Piezoelectric Signals in Vascularized Bone Regeneration
Abstract
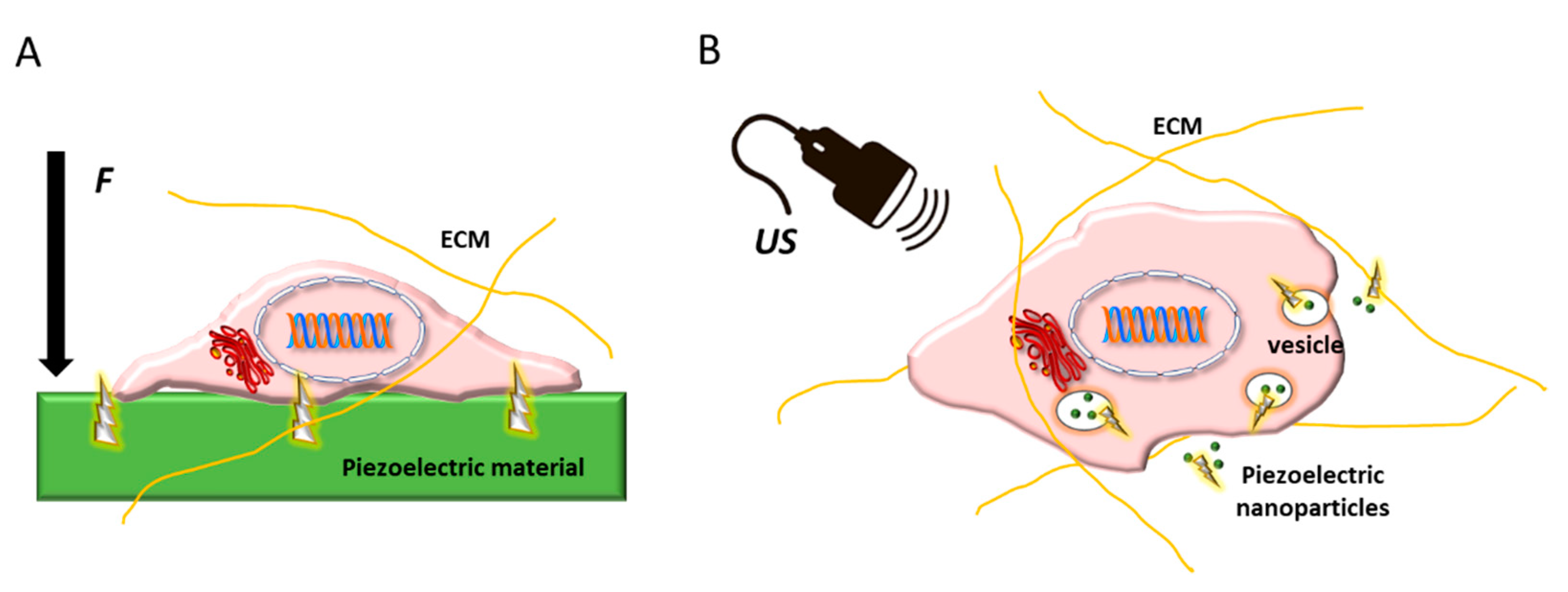
:1. Introduction
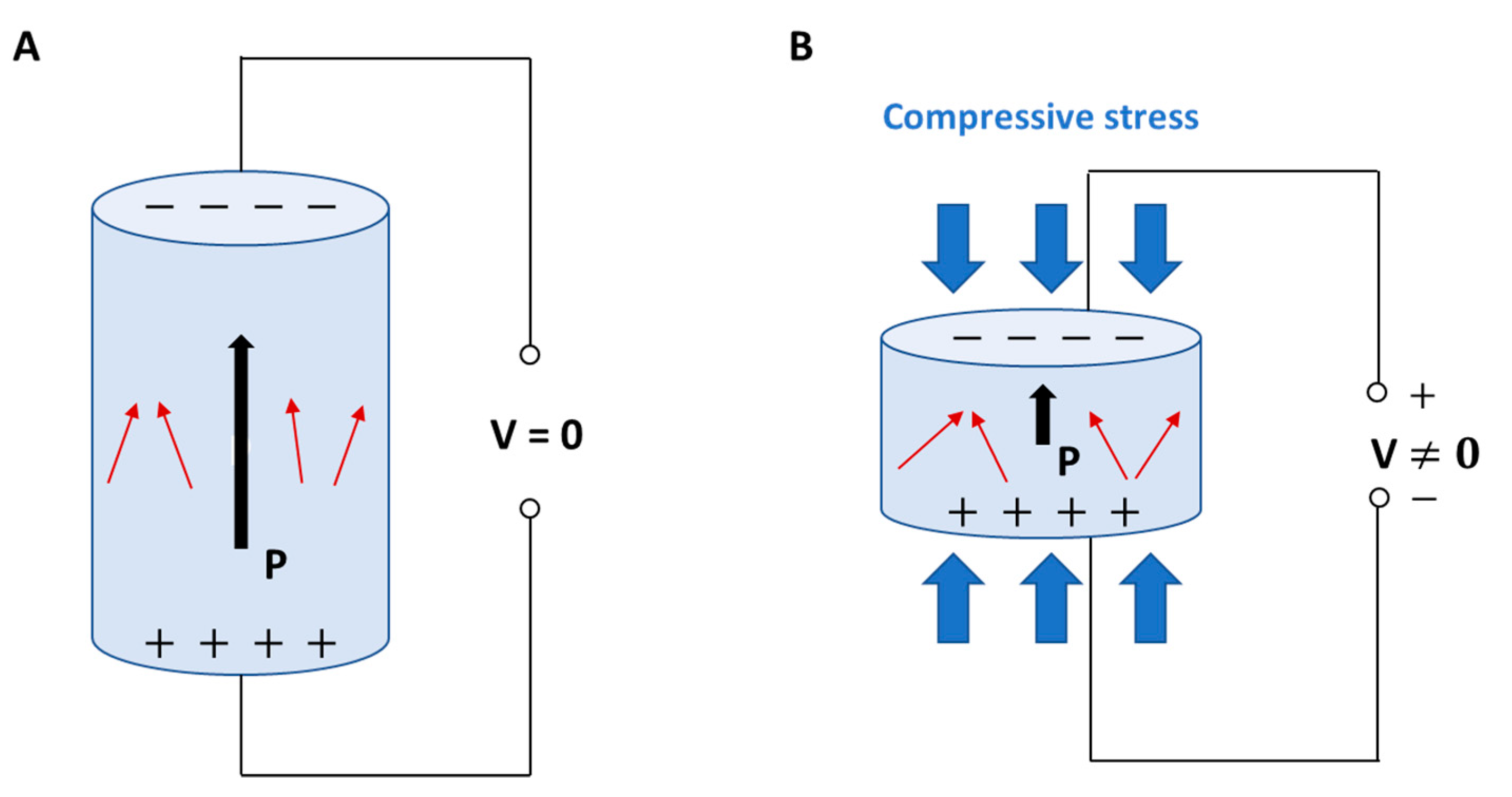
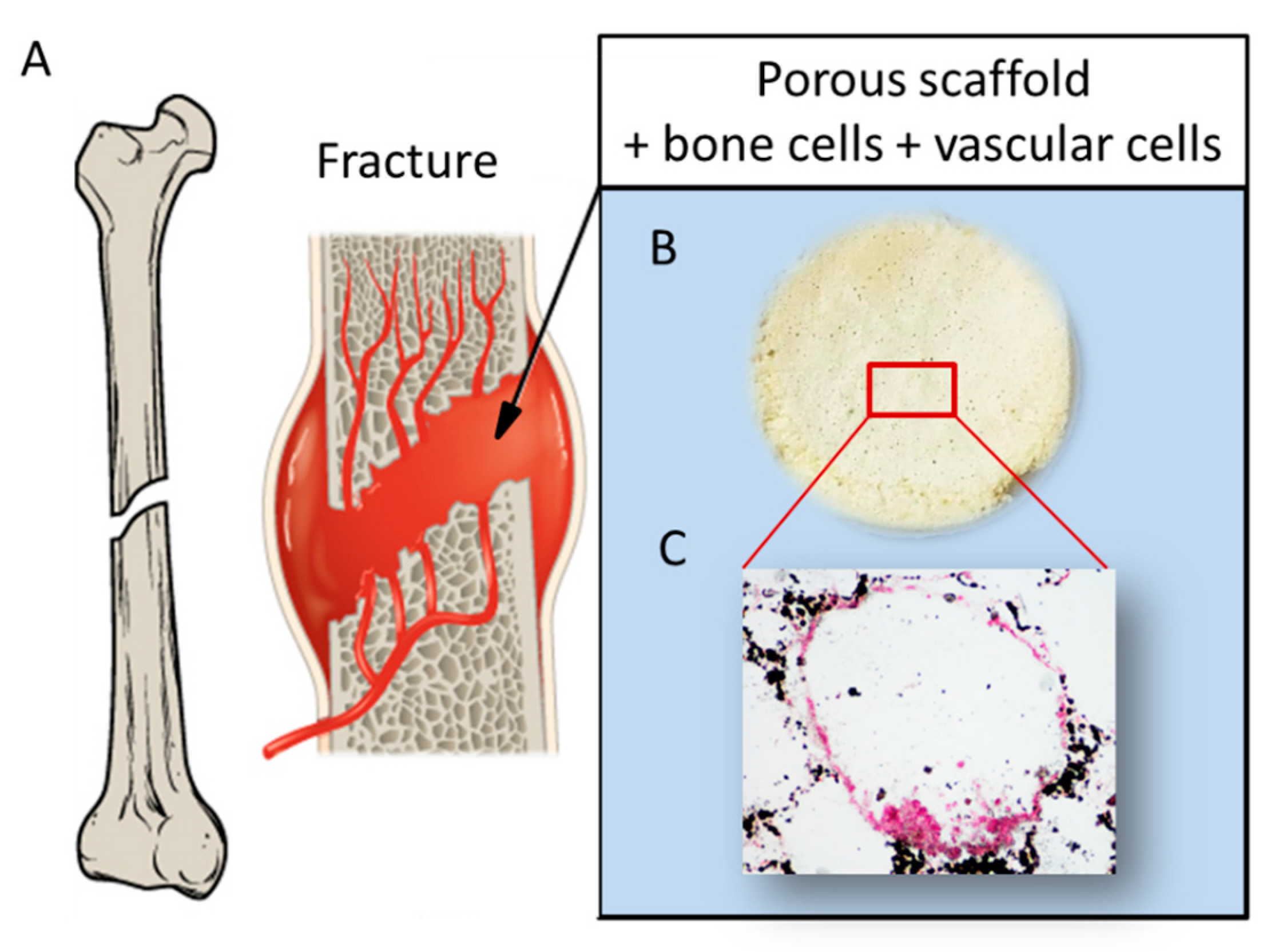
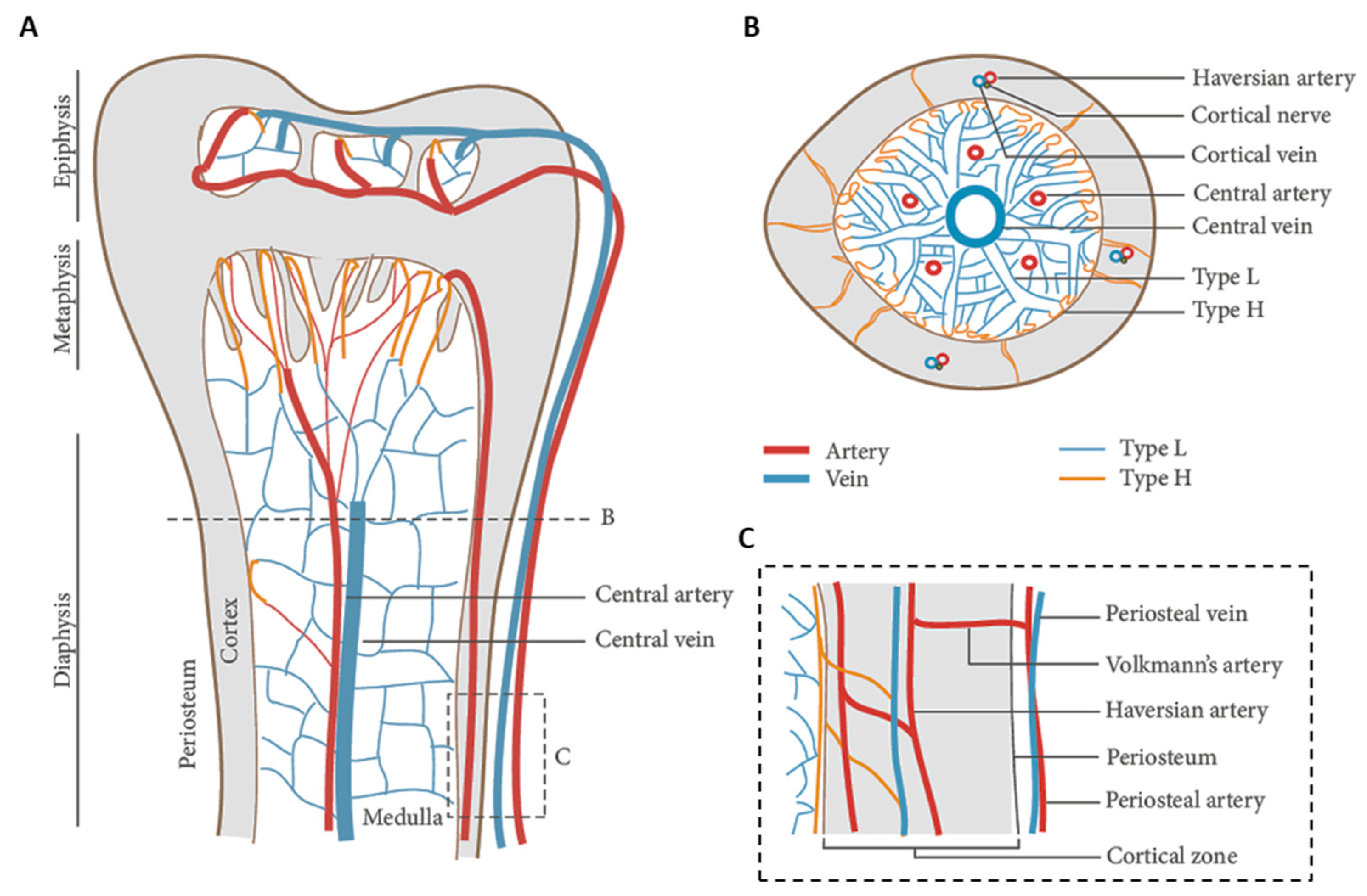
2. Vascularized Bone as a “Piezoelectric” Regenerative Target
3. Cell Sources Used to Engineer Vascularized Bone Substitutes, and Cellular Susceptivity to (Piezo)electric Stimuli
4. Piezoelectricity in Vascular Grafts and Endothelial Regeneration
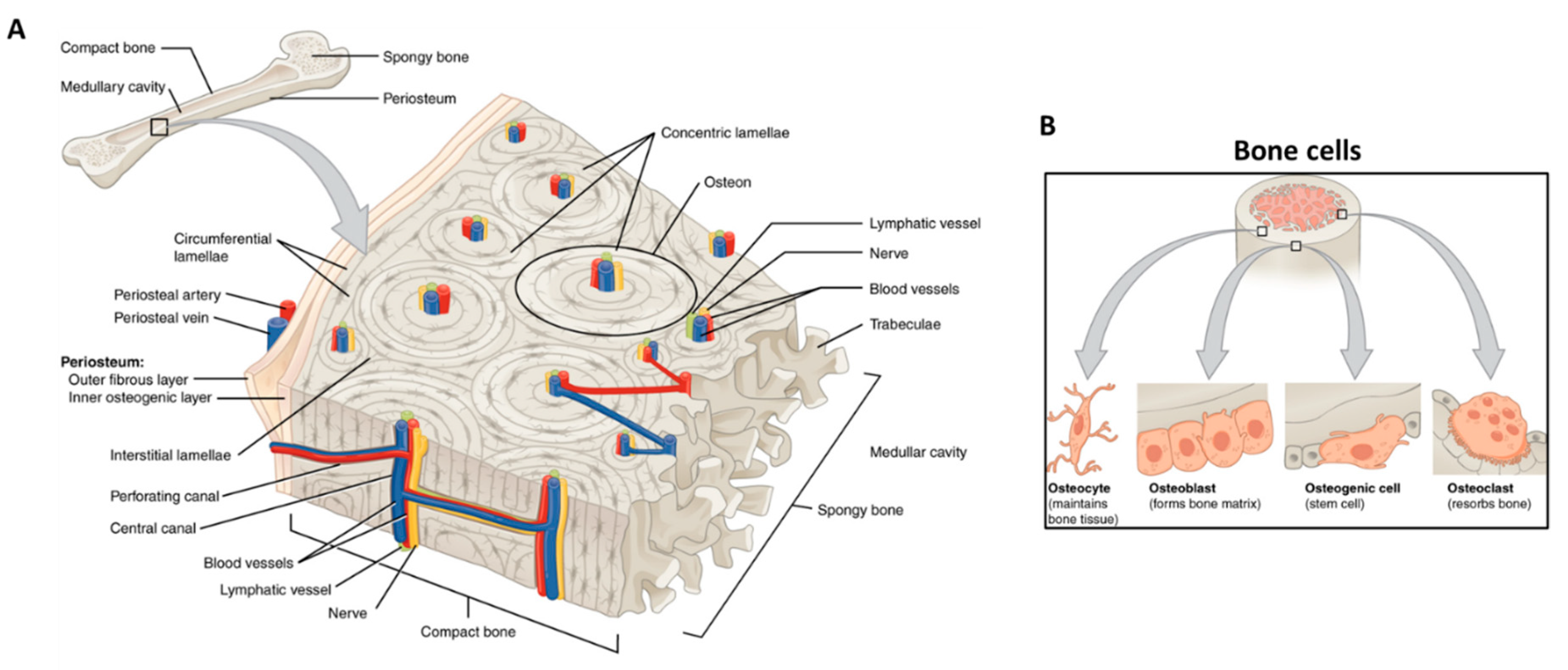
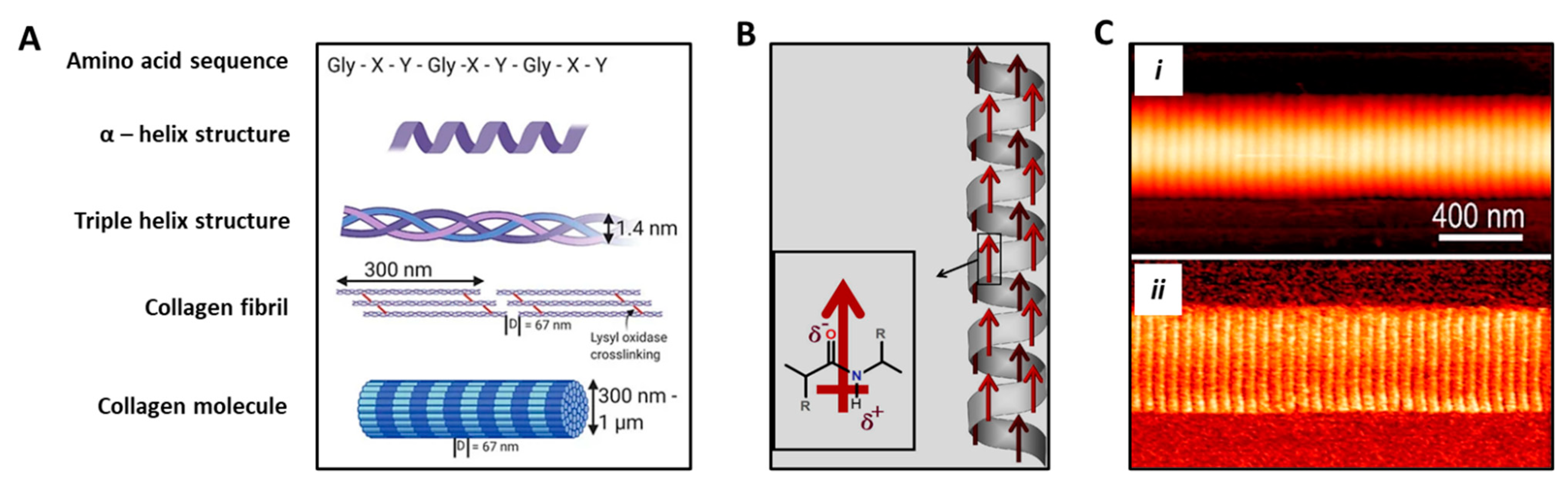
5. Piezoelectricity in Bone Tissue Regeneration
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beldjilali-Labro, M.; Garcia Garcia, A.; Farhat, F.; Bedoui, F.; Grosset, J.-F.; Dufresne, M.; Legallais, C. Biomaterials in tendon and skeletal muscle tissue engineering: Current trends and challenges. Materials 2018, 11, 1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyburz, K.A.; Anseth, K.S. Synthetic mimics of the extracellular matrix: How simple is complex enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020, 258, 120280. [Google Scholar]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef] [PubMed]
- Cros, D.; Gominak, S.; Shahani, B.; Fang, J.; Day, B. Comparison of electric and magnetic coil stimulation in the supraclavicular region. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1992, 15, 587–590. [Google Scholar] [CrossRef]
- Ikeda, T. Fundamentals of Piezoelectricity; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Yasuda, I. On the piezoelectric activity of bone. J. Jpn Orthop Surg Soc. 1954, 28, 267. [Google Scholar]
- Arnau, A.; Soares, D. Fundamentals of Piezoelectricity. In Piezoelectric Transducers and Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–38. [Google Scholar]
- Caliò, R.; Rongala, U.; Camboni, D.; Milazzo, M.; Stefanini, C.; de Petris, G.; Oddo, C. Piezoelectric Energy Harvesting Solutions. Sensors 2014, 14, 4755–4790. [Google Scholar] [CrossRef] [Green Version]
- Fukada, E.; Furukawa, T. Piezoelectricity and ferroelectricity in polyvinylidene fluoride. Ultrasonics 1981, 19, 31–39. [Google Scholar] [CrossRef]
- Fukada, E. Piezoelectricity in polymers and biological materials. Ultrasonics 1968, 6, 229–234. [Google Scholar] [CrossRef]
- da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric phenomenon: A disregarded tool in tissue engineering and regenerative medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wang, X.; Chen, X.-Z.; Mushtaq, F.; Deng, S.; Zhu, C.; Torlakcik, H.; Terzopoulou, A.; Qin, X.-H.; Xiao, X.; et al. 3D-Printed Soft Magnetoelectric Microswimmers for Delivery and Differentiation of Neuron-Like Cells. Adv. Funct. Mater. 2020, 30, 1910323. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, H.-L.; Zelisko, M.; Wang, Y.; Sun, J.; Yan, F.; Ma, F.; Wang, P.; Chen, Q.N.; Zheng, H.; et al. Ferroelectric switching of elastin. Proc. Natl. Acad. Sci. USA 2014, 111, E2780–E2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukada, E.; Yasuda, I. Piezoelectric effects in collagen. Jpn. J. Appl. Phys. 1964, 3, 117. [Google Scholar] [CrossRef]
- Kitsara, M.; Blanquer, A.; Murillo, G.; Humblot, V.; Vieira, S.D.B.; Nogués, C.; Ibáñez, E.; Esteve, J.; Barrios, L. Permanently hydrophilic, piezoelectric PVDF nanofibrous scaffolds promoting unaided electromechanical stimulation on osteoblasts. Nanoscale 2019, 11, 8906–8917. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, P.K.; Metwally, S.; Karbowniczek, J.E.; Marzec, M.M.; Stodolak-Zych, E.; Gruszczyński, A.; Bernasik, A.; Stachewicz, U. Surface-potential-controlled cell proliferation and collagen mineralization on electrospun polyvinylidene fluoride (PVDF) fiber scaffolds for bone regeneration. ACS Biomater. Sci. Eng. 2018, 5, 582–593. [Google Scholar] [CrossRef]
- Martins, P.M.; Ribeiro, S.; Ribeiro, C.; Sencadas, V.; Gomes, A.C.; Gama, F.M.; Lanceros-Méndez, S. Effect of poling state and morphology of piezoelectric poly (vinylidene fluoride) membranes for skeletal muscle tissue engineering. Rsc Adv. 2013, 3, 17938–17944. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Collins, G.; Arinzeh, T.L. Neurite extension of primary neurons on electrospun piezoelectric scaffolds. Acta Biomater. 2011, 7, 3877–3886. [Google Scholar] [CrossRef]
- Hitscherich, P.; Wu, S.; Gordan, R.; Xie, L.H.; Arinzeh, T.; Lee, E.J. The effect of PVDF-TrFE scaffolds on stem cell derived cardiovascular cells. Biotechnol. Bioeng. 2016, 113, 1577–1585. [Google Scholar] [CrossRef]
- Guo, H.F.; Li, Z.S.; Dong, S.W.; Chen, W.J.; Deng, L.; Wang, Y.F.; Ying, D.J. Piezoelectric PU/PVDF electrospun scaffolds for wound healing applications. Colloids Surf. B Biointerfaces 2012, 96, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning piezoelectric fibers for biocompatible devices. Adv. Healthc. Mater. 2020, 9, 1901287. [Google Scholar] [CrossRef]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Berlincourt, D.A.; Cmolik, C.; Jaffe, H. Piezoelectric properties of polycrystalline lead titanate zirconate compositions. Proc. IRE 1960, 48, 220–229. [Google Scholar] [CrossRef]
