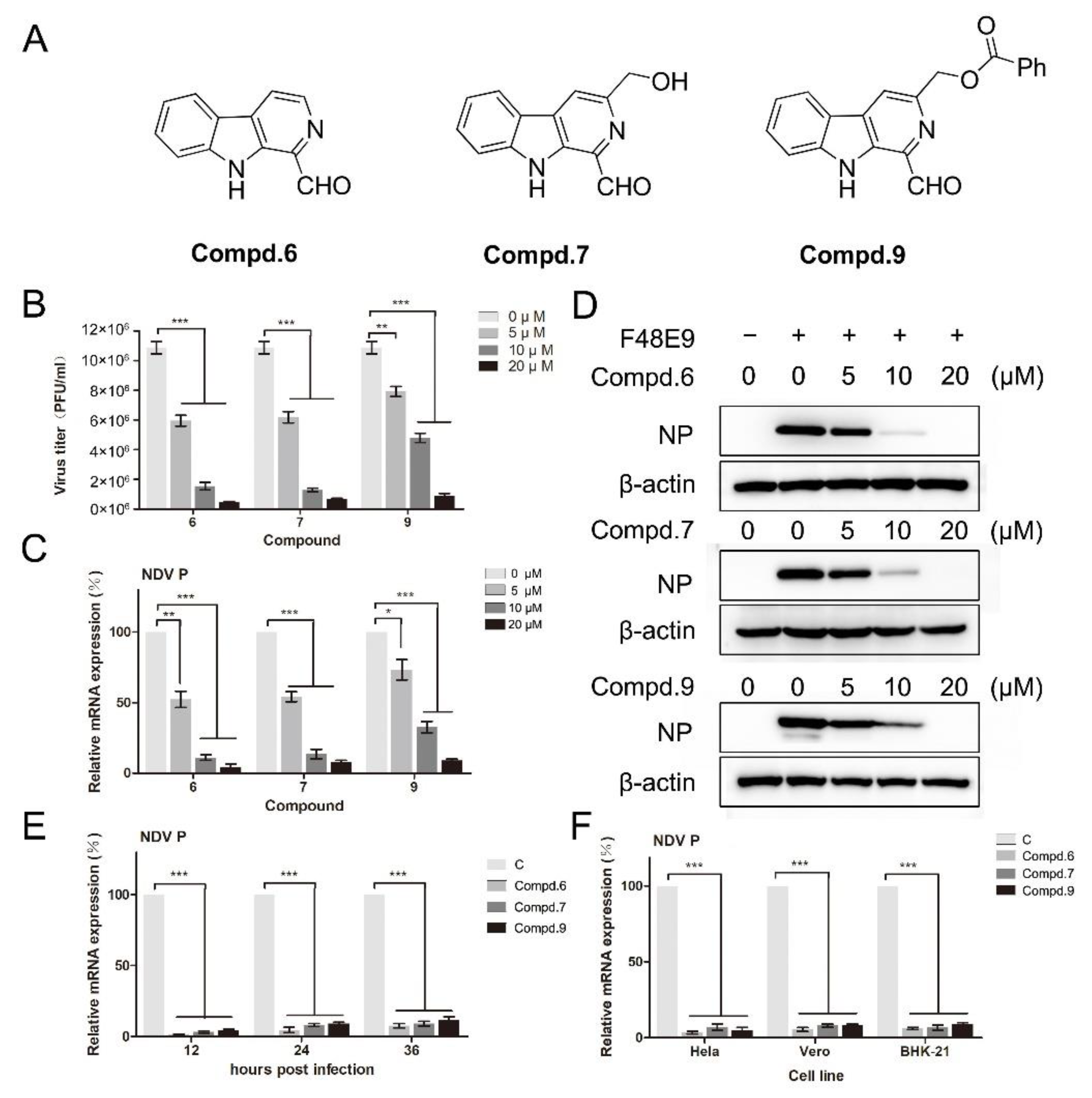
3.1. Cytotoxic and Anti-Viral Activity of β-Carbolines In Vitro
3.2. 1-Formyl-β-Carboline Derivatives Disrupt the Early Stages of NDV Life Cycle
3.3. 1-Formyl-β-Carboline Derivatives Inhibit NDV Adsorption through Interacting with HN
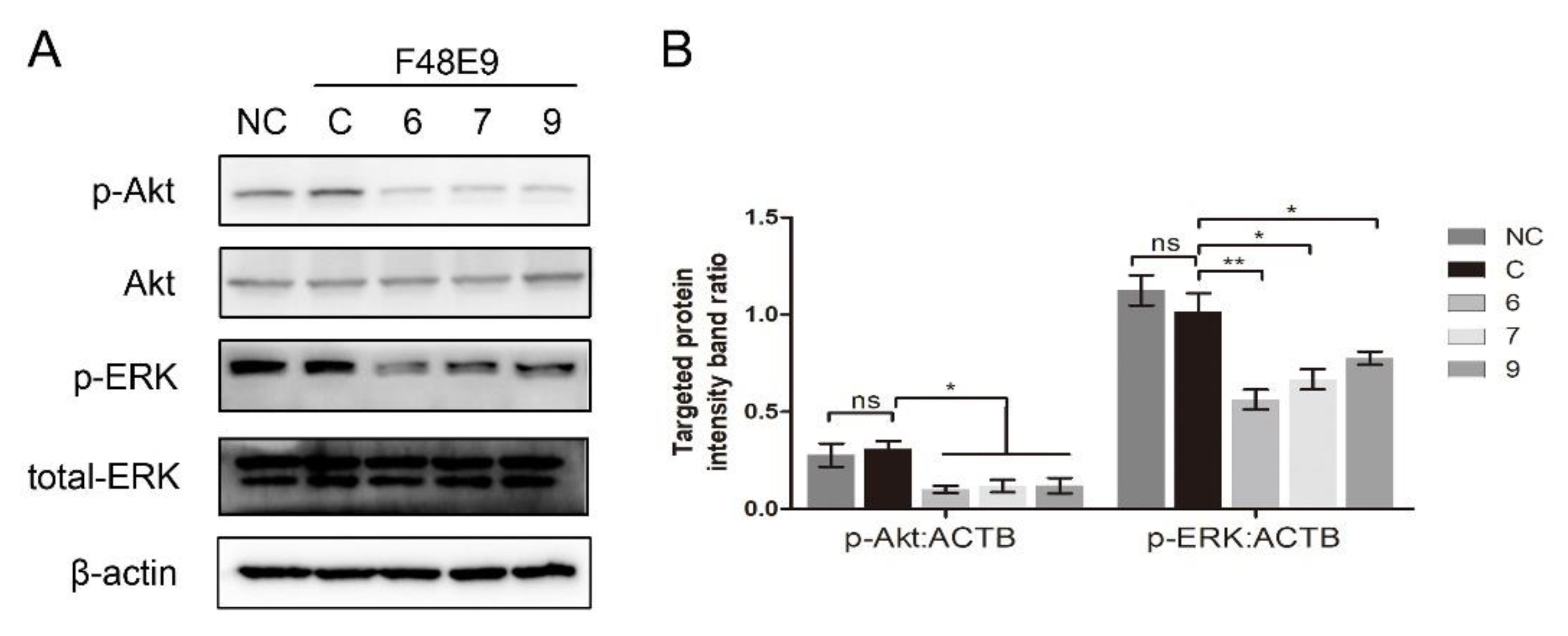
3.4. 1-Formyl-β-Carboline Derivatives Inhibited NDV Entry through Suppressing the PI3K/Akt Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexander, D.J. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech. 2000, 19, 443–462. [Google Scholar] [CrossRef]
- Miller, P.J.; Decanini, E.L.; Afonso, C.L. Newcastle disease: Evolution of genotypes and the related diagnostic challenges. Infect. Genet. Evol. 2010, 10, 26–35. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Yin, Z.; Zhao, X.; Liang, X.; He, C.; Yin, L.; Cheng, L.; Ling, Z.; Gang, Y.; et al. Antiviral effect of sulfated Chuanmingshen violaceum polysaccharide in chickens infected with virulent Newcastle disease virus. Virology 2015, 476, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Dan, W.; Schneider, U.; Wang, J. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef]
- Chen, Q.; Chao, R.; Chen, H.; Hou, X.; Yan, H.; Zhou, S.; Peng, W.; Xu, A. Antitumor and neurotoxic effects of novel harmine derivatives and structure-activity relationship analysis. Int. J. Cancer 2005, 114, 675–682. [Google Scholar] [CrossRef]
- Moura, D.J.; Richter, M.F.; Boeira, J.M.; Pêgas Henriques, J.A.; Saffi, J. Antioxidant properties of beta-carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis 2007, 22, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Gao, Z.; Jiao, W.; Chen, L.; Chen, L.; Yao, X. In vitro anti-inflammatory effects of beta-carboline alkaloids, isolated from Picrasma quassioides, through inhibition of the iNOS pathway. Planta Med. 2012, 78, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Otto, R.; Penzis, R.; Gaube, F.; Winckler, T.; Appenroth, D.; Fleck, C.; Tränkle, C.; Lehmann, J.; Enzensperger, C. Beta and gamma carboline derivatives as potential anti-Alzheimer agents: A comparison. Eur. J. Med. Chem. 2014, 87, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Su, A.; Fu, Y.; Wang, X.; Lv, X.; Xu, W.; Xu, S.; Wang, H.; Wu, Z. Harmine