Quantitative Morphological Analysis of Filamentous Microorganisms in Cocultures and Monocultures: Aspergillus terreus and Streptomyces rimosus Warfare in Bioreactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Bioreactor Experiments
- (1)
- coculture of A. terreus and S. rimosus,
- (2)
- monoculture of A. terreus,
- (3)
- monoculture of S. rimosus.
2.3. Coculture Initiation Strategies and Cultivation Medium Compositions
2.4. Media Compositions
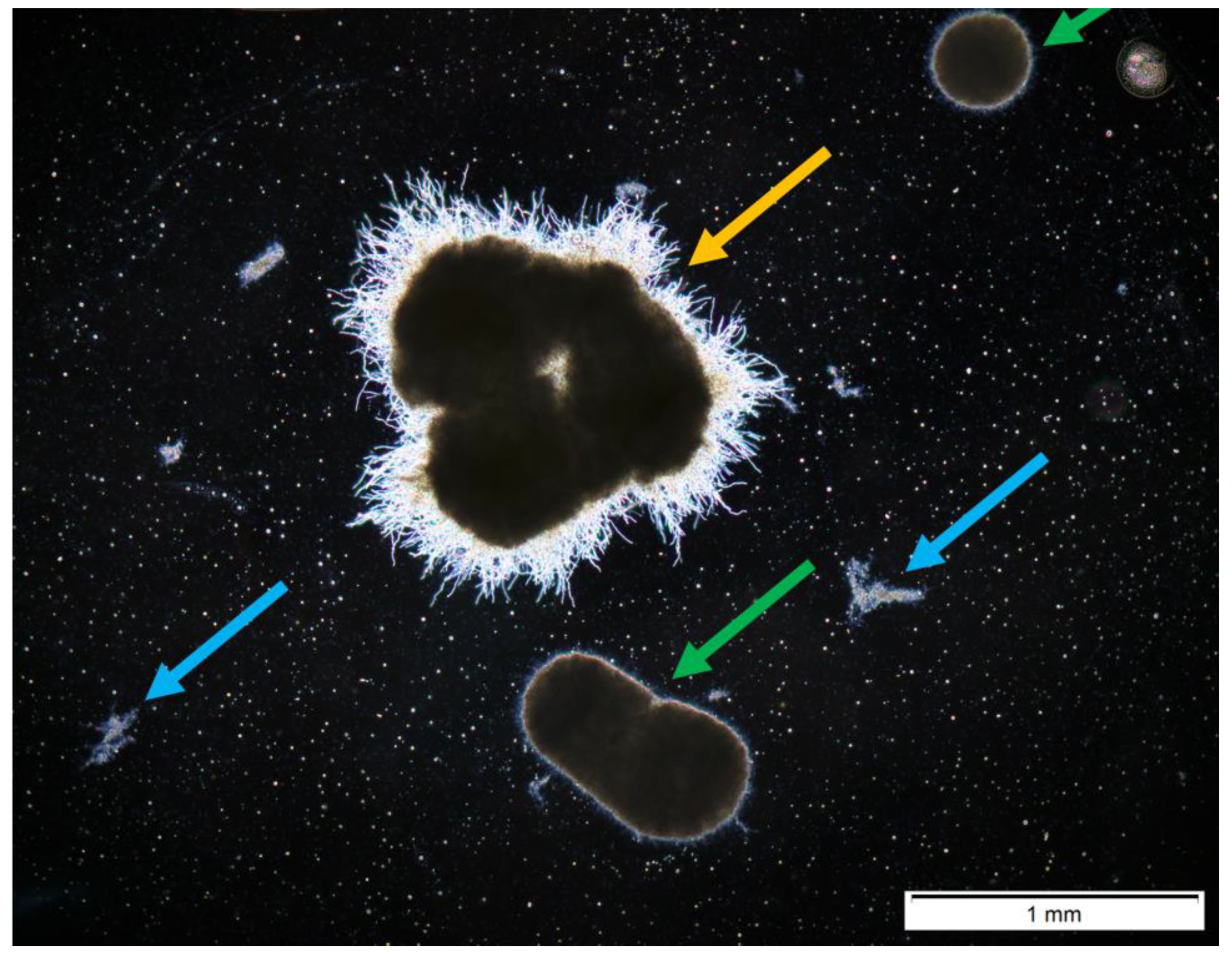
2.5. Morphological Analysis
Semiautomatic Image Processing
3. Results
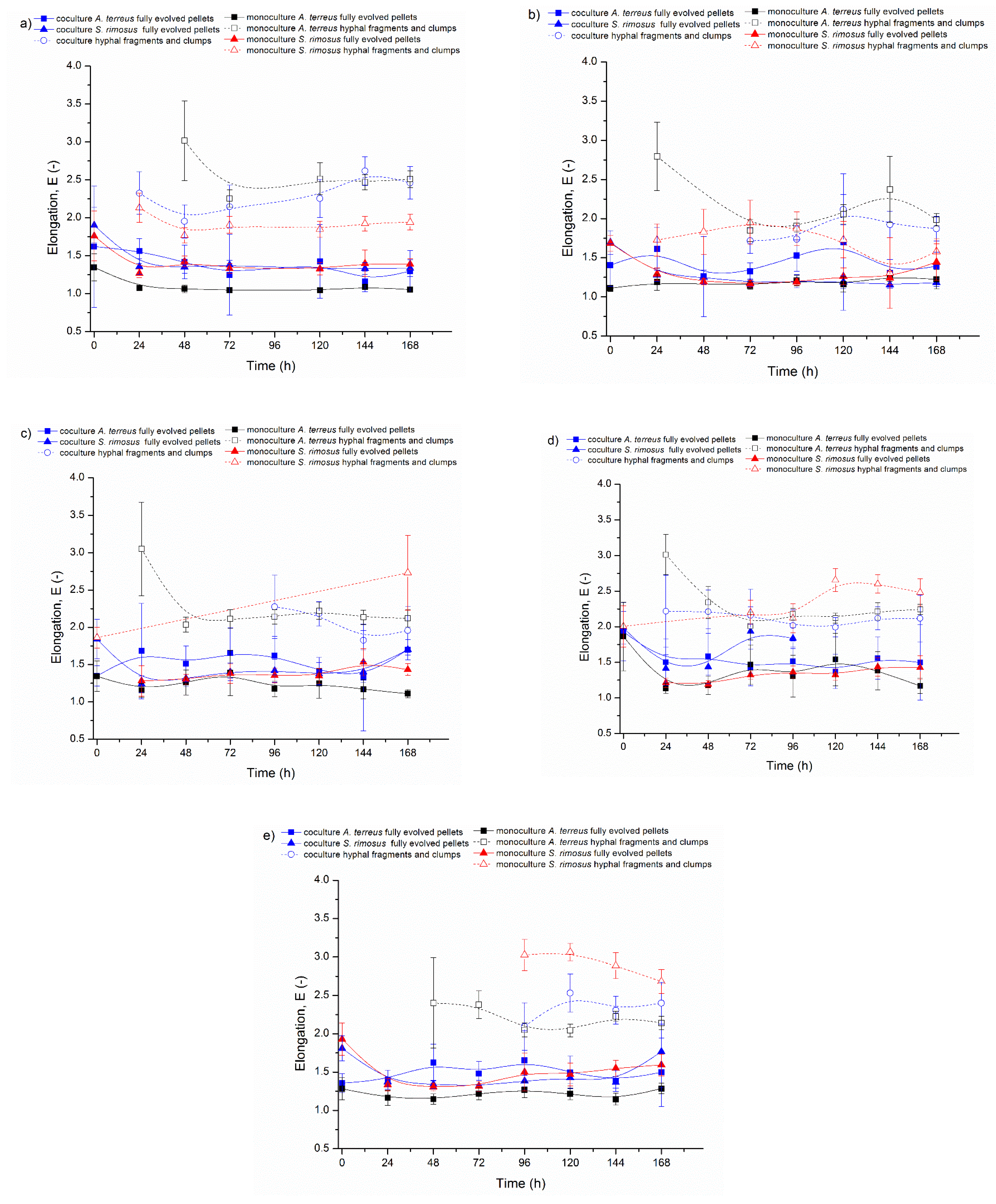