- Mokhtari, F.; Azimi, B.; Salehi, M.; Hashemikia, S.; Danti, S. Recent advances of polymer-based piezoelectric composites for biomedical applications. J. Mech. Behav. Biomed. Mater. 2021, 122, 104669. [Google Scholar] [CrossRef] [PubMed]
- Fousek, J.; Cross, L.E.; Litvin, D.B. Possible piezoelectric composites based on the flexoelectric effect. Mater. Lett. 1999, 39, 287–291. [Google Scholar] [CrossRef]
- Ekwueme, E.C.; Patel, J.M.; Freeman, J.W.; Danti, S. Applications of bioresorbable polymers in the skeletal systems (cartilages, tendons, bones). In Bioresorbable Polymers for Biomedical Applications; Perale, G., Hilborn, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 391–422. [Google Scholar]
- Milazzo, M.; David, A.; Jung, G.S.; Danti, S.; Buehler, M.J. Molecular origin of viscoelasticity in mineralized collagen fibrils. Biomater. Sci. 2021, 9, 3390–3400. [Google Scholar] [CrossRef]
- Milazzo, M.; Jung, G.S.; Danti, S.; Buehler, M.J. Mechanics of mineralized collagen fibrils upon transient loads. ACS Nano 2020, 14, 8307–8316. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, B.; Liu, X. Osteon: Structure, Turnover, and Regeneration. Tissue Eng. Part. B Rev. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Prisby, R.D. Bone Marrow Microvasculature. Compr. Physiol. 2011, 10, 1009–1046. [Google Scholar]
- Surhone, L.M.; Timpledon, M.T.; Marseken, S.F. (Eds.) Volkmann’s Canals: Osteons, Haversian Canals, Alfred Volkmann, Clopton Havers, Cortical Bone; Betascript Publishing: Riga, Latvia, 2010. [Google Scholar]
- Sawa, N.; Fujimoto, H.; Sawa, Y.; Yamashita, J. Alternating differentiation and dedifferentiation between mature osteoblasts and osteocytes. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubin, J.E. Regulation of osteoblast formation and function. Rev. Endocr. Metab. Disord. 2001, 2, 81–94. [Google Scholar] [CrossRef]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Matic, I.; Matthews, B.G.; Wang, X.; Dyment, N.A.; Worthley, D.L.; Rowe, D.W.; Grcevic, D.; Kalajzic, I. Quiescent bone lining cells are a major source of osteoblasts during adulthood. Stem Cells 2016, 34, 2930–2942. [Google Scholar] [CrossRef] [Green Version]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Xu, F.; Teitelbaum, S.L. Osteoclasts: New insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Aubin, J.E. Advances in the osteoblast lineage. Biochem. Cell Biol. 1998, 76, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. Collagen fibrillogenesis. Connect. Tissue Res. 1982, 10, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Walimbe, T.; Panitch, A. Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels. Bioengineering 2020, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Jung, G.S.; Danti, S.; Buehler, M.J. Wave propagation and energy dissipation in collagen molecules. ACS Biomater. Sci. Eng. 2020, 6, 1367–1374. [Google Scholar] [CrossRef]
- Xu, J.; Shi, G.-P. Vascular wall extracellular matrix proteins and vascular diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 2106–2119. [Google Scholar] [CrossRef] [Green Version]
- Minary-Jolandan, M.; Yu, M.F. Uncovering nanoscale electromechanical heterogeneity in the subfibrillar structure of collagen fibrils responsible for the piezoelectricity of bone. ACS Nano 2009, 3, 1859–1863. [Google Scholar] [CrossRef]
- Minary-Jolandan, M.; Yu, M.-F. Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromolecules 2009, 10, 2565–2570. [Google Scholar] [CrossRef] [Green Version]
- Becker, R.O.; Spadaro, J.A. Electrical stimulation of partial limb regeneration in mammals. Bull. N. Y. Acad. Med. 1972, 48, 627. [Google Scholar]
- Lang, S.B.; Tofail, S.A.M.; Kholkin, A.L.; Wojtaś, M.; Gregor, M.; Gandhi, A.A.; Wang, Y.; Bauer, S.; Krause, M.; Plecenik, A. Ferroelectric polarization in nanocrystalline hydroxyapatite thin films on silicon. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Haverty, D.; Tofail, S.A.M.; Stanton, K.T.; McMonagle, J.B. Structure and stability of hydroxyapatite: Density functional calculation and Rietveld analysis. Phys. Rev. B 2005, 71, 94103. [Google Scholar] [CrossRef]
- Larrouture, Q.C.; Nelson, D.J.; Robinson, L.J.; Liu, L.; Tourkova, I.; Schlesinger, P.H.; Blair, H.C. Chloride--hydrogen antiporters ClC-3 and ClC-5 drive osteoblast mineralization and regulate fine-structure bone patterning in vitro. Physiol. Rep. 2015, 3, e12607. [Google Scholar] [CrossRef]
- Fukada, E.; Hara, K. Piezoelectric effect in blood vessel walls. J. Phys. Soc. Jpn. 1969, 26, 777–780. [Google Scholar] [CrossRef]
- Robert, L.; Jacob, M.P.; Fulop, T. Elastin in blood vessels. Mol. Biol Pathol Elastic Tissues 1995, 192, 286–303. [Google Scholar]
- Caplan, A.I. The mesengenic process. Clin. Plast. Surg. 1994, 21, 429–435. [Google Scholar] [CrossRef]
- Aubin, J.E. Bone stem cells. J. Cell. Biochem. 1998, 72, 73–82. [Google Scholar] [CrossRef]
- Phinney, D.G. Building a consensus regarding the nature and origin of mesenchymal stem cells. J. Cell. Biochem. 2002, 85, 7–12. [Google Scholar] [CrossRef]