blocks herpes simplex virus infection through downregulating cellular NF-κB and MAPK pathways induced by oxidative stress. Antivir. Res. 2015, 123, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kong, F.; Wei, J.; Wang, Y.; Wang, W.; Hong, K.; Zhu, W. Alkaloids from the Mangrove-Derived ActinomyceteJishengella endophytica161111. Marine Drugs 2014, 12, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Tian, X.; Zou, X.; Xu, S.; Wang, H.; Zheng, N.; Wu, Z. Harmine, a small molecule derived from natural sources, inhibits enterovirus 71 replication by targeting NF-κB pathway. Int. Immunopharmacol. 2018, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ashok, P.; Chander, S.; Balzarini, J.; Pannecouque, C.; Murugesan, S. Design, synthesis of new β-carboline derivatives and their selective anti-HIV-2 activity. Bioorg. Med. Chem. Lett. 2015, 25, 1232–1235. [Google Scholar] [CrossRef]
- Quintana, V.M.; Piccini, L.E.; Panozzo Zénere, J.D.; Damonte, E.B.; Ponce, M.A.; Castilla, V. Antiviral activity of natural and synthetic β-carbolines against dengue virus. Antivir. Res. 2016, 134, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Porotto, M.; Salah, Z.; DeVito, I.; Talekar, A.; Palmer, S.G.; Xu, R.; Wilson, I.A.; Moscona, A. The second receptor binding site of the globular head of the Newcastle disease virus hemagglutinin-neuraminidase activates the stalk of multiple paramyxovirus receptor binding proteins to trigger fusion. J. Virol. 2012, 86, 5730–5741. [Google Scholar] [CrossRef] [Green Version]
- Yuan, P.; Paterson, R.G.; Leser, G.P.; Lamb, R.A.; Jardetzky, T.S. Structure of the ulster strain newcastle disease virus hemagglutinin-neuraminidase reveals auto-inhibitory interactions associated with low virulence. PLoS Pathog. 2012, 8, e1002855. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Sun, Y.; Rao, Z. Current progress in antiviral strategies. Trends Pharmacol. Sci. 2014, 35, 86–102. [Google Scholar] [CrossRef]
- Jardetzky, T.S.; Lamb, R.A. Activation of paramyxovirus membrane fusion and virus entry. Curr. Opin. Virol. 2014, 5, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Jardetzky, T.S.; Lamb, R.A. Timing is everything: Fine-tuned molecular machines orchestrate paramyxovirus entry. Virology 2015, 479–480, 518–531. [Google Scholar] [CrossRef] [Green Version]