- Risbud, M.V.; Sittinger, M. Tissue engineering: Advances in in vitro cartilage generation. TRENDS Biotechnol. 2002, 20, 351–356. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. Others In search of an osteoblast cell model for in vitro research. Eur Cell Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, Y.; Wang, Y.; Ge, L.; Zhai, N.; Han, J. A protocol for isolation and identification and comparative characterization of primary osteoblasts from mouse and rat calvaria. Cell Tissue Bank. 2019, 20, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robey, P.G. Cell sources for bone regeneration: The good, the bad, and the ugly (but promising). Tissue Eng. Part. B Rev. 2011, 17, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef] [PubMed]
- Zvaifler, N.J.; Marinova-Mutafchieva, L.; Adams, G.; Edwards, C.J.; Moss, J.; Burger, J.A.; Maini, R.N. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. Ther. 2000, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Deans, R.J.; Moseley, A.B. Mesenchymal stem cells: Biology and potential clinical uses. Exp. Hematol. 2000, 28, 875–884. [Google Scholar] [CrossRef]
- Barry, F.P.; Boynton, R.E.; Haynesworth, S.; Murphy, J.M.; Zaia, J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem. Biophys. Res. Commun. 1999, 265, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Lund, T.; Lenvik, T.; Aguiar, D.; Koodie, L.; Verfaillie, C.M. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood J. Am. Soc. Hematol. 2001, 98, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Mesimäki, K.; Lindroos, B.; Törnwall, J.; Mauno, J.; Lindqvist, C.; Kontio, R.; Miettinen, S.; Suuronen, R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 2009, 38, 201–209. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Hu, L.; Gu, W.; Zhu, L. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-Exp. Cell Res. 2018, 370, 13–23. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Amantea, M.; Rengo, C.; Rengo, S.; Goldberg, M.; Spagnuolo, G.; Tatullo, M. Adipose tissue as a strategic source of mesenchymal stem cells in bone regeneration: A topical review on the most promising craniomaxillofacial applications. Int. J. Mol. Sci. 2017, 18, 2140. [Google Scholar] [CrossRef] [Green Version]
- Barachini, S.; Danti, S.; Pacini, S.; D’Alessandro, D.; Carnicelli, V.; Trombi, L.; Moscato, S.; Mannari, C.; Cei, S.; Petrini, M. Plasticity of human dental pulp stromal cells with bioengineering platforms: A versatile tool for regenerative medicine. Micron 2014, 67, 155–168. [Google Scholar] [CrossRef]
- Amghar-Maach, S.; Gay-Escoda, C.; Sánchez-Garcés, M. Regeneration of periodontal bone defects with dental pulp stem cells grafting: Systematic Review. J. Clin. Exp. Dent. 2019, 11, e373. [Google Scholar] [CrossRef]
- Trubiani, O.; Pizzicannella, J.; Caputi, S.; Marchisio, M.; Mazzon, E.; Paganelli, R.; Paganelli, A.; Diomede, F. Periodontal ligament stem cells: Current knowledge and future perspectives. Stem Cells Dev. 2019, 28, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Diomede, F.; Gugliandolo, A.; Scionti, D.; Giudice, F.L.; Cariccio, V.L.; Iori, R.; Bramanti, P.; Trubiani, O.; Mazzon, E. Moringin induces neural differentiation in the stem cell of the human periodontal ligament. Sci. Rep. 2018, 8, 1–12. [Google Scholar]
- Pizzicannella, J.; Pierdomenico, S.D.; Piattelli, A.; Varvara, G.; Fonticoli, L.; Trubiani, O.; Diomede, F. 3D human periodontal stem cells and endothelial cells promote bone development in bovine pericardium-based tissue biomaterial. Materials 2019, 12, 2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Thomson, J.A. Induced pluripotent stem cells. In Principles of Tissue Engineering; Lanza, R., Langer, R., Vacanti, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 581–594. [Google Scholar]
- Bastami, F.; Nazeman, P.; Moslemi, H.; Rezai Rad, M.; Sharifi, K.; Khojasteh, A. Induced pluripotent stem cells as a new getaway for bone tissue engineering: A systematic review. Cell Prolif. 2017, 50, e12321. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, A.E.; Kennedy, M.; Bozec, A.; Brunton, F.; Stenbeck, G.; Park, I.-H.; Wagner, E.F.; Keller, G.M. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood J. Am. Soc. Hematol. 2010, 115, 2769–2776. [Google Scholar] [CrossRef] [Green Version]
- Isner, J.M.; Kalka, C.; Kawamoto, A.; Asahara, T. Bone marrow as a source of endothelial cells for natural and iatrogenic vascular repair. Ann. N. Y. Acad. Sci. 2001, 953, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Haendeler, J.; Reinhold, J.; Rochwalsky, U.; Seeger, F.; Honold, J.; Hoffmann, J.; Urbich, C.; Lehmann, R.; Arenzana-Seisdesdos, F.; et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ. Res. 2005, 97, 1142–1151. [Google Scholar] [CrossRef] [Green Version]
- Stolk, L.M.L.; der Geest, S. Plasma concentrations after a clomipramine intoxication. J. Anal. Toxicol. 1998, 22, 612–613. [Google Scholar] [CrossRef] [Green Version]
- Goerke, S.M.; Obermeyer, J.; Plaha, J.; Stark, G.B.; Finkenzeller, G. Endothelial progenitor cells from peripheral blood support bone regeneration by provoking an angiogenic response. Microvasc. Res. 2015, 98, 40–47. [Google Scholar] [CrossRef]