- Cantín, C.; Holguera, J.; Ferreira, L.; Villar, E.; Muñoz-Barroso, I. Newcastle disease virus may enter cells by caveolae-mediated endocytosis. J. Gen. Virol. 2007, 88 Pt 2, 559–569. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, Y.; Zhan, Y.; Yuan, Y.; Sun, Y.; Qiu, X.; Meng, C.; Song, C.; Liao, Y.; Ding, C. Newcastle disease virus employs macropinocytosis and Rab5a-dependent intracellular trafficking to infect DF-1 cells. Oncotarget 2016, 7, 86117–86133. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Huang, W.R.; Chi, P.I.; Chiu, H.C.; Liu, H.J. Cell entry of bovine ephemeral fever virus requires activation of Src-JNK-AP1 and PI3K-Akt-NF-κB pathways as well as Cox-2-mediated PGE2/EP receptor signalling to enhance clathrin-mediated virus endocytosis. Cell Microbiol. 2015, 17, 967–987. [Google Scholar] [CrossRef] [PubMed]
- McPherson, P.S.; Kay, B.K.; Hussain, N.K. Signaling on the endocytic pathway. Traffic 2001, 2, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, N.; Dai, J.; Xi, Y.; Wang, S.; Wang, J. Synthesis and In Vitro Antitumor Activity of Novel Bivalent β-Carboline-3-carboxylic Acid Derivatives with DNA as a Potential Target. Int. J. Mol. Sci. 2018, 19, 3179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Dan, W.; Ren, S.; Shang, C.; Wang, J. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur. J. Med. Chem. 2018, 160, 23–36. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Hu, R.; Dai, J.; Liu, H.; Li, N.; Schneider, U.; Yang, Z.; Wang, J. Cyclooxygenase-2 Facilitates Newcastle Disease Virus Proliferation and Is as a Target for Canthin-6-One Antiviral Activity. Front. Microbiol. 2020, 11, 987. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.G.; Wang, Y.H.; Wang, R.R.; Dong, Z.J.; Yang, L.M.; Zheng, Y.T.; Liu, J.K. Synthesis of analogues of flazin, in particular, flazinamide, as promising anti-HIV agents. Chem. Biodivers 2008, 5, 447–460. [Google Scholar] [CrossRef]
- Dai, J.; Dan, W.; Li, N.; Wang, R.; Zhang, Y.; Li, N.; Wang, R.; Wang, J. Synthesis and antibacterial activity of C2 or C5 modified and D ring rejiggered canthin-6-one analogues. Food Chem. 2018, 253, 211–220. [Google Scholar] [CrossRef]