- Rana, D.; Kumar, A.; Sharma, S. Endothelial progenitor cells as molecular targets in vascular senescence and repair. Curr. Stem Cell Res. Ther. 2018, 13, 438–446. [Google Scholar] [CrossRef]
- Tamari, T.; Kawar-Jaraisy, R.; Doppelt, O.; Giladi, B.; Sabbah, N.; Zigdon-Giladi, H. The paracrine role of endothelial cells in bone formation via CXCR4/SDF-1 pathway. Cells 2020, 9, 1325. [Google Scholar] [CrossRef]
- Pang, H.; Wu, X.-H.; Fu, S.-L.; Luo, F.; Zhang, Z.-H.; Hou, T.-Y.; Li, Z.-Q.; Chang, Z.-Q.; Yu, B.; Xu, J.-Z. Prevascularisation with endothelial progenitor cells improved restoration of the architectural and functional properties of newly formed bone for bone reconstruction. Int. Orthop. 2013, 37, 753–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Zhu, Y.; Qiu, S.; Xu, J.; Chai, Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wu, V.; Helder, M.N.; Bravenboer, N.; Ten Bruggenkate, C.M.; Jin, J.; Klein-Nulend, J.; Schulten, E.A.J.M. Bone tissue regeneration in the oral and maxillofacial region: A review on the application of stem cells and new strategies to improve vascularization. Stem Cells Int. 2019, 2019, 6279721. [Google Scholar] [CrossRef] [Green Version]
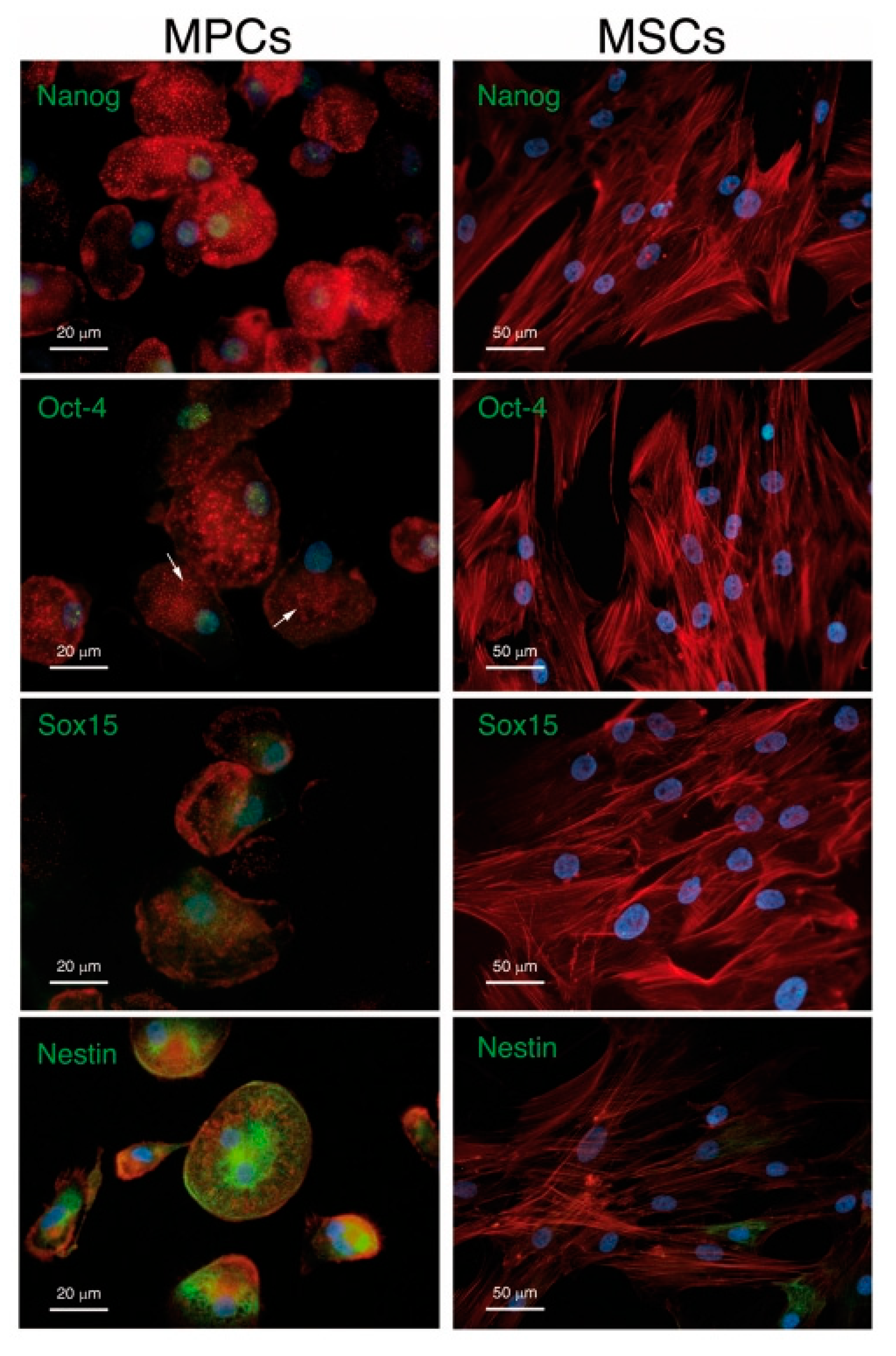
- Petrini, M.; Pacini, S.; Trombi, L.; Fazzi, R.; Montali, M.; Ikehara, S.; Abraham, N.G. Identification and purification of mesodermal progenitor cells from human adult bone marrow. Stem Cells Dev. 2009, 18, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Pacini, S.; Carnicelli, V.; Trombi, L.; Montali, M.; Fazzi, R.; Lazzarini, E.; Giannotti, S.; Petrini, M. Constitutive expression of pluripotency-associated genes in mesodermal progenitor cells (MPCs). PLoS ONE 2010, 5, e9861. [Google Scholar] [CrossRef] [PubMed]
- Montali, M.; Barachini, S.; Pacini, S.; Panvini, F.M.; Petrini, M. Isolating mesangiogenic progenitor cells (MPCs) from human bone marrow. J. Vis. Exp. 2016, 15, 10–3791. [Google Scholar] [CrossRef] [Green Version]
- Fazzi, R.; Pacini, S.; Carnicelli, V.; Trombi, L.; Montali, M.; Lazzarini, E.; Petrini, M. Mesodermal progenitor cells (MPCs) differentiate into mesenchymal stromal cells (MSCs) by activation of Wnt5/calmodulin signalling pathway. PLoS ONE 2011, 6, e25600. [Google Scholar] [CrossRef] [Green Version]
- Montali, M.; Panvini, F.M.; Barachini, S.; Ronca, F.; Carnicelli, V.; Mazzoni, S.; Petrini, I.; Pacini, S. Human adult mesangiogenic progenitor cells reveal an early angiogenic potential, which is lost after mesengenic differentiation. Stem Cell Res. Ther. 2017, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pacini, S.; Barachini, S.; Montali, M.; Carnicelli, V.; Fazzi, R.; Parchi, P.; Petrini, M. Mesangiogenic progenitor cells derived from one novel CD64brightCD31brightCD14neg population in human adult bone marrow. Stem Cells Dev. 2016, 25, 661–673. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, D.; Moscato, S.; Fusco, A.; la Ossa, J.G.; D’Acunto, M.; Trombi, L.; Feula, M.; Serino, L.P.; Donnarumma, G.; Petrini, M.; et al. Poly (vinyl alcohol)/Gelatin Scaffolds Allow Regeneration of Nasal Tissues. Appl. Sci. 2021, 11, 3651. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Hashimoto, M.; Yamaguchi, S.; Itoh, Y.; Yoshimoto, I.; Matsumoto, T.; Imazato, S. Fabrication of biomimetic bone tissue using mesenchymal stem cell-derived three-dimensional constructs incorporating endothelial cells. PLoS ONE 2015, 10, e0129266. [Google Scholar]
- Bidarra, S.J.; Barrias, C.C.; Barbosa, M.A.; Soares, R.; Amédée, J.; Granja, P.L. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res. 2011, 7, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Genova, T.; Munaron, L.; Carossa, S.; Mussano, F. Overcoming physical constraints in bone engineering:‘the importance of being vascularized. J. Biomater. Appl. 2016, 30, 940–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Ossa, J.G.; Trombi, L.; D’Alessandro, D.; Coltelli, M.B.; Serino, L.P.; Pini, R.; Lazzeri, A.; Petrini, M.; Danti, S. Pore size distribution and blend composition affect in vitro prevascularized bone matrix formation on poly (vinyl alcohol)/gelatin sponges. Macromol. Mater. Eng. 2017, 302, 1700300. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2008, 3, e3737. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, K.; Boda, S.K.; Basu, B. Synergy of substrate conductivity and intermittent electrical stimulation towards osteogenic differentiation of human mesenchymal stem cells. Bioelectrochemistry 2017, 116, 52–64. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Banks, T.A.; Luckman, P.S.B.; Frith, J.E.; Cooper-White, J.J. Effects of electric fields on human mesenchymal stem cell behaviour and morphology using a novel multichannel device. Integr. Biol. 2015, 7, 693–712. [Google Scholar] [CrossRef]
- Tzoneva, R. Influence of electric field on cell behavior. Electrotreatment of cells for biomedical applications. Asian J. Phys. 2014, 23, 789–814. [Google Scholar]
- Creecy, C.M.; O’Neill, C.F.; Arulanandam, B.P.; Sylvia, V.L.; Navara, C.S.; Bizios, R. Mesenchymal stem cell osteodifferentiation in response to alternating electric current. Tissue Eng. Part. A 2013, 19, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.-C.; Lim, J.; Hwang, W.-H.; Lin, Y.-X.; Wang, J.-L. Piezoelectric stimulation by ultrasound facilitates chondrogenesis of mesenchymal stem cells. J. Acoust. Soc. Am. 2020, 148, EL58–EL64. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 2017, 149, 51–62. [Google Scholar] [CrossRef]
- Steward, A.J.; Kelly, D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015, 227, 717–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, S.K. Structure and functions of blood vessels and vascular niches in bone. Stem Cells Int. 2017, 2017, 5046953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.I.; Reis, R.L. Vascularization in bone tissue engineering: Physiology, current strategies, major hurdles and future challenges. Macromol. Biosci. 2010, 10, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Kneser, U.; Schaefer, D.J.; Polykandriotis, E.; Horch, R.E. Tissue engineering of bone: The reconstructive surgeon’s point of view. J. Cell. Mol. Med. 2006, 10, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, K.; Gopalakrishnan, S. Nanomedicine in the diagnosis and treatment of atherosclerosis-a systematic review. Cardiovasc. Haematol. Disord. Targets. 2017, 17, 119–131. [Google Scholar] [CrossRef]
- Ziegler, K.R.; Muto, A.; Eghbalieh, S.D.D.; Dardik, A. Basic data related to surgical infrainguinal revascularization procedures: A twenty year update. Ann. Vasc. Surg. 2011, 25, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Sidawy, A.N.; DeZee, K.J.; Neville, R.F.; Akbari, C.; Henderson, W. Factors associated with early failure of infrainguinal lower extremity arterial bypass. J. Vasc. Surg. 2008, 47, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Cheong, J.H.; Ng, S.S.Y.; Liu, X.; Xue, R.-F.; Lim, H.J.; Khannur, P.B.; Chan, K.L.; Lee, A.A.; Kang, K.; Lim, L.S.; et al. An inductively powered implantable blood flow sensor microsystem for vascular grafts. IEEE Trans. Biomed. Eng. 2012, 59, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Koobatian, M.T.; Koenigsknecht, C.; Row, S.; Andreadis, S.; Swartz, D. Surgical technique for the implantation of tissue engineered vascular grafts and subsequent in vivo monitoring. J. Vis. Exp. 2015, 98, e52354. [Google Scholar] [CrossRef] [PubMed]
- Oresanya, L.; Makam, A.N.; Belkin, M.; Moneta, G.L.; Conte, M.S. Factors associated with primary vein graft occlusion in a multicenter trial with mandated ultrasound surveillance. J. Vasc. Surg. 2014, 59, 996–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [Green Version]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef]
- Majerus, S.J.A.; Dunning, J.; Potkay, J.A.; Bogie, K.M. Flexible, structured MWCNT/PDMS sensor for chronic vascular access monitoring. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Neville, R.F.; Gupta, S.K.; Kuraguntla, D.J. Initial in vitro and in vivo evaluation of a self-monitoring prosthetic bypass graft. J. Vasc. Surg. 2017, 65, 1793–1801. [Google Scholar] [CrossRef] [Green Version]
- Park, D.Y.; Joe, D.J.; Kim, D.H.; Park, H.; Han, J.H.; Jeong, C.K.; Park, H.; Park, J.G.; Joung, B.; Lee, K.J. Self-powered real-time arterial pulse monitoring using ultrathin epidermal piezoelectric sensors. Adv. Mater. 2017, 29, 1702308. [Google Scholar] [CrossRef]
- Sekine, T.; Sugano, R.; Tashiro, T.; Sato, J.; Takeda, Y.; Matsui, H.; Kumaki, D.; Dos Santos, F.D.; Miyabo, A.; Tokito, S. Fully printed wearable vital sensor for human pulse rate monitoring using ferroelectric polymer. Sci. Rep. 2018, 8, 1–10. [Google Scholar]
- Natta, L.; Mastronardi, V.M.; Guido, F.; Algieri, L.; Puce, S.; Pisano, F.; Rizzi, F.; Pulli, R.; Qualtieri, A.; De Vittorio, M. Soft and flexible piezoelectric smart patch for vascular graft monitoring based on aluminum nitride thin film. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Sharma, T.; Naik, S.; Langevine, J.; Gill, B.; Zhang, J.X.J. Aligned PVDF-TrFE nanofibers with high-density PVDF nanofibers and PVDF core--shell structures for endovascular pressure sensing. IEEE Trans. Biomed. Eng. 2014, 62, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue engineering at the blood-contacting surface: A review of challenges and strategies in vascular graft development. Adv. Healthc. Mater. 2018, 7, 1701461. [Google Scholar] [CrossRef]
- Gostev, A.A.; Karpenko, A.A.; Laktionov, P.P. Polyurethanes in cardiovascular prosthetics. Polym. Bull. 2018, 75, 4311–4325. [Google Scholar] [CrossRef]
- Soldani, G.; Losi, P.; Bernabei, M.; Burchielli, S.; Chiappino, D.; Kull, S.; Briganti, E.; Spiller, D. Long term performance of small-diameter vascular grafts made of a poly (ether) urethane--polydimethylsiloxane semi-interpenetrating polymeric network. Biomaterials 2010, 31, 2592–2605. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; De Groot, J.; Clatworthy, I.; Bozec, L.; Horton, M.; Butler, P.E.; Seifalian, A.M. The antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocomposite. Biomacromolecules 2006, 7, 215–223. [Google Scholar] [CrossRef] [PubMed]