- Dai, J.; Dan, W.; Zhang, Y.; He, M.; Wang, J. Design and synthesis of C10 modified and ring-truncated canthin-6-one analogues as effective membrane-active antibacterial agents. Bioorg. Med. Chem. Lett. 2018, 28, 3123–3128. [Google Scholar] [CrossRef]
- Pourianfar, H.R.; Poh, C.L.; Fecondo, J.; Grollo, L. In vitro evaluation of the antiviral activity of heparan sulfate mimetic compounds against Enterovirus 71. Virus Res. 2012, 169, 22–29. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, F.; Arnold, K.; Künzli, M.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009, 37, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomenick, B.; Hao, R.; Jonai, N.; Chin, R.M.; Aghajan, M.; Warburton, S.; Wang, J.; Wu, R.P.; Gomez, F.; Loo, J.A.; et al. Target identification using drug affinity responsive target stability (DARTS). Proc. Natl. Acad. Sci. USA 2009, 106, 21984–21989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, M.Y.; Lomenick, B.; Hwang, H.; Schiestl, R.; McBride, W.; Loo, J.A.; Huang, J. Drug affinity responsive target stability (DARTS) for small-molecule target identification. Methods Mol. Biol. 2015, 1263, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Jafari, R.; Almqvist, H.; Axelsson, H.; Ignatushchenko, M.; Lundbäck, T.; Nordlund, P.; Martinez, M.D. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 2014, 9, 2100–2122. [Google Scholar] [CrossRef]
- Dziekan, J.M.; Wirjanata, G.; Dai, L.; Go, K.D.; Yu, H.; Lim, Y.T.; Chen, L.; Wang, L.C.; Puspita, B.; Prabhu, N.; et al. Cellular thermal shift assay for the identification of drug-target interactions in the Plasmodium falciparum proteome. Nat. Protoc. 2020, 15, 1881–1921. [Google Scholar] [CrossRef]
- Nazari Formagio, A.S.; Santos, P.R.; Zanoli, K.; Ueda-Nakamura, T.; Düsman Tonin, L.T.; Nakamura, C.V.; Sarragiotto, M.H. Synthesis and antiviral activity of beta-carboline derivatives bearing a substituted carbohydrazide at C-3 against poliovirus and herpes simplex virus (HSV-1). Eur. J. Med. Chem. 2009, 44, 4695–4701. [Google Scholar] [CrossRef]