- de Mel, A.; Chaloupka, K.; Malam, Y.; Darbyshire, A.; Cousins, B.; Seifalian, A.M. A silver nanocomposite biomaterial for blood-contacting implants. J. Biomed. Mater. Res. Part A 2012, 100, 2348–2357. [Google Scholar] [CrossRef]
- Whitfle, B.J.R.; Moncada, S. Pharmacological interactions between prostacyclin and thromboxanes. Br. Med. Bull. 1983, 39, 232–238. [Google Scholar] [CrossRef]
- Colowick, S.P.; Kaplan, N.O. Structural and contractile proteins, extra-cellular matrix. Methods Enzymol. 1983, 82, 33–61. [Google Scholar]
- Setter, N.; Damjanovic, D.; Eng, L.; Fox, G.; Gevorgian, S.; Hong, S.; Kingon, A.; Kohlstedt, H.; Park, N.Y.; Stephenson, G.B.; et al. Ferroelectric thin films: Review of materials, properties, and applications. J. Appl. Phys. 2006, 100, 51606. [Google Scholar] [CrossRef]
- Mindlin, R.D. Elasticity, piezoelectricity and crystal lattice dynamics. J. Elast. 1972, 2, 217–282. [Google Scholar] [CrossRef] [Green Version]
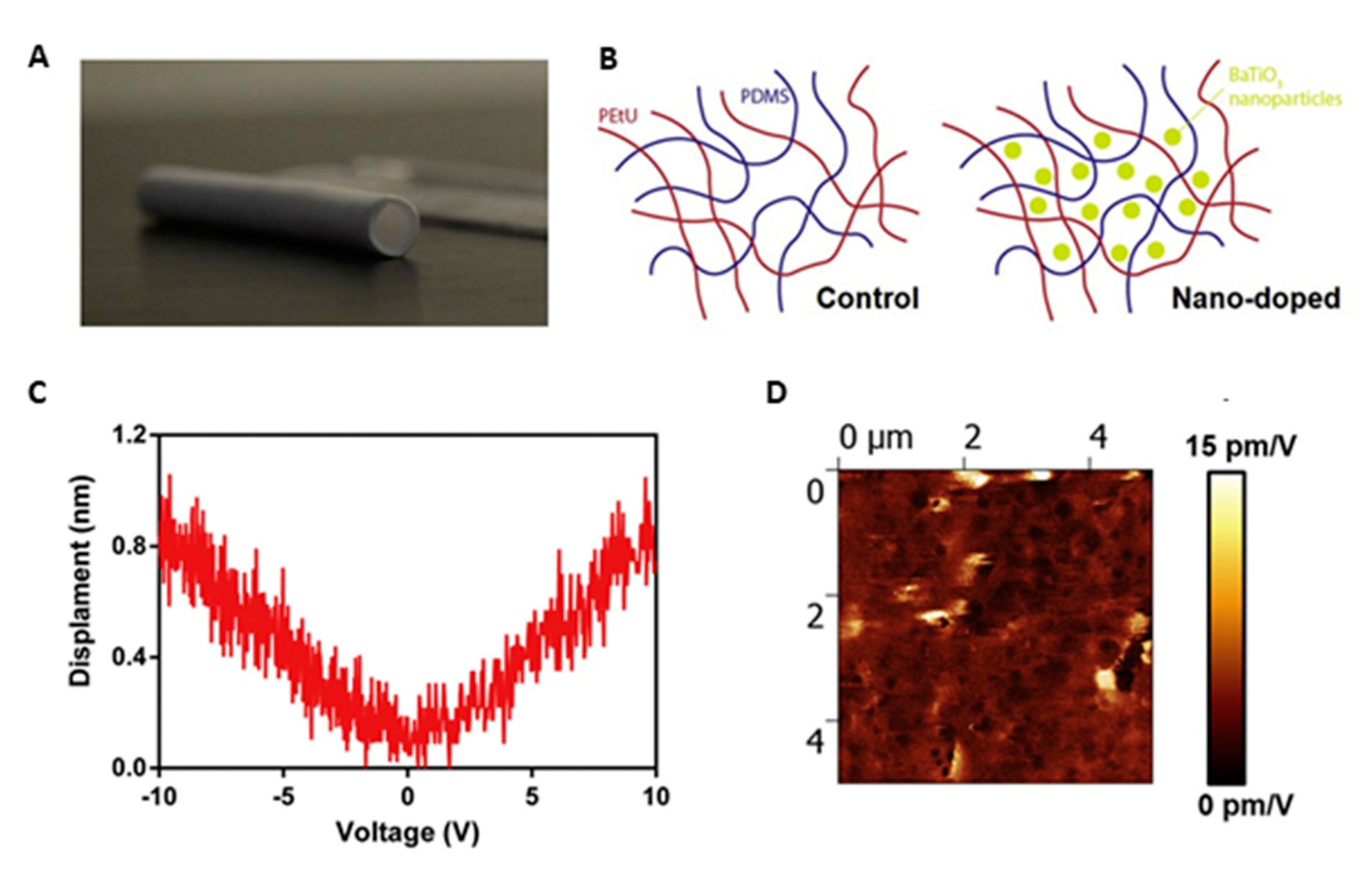
- Cafarelli, A.; Losi, P.; Salgarella, A.R.; Barsotti, M.C.; Di Cioccio, I.B.; Foffa, I.; Vannozzi, L.; Pingue, P.; Soldani, G.; Ricotti, L. Small-caliber vascular grafts based on a piezoelectric nanocomposite elastomer: Mechanical properties and biocompatibility. J. Mech. Behav. Biomed. Mater. 2019, 97, 138–148. [Google Scholar] [CrossRef]
- Bouaziz, A.; Vacher, M.; Caprani, A. Effect of constant and modulated electrical charges applied to the culture material on PGI2 and TXA2 secretion by endothelial cells. Biomaterials 1995, 16, 727–734. [Google Scholar] [CrossRef]
- Baxter, F.R.; Bowen, C.R.; Turner, I.G.; Dent, A.C.E. Electrically active bioceramics: A review of interfacial responses. Ann. Biomed. Eng. 2010, 38, 2079–2092. [Google Scholar] [CrossRef]
- Jensen, D.B.; Li, Z.; Pavel, I.; Dervishi, E.; Biris, A.S.; Biris, A.R.; Lupu, D.; Jensen, P.J. Bone tissue: A relationship between micro and nano structural composition and its corresponding electrostatic properties with applications in tissue engineering. In Proceedings of the 2007 IEEE Industry Applications Annual Meeting, New Orleans, LA, USA, 23–27 September 2007; pp. 55–59. [Google Scholar]
- Zhu, Y.; Li, Z.; Zhang, Y.; Lan, F.; He, J.; Wu, Y. The essential role of osteoclast-derived exosomes in magnetic nanoparticle-infiltrated hydroxyapatite scaffold modulated osteoblast proliferation in an osteoporosis model. Nanoscale 2020, 12, 8720–8726. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Kini, U.; Nandeesh, B.N. Physiology of Bone Formation, Remodeling, and Metabolism. In Radionuclide and Hybrid Bone Imaging; Fogelman, I., Gnanasegaran, G., van der Wall, H., Eds.; Springer: Berlin, Germany, 2012; pp. 29–57. [Google Scholar]
- Goonoo, N.; Bhaw-Luximon, A.; Passanha, P.; Esteves, S.R.; Jhurry, D. Third generation poly (hydroxyacid) composite scaffolds for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernozem, R.V.; Guselnikova, O.; Surmeneva, M.A.; Postnikov, P.S.; Abalymov, A.A.; Parakhonskiy, B.V.; De Roo, N.; Depla, D.; Skirtach, A.G.; Surmenev, R.A. Diazonium chemistry surface treatment of piezoelectric polyhydroxybutyrate scaffolds for enhanced osteoblastic cell growth. Appl. Mater. Today 2020, 20, 100758. [Google Scholar] [CrossRef]
- Correia, C.; Bhumiratana, S.; Yan, L.-P.; Oliveira, A.L.; Gimble, J.M.; Rockwood, D.; Kaplan, D.L.; Sousa, R.A.; Reis, R.L.; Vunjak-Novakovic, G. Development of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cells. Acta Biomater. 2012, 8, 2483–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Fukada, E.; Ando, Y. Piezoelectric properties of poly-β-hydroxybutyrate and copolymers of β-hydroxybutyrate and β-hydroxyvalerate. Int. J. Biol. Macromol. 1986, 8, 361–366. [Google Scholar] [CrossRef]
- Knowles, J.C.; Hastings, G.W.; Ohta, H.; Niwa, S.; Boeree, N. Development of a degradable composite for orthopaedic use: In vivo biomechanical and histological evaluation of two bioactive degradable composites based on the polyhydroxybutyrate polymer. Biomaterials 1992, 13, 491–496. [Google Scholar] [CrossRef]
- Antonini, S.; Montali, M.; Jacchetti, E.; Meucci, S.; Parchi, P.D.; Barachini, S.; Panvini, F.M.; Pacini, S.; Petrini, I.; Cecchini, M. Nanotopography Induced Human Bone Marrow Mesangiogenic Progenitor Cells (MPCs) to Mesenchymal Stromal Cells (MSCs) Transition. Front. Cell Dev. Biol. 2016, 4, 144. [Google Scholar] [CrossRef] [Green Version]
- El Messiery, M.A.; Hastings, G.W.; Rakowski, S. Ferro-electricity of dry cortical bone. J. Biomed. Eng. 1979, 1, 63–65. [Google Scholar] [CrossRef]
- Lang, S.B. Pyroelectricity: Occurrence in biological materials and ossible physiological implications. Ferroelectrics 1981, 34, 3–9. [Google Scholar] [CrossRef]
- McDowell, C.S. Implanted Bone Stimulator and Prosthesis System and Method of Enhancing Bone Growth. U.S. Patent 6,143, 28 January 1999. [Google Scholar]
- Danti, S.; Ciofani, G.; Moscato, S.; D’Alessandro, D.; Ciabatti, E.; Nesti, C.; Brescia, R.; Bertoni, G.; Pietrabissa, A.; Lisanti, M.; et al. Boron nitride nanotubes and primary human osteoblasts: In vitro compatibility and biological interactions under low frequency ultrasound stimulation. Nanotechnology 2013, 24, 465102. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.T.; Gomes, M.E.; Mano, J.F.; Reis, R.L. β-PVDF membranes induce cellular proliferation and differentiation in static and dynamic conditions. In Materials Science Forum; Trans Tech Publication Ltd.: Bäch, Switzerland, 2008; Volume 587, pp. 72–76. [Google Scholar]
- Marques-Almeida, T.; Cardoso, V.F.; Gama, M.; Lanceros-Mendez, S.; Ribeiro, C. Patterned Piezoelectric Scaffolds for Osteogenic Differentiation. Int. J. Mol. Sci. 2020, 21, 8352. [Google Scholar] [CrossRef]
- Gimenes, R.; Zaghete, M.A.; Bertolini, M.; Varela, J.A.; Coelho, L.O.; Silva Jr, N.F. Composites PVDF-TrFE/BT used as bioactive membranes for enhancing bone regeneration. In Proceedings of the Smart Structures and Materials 2004: Electroactive Polymer Actuators and Devices (EAPAD); SPIE: San Diego, CA, USA, 2004; Volume 5385, pp. 539–547. [Google Scholar]
- Zhang, X.; Zhang, C.; Lin, Y.; Hu, P.; Shen, Y.; Wang, K.; Meng, S.; Chai, Y.; Dai, X.; Liu, X.; et al. Nanocomposite membranes enhance bone regeneration through restoring physiological electric microenvironment. ACS Nano 2016, 10, 7279–7286. [Google Scholar] [CrossRef] [PubMed]
- Saburi, E.; Islami, M.; Hosseinzadeh, S.; Moghadam, A.S.; Mansour, R.N.; Azadian, E.; Joneidi, Z.; Nikpoor, A.R.; Ghadiani, M.H.; Khodaii, Z.; et al. In vitro osteogenic differentiation potential of the human induced pluripotent stem cells augments when grown on Graphene oxide-modified nanofibers. Gene 2019, 696, 72–79. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P. Applications of PHAs in medicine and pharmacy. Biopolymers 2002, 4, 91–127. [Google Scholar]
- Chen, G.-Q. A microbial polyhydroxyalkanoates (PHA) based bio-and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Kumarasuriyar, A.; Jackson, R.A.; Grøndahl, L.; Trau, M.; Nurcombe, V.; Cool, S.M. Poly (β-hydroxybutyrate-co-β-hydroxyvalerate) supports in vitro osteogenesis. Tissue Eng. 2005, 11, 1281–1295. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Pancholi, M.; Stubbs Iii, J.; Raghavan, D. Influence of hydroxyvalerate composition of polyhydroxy butyrate valerate (PHBV) copolymer on bone cell viability and in vitro degradation. J. Appl. Polym. Sci. 2010, 116, 3225–3231. [Google Scholar]
- Reusch, R.N. Low molecular weight complexed poly (3-hydroxybutyrate): A dynamic and versatile molecule in vivo. Can. J. Microbiol. 1995, 41, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Hazer, D.B.; Kiliçay, E.; Hazer, B. Poly (3-hydroxyalkanoate) s: Diversification and biomedical applications: A state of the art review. Mater. Sci. Eng. C 2012, 32, 637–647. [Google Scholar] [CrossRef]
- Liu, Y.; Lim, J.; Teoh, S.-H. Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef]
- Qu, X.-H.; Wu, Q.; Zhang, K.-Y.; Chen, G.Q. In vivo studies of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) based polymers: Biodegradation and tissue reactions. Biomaterials 2006, 27, 3540–3548. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Wu, Q.; Chen, G.-Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef]
- Doyle, C.; Tanner, E.T.; Bonfield, W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef]
- Hayati, A.N.; Rezaie, H.R.; Hosseinalipour, S.M. Preparation of poly (3-hydroxybutyrate)/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Lett. 2011, 65, 736–739. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Medvecky, L. Microstructure and properties of polyhydroxybutyrate-chitosan-nanohydroxyapatite composite scaffolds. Sci. World J. 2012, 2012, 537973. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Biocomposite scaffolds for bone regeneration: Role of chitosan and hydroxyapatite within poly-3-hydroxybutyrate-co-3-hydroxyvalerate on mechanical properties and in vitro evaluation. J. Mech. Behav. Biomed. Mater. 2015, 51, 88–98. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Shkarina, S.N.; Loza, K.; Epple, M.; Ulbricht, M.; Cecilia, A.; Krause, B.; Baumbach, T.; Abalymov, A.A.; et al. Piezoelectric 3-D fibrous poly (3-hydroxybutyrate)-based scaffolds ultrasound-mineralized with calcium carbonate for bone tissue engineering: Inorganic phase formation, osteoblast cell adhesion, and proliferation. ACS Appl. Mater. Interfaces 2019, 11, 19522–19533. [Google Scholar] [CrossRef]
- Naderi, P.; Zarei, M.; Karbasi, S.; Salehi, H. Evaluation of the effects of keratin on physical, mechanical and biological properties of poly (3-hydroxybutyrate) electrospun scaffold: Potential application in bone tissue engineering. Eur. Polym. J. 2020, 124, 109502. [Google Scholar] [CrossRef]
- Ke, S.; Huang, H.; Ren, L.; Wang, Y. Nearly constant dielectric loss behavior in poly (3-hydroxybutyrate-co-3-hydroxyvalerate) biodegradable polyester. J. Appl. Phys. 2009, 105, 096103. [Google Scholar] [CrossRef] [Green Version]