- Gonzalez, M.M.; Cabrerizo, F.M.; Baiker, A.; Erra-Balsells, R.; Osterman, A.; Nitschko, H.; Vizoso-Pinto, M.G. β-Carboline derivatives as novel antivirals for herpes simplex virus. Int. J. Antimicrob. Agents 2018, 52, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Zaitsev, V.; Von Itzstein, M.; Groves, D.; Kiefel, M.; Takimoto, T.; Portner, A.; Taylor, G. Second Sialic Acid Binding Site in Newcastle Disease Virus Hemagglutinin-Neuraminidase: Implications for Fusion. J. Virol. 2004, 78, 3733–3741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connaris, H.; Takimoto, T.; Russell, R.; Crennell, S.; Moustafa, I.; Portner, A.; Taylor, G. Probing the sialic acid binding site of the hemagglutinin-neuraminidase of newcastle disease virus: Identification of key amino acids involved in cell binding, catalysis, and fusion. J. Virol. 2002, 76, 1816–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corey, E.A.; Mirza, A.M.; Levandowsky, E.; Iorio, R.M. Fusion deficiency induced by mutations at the dimer interface in the Newcastle disease virus hemagglutinin-neuraminidase is due to a temperature-dependent defect in receptor binding. J. Virol. 2003, 77, 6913–6922. [Google Scholar] [CrossRef] [Green Version]
- Crennell, S.; Takimoto, T.; Portner, A.; Taylor, G. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat. Struct. Biol. 2000, 7, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, K.G.; Ahmed, N.; Sabde, S.; Mitra, D.; Singh, I.P.; Bhutani, K.K. Synthesis and evaluation of beta-carboline derivatives as inhibitors of human immunodeficiency virus. Bioorg. Med. Chem. Lett. 2010, 20, 4416–4419. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.R.; Mani, P.; Nandwani, N.; Mishra, R.; Rana, A.; Sarkar, D.P. Reciprocal regulation of AKT and MAP kinase dictates virus-host cell fusion. J. Virol. 2010, 84, 4366–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, J.E.; Foscaldi, S.; Quintana, V.M.; Scolaro, L.A.; López, N.; Castilla, V. Role of the ERK1/2 Signaling Pathway in the Replication of Junín and Tacaribe Viruses. Viruses 2018, 10, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]




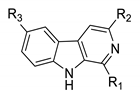
| Compd. | R1 | R2 | R3 |
|---|---|---|---|
| 1 | H | H | H |
| 2 | CH(OCH3)2 | H | H |
| 3 | CH(OCH3)2 | H | OCH3 |
| 4 | CH(OCH3)2 | COOCH3 | H |
| 5 | CH(OCH3)2 | OH | H |
| 6 | CHO | H | H |
| 7 | CHO | OH | H |
| 8 | CHO | COOCH3 | H |
| 9 | CHO | CH2OCOPh | H |
| 10 | CH2NHBn | COOCH3 | H |
| 11 | (CH2)3OH | H | H |