- Zonari, A.; Novikoff, S.; Electo, N.R.P.; Breyner, N.M.; Gomes, D.A.; Martins, A.; Neves, N.M.; Reis, R.L.; Goes, A.M. Endothelial differentiation of human stem cells seeded onto electrospun polyhydroxybutyrate/polyhydroxybutyrate-co-hydroxyvalerate fiber mesh. PLoS ONE 2012, 7, e35422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rentsch, C.; Rentsch, B.; Breier, A.; Hofmann, A.; Manthey, S.; Scharnweber, D.; Biewener, A.; Zwipp, H. Evaluation of the osteogenic potential and vascularization of 3D poly (3) hydroxybutyrate scaffolds subcutaneously implanted in nude rats. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 185–195. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Abalymov, A.A.; Parakhonskiy, B.V.; Rigole, P.; Coenye, T.; Surmenev, R.A.; Skirtach, A.G. Piezoelectric hybrid scaffolds mineralized with calcium carbonate for tissue engineering: Analysis of local enzyme and small-molecule drug delivery, cell response and antibacterial performance. Mater. Sci. Eng. C 2021, 122, 111909. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E. Piezoelectricity of biopolymers. Biorheology 1995, 32, 593–609. [Google Scholar] [CrossRef]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Tsuji, T.; Motohara, S.; Uehata, H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 2000, 102, 399–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, C.S.; Fitz-Gerald, J.M.; Park, C. Decoupling the effects of crystallinity and orientation on the shear piezoelectricity of polylactic acid. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1555–1562. [Google Scholar] [CrossRef]
- Tajitsu, Y. Fundamental study on improvement of piezoelectricity of poly (ι-lactic acid) and its application to film actuators. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013, 60, 1625–1629. [Google Scholar] [CrossRef]
- Bernard, F.; Gimeno, L.; Viala, B.; Gusarov, B.; Cugat, O. Direct piezoelectric coefficient measurements of PVDF and PLLA under controlled strain and stress. In Proceedings of the Multidisciplinary Digital Publishing Institute Proceedings, Valencia, Spain, 6–8 March 2017; Volume 1, p. 335. [Google Scholar]
- Chen, Y.; Mak, A.F.T.; Wang, M.; Li, J.; Wong, M.S. PLLA scaffolds with biomimetic apatite coating and biomimetic apatite/collagen composite coating to enhance osteoblast-like cells attachment and activity. Surf. Coat. Technol. 2006, 201, 575–580. [Google Scholar] [CrossRef]
- Li, Y.; Dai, X.; Bai, Y.; Liu, Y.; Wang, Y.; Liu, O.; Yan, F.; Tang, Z.; Zhang, X.; Deng, X. Electroactive BaTiO3 nanoparticle-functionalized fibrous scaffolds enhance osteogenic differentiation of mesenchymal stem cells. Int. J. Nanomed. 2017, 12, 4007. [Google Scholar] [CrossRef] [Green Version]
- Rocha, L.B.; Goissis, G.; Rossi, M.A. Biocompatibility of anionic collagen matrix as scaffold for bone healing. Biomaterials 2002, 23, 449–456. [Google Scholar] [CrossRef]
- Moreira, P.L.; An, Y.H.; Santos Jr, A.R.; Genari, S.C. In vitro analysis of anionic collagen scaffolds for bone repair. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 71, 229–237. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Shamma, R.N. Determination of cytocompatibility and osteogenesis properties of in situ forming collagen-based scaffolds loaded with bone synthesizing drug for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 494–500. [Google Scholar] [CrossRef]
- Lazzeri, L.; Cascone, M.G.; Danti, S.; Serino, L.P.; Moscato, S.; Bernardini, N. Gelatine/PLLA sponge-like scaffolds: Morphological and biological characterization. J. Mater. Sci. Mater. Med. 2007, 18, 1399–1405. [Google Scholar] [CrossRef]
- Danti, S.; Serino, L.P.; D’Alessandro, D.; Moscato, S.; Danti, S.; Trombi, L.; Dinucci, D.; Chiellini, F.; Pietrabissa, A.; Lisanti, M.; et al. Growing bone tissue-engineered niches with graded osteogenicity: An in vitro method for biomimetic construct assembly. Tissue Eng. Part C Methods 2013, 19, 911–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Martino, A.; Sittinger, M.; Risbud, M. V Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, D.; Ricci, C.; Milazzo, M.; Strangis, G.; Forli, F.; Buda, G.; Petrini, M.; Berrettini, S.; Uddin, M.J.; Danti, S.; et al. Piezoelectric Signals in Vascularized Bone Regeneration. Biomolecules 2021, 11, 1731. https://doi.org/10.3390/biom11111731
D’Alessandro D, Ricci C, Milazzo M, Strangis G, Forli F, Buda G, Petrini M, Berrettini S, Uddin MJ, Danti S, et al. Piezoelectric Signals in Vascularized Bone Regeneration. Biomolecules. 2021; 11(11):1731. https://doi.org/10.3390/biom11111731
Chicago/Turabian StyleD’Alessandro, Delfo, Claudio Ricci, Mario Milazzo, Giovanna Strangis, Francesca Forli, Gabriele Buda, Mario Petrini, Stefano Berrettini, Mohammed Jasim Uddin, Serena Danti, and et al. 2021. "Piezoelectric Signals in Vascularized Bone Regeneration" Biomolecules 11, no. 11: 1731. https://doi.org/10.3390/biom11111731
APA StyleD’Alessandro, D., Ricci, C., Milazzo, M., Strangis, G., Forli, F., Buda, G., Petrini, M., Berrettini, S., Uddin, M. J., Danti, S., & Parchi, P. (2021). Piezoelectric Signals in Vascularized Bone Regeneration. Biomolecules, 11(11), 1731. https://doi.org/10.3390/biom11111731