| 12 | (CH2)3OH | CH2OH | H |
| RNA Target | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| NDV P | TCATGCCCAGCTACCTGTCG | CTGTTGGATTTCAGACCGCATC |
| β-actin (DF-1) | TGGTGATGAAGCCCAGAGCAA | TGGCTTTGGGGTTCAGGGGAG |
| β-actin (Hela) | TGGACATCCGCAAAGACCTGT | GGAGTACTTGCGCTCAGGAGG |
| β-actin (BHK-21) | GAGAAGATCTGGCACCACACC | TACGACCAGAGGCATACAGGGA |
| β-actin (Vero) | GCGCGGCTACAGCTTCACCAC | GCCATCTCCTGCTCGAAGTCCA |
| Compd. | CC50 a (μM) | IC50 b (μM) | SI c |
|---|---|---|---|
| 6 | 67.1 ± 6.2 | 7.5 ± 1.8 | 8.9 |
| 7 | 51.7 ± 4.5 | 8.2 ± 1.9 | 6.3 |
| 9 | 82.9 ± 7.3 | 10.7 ± 2.3 | 7.7 |
| Ribavirin | – d | 8.6 ± 1.4 | – d |
| Compd. | Blackbird/China/08 (IX) | PPMV-1/SX-01/Ch/15 (VI) | La Sota (II) |
|---|---|---|---|
| 6 | 7.3 ± 1.2 | 9.4 ± 1.8 | 8.1 ± 1.3 |
| 7 | 12.8 ± 1.1 | 10.2 ± 1.3 | 8.5 ± 1.6 |
| 9 | 13.9 ± 2.5 | 15.7 ± 1.4 | 12.2 ± 3.4 |
| Ribavirin | 10.4 ± 2.8 | 11.6 ± 2.1 | 7.9 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, T.; Dai, J.; An, Z.; Hu, R.; Duan, L.; Chen, H.; Wang, X.; Chu, Z.; Liu, H.; et al. 1-Formyl-β-carboline Derivatives Block Newcastle Disease Virus Proliferation through Suppressing Viral Adsorption and Entry Processes. Biomolecules 2021, 11, 1687. https://doi.org/10.3390/biom11111687
Wang C, Wang T, Dai J, An Z, Hu R, Duan L, Chen H, Wang X, Chu Z, Liu H, et al. 1-Formyl-β-carboline Derivatives Block Newcastle Disease Virus Proliferation through Suppressing Viral Adsorption and Entry Processes. Biomolecules. 2021; 11(11):1687. https://doi.org/10.3390/biom11111687
Chicago/Turabian StyleWang, Chongyang, Ting Wang, Jiangkun Dai, Zhiyuan An, Ruochen Hu, Liuyuan Duan, Hui Chen, Xiangwei Wang, Zhili Chu, Haijin Liu, and et al. 2021. "1-Formyl-β-carboline Derivatives Block Newcastle Disease Virus Proliferation through Suppressing Viral Adsorption and Entry Processes" Biomolecules 11, no. 11: 1687. https://doi.org/10.3390/biom11111687
APA StyleWang, C., Wang, T., Dai, J., An, Z., Hu, R., Duan, L., Chen, H., Wang, X., Chu, Z., Liu, H., Wang, J., Li, N., Yang, Z., & Wang, J. (2021). 1-Formyl-β-carboline Derivatives Block Newcastle Disease Virus Proliferation through Suppressing Viral Adsorption and Entry Processes. Biomolecules, 11(11), 1687. https://doi.org/10.3390/biom11111687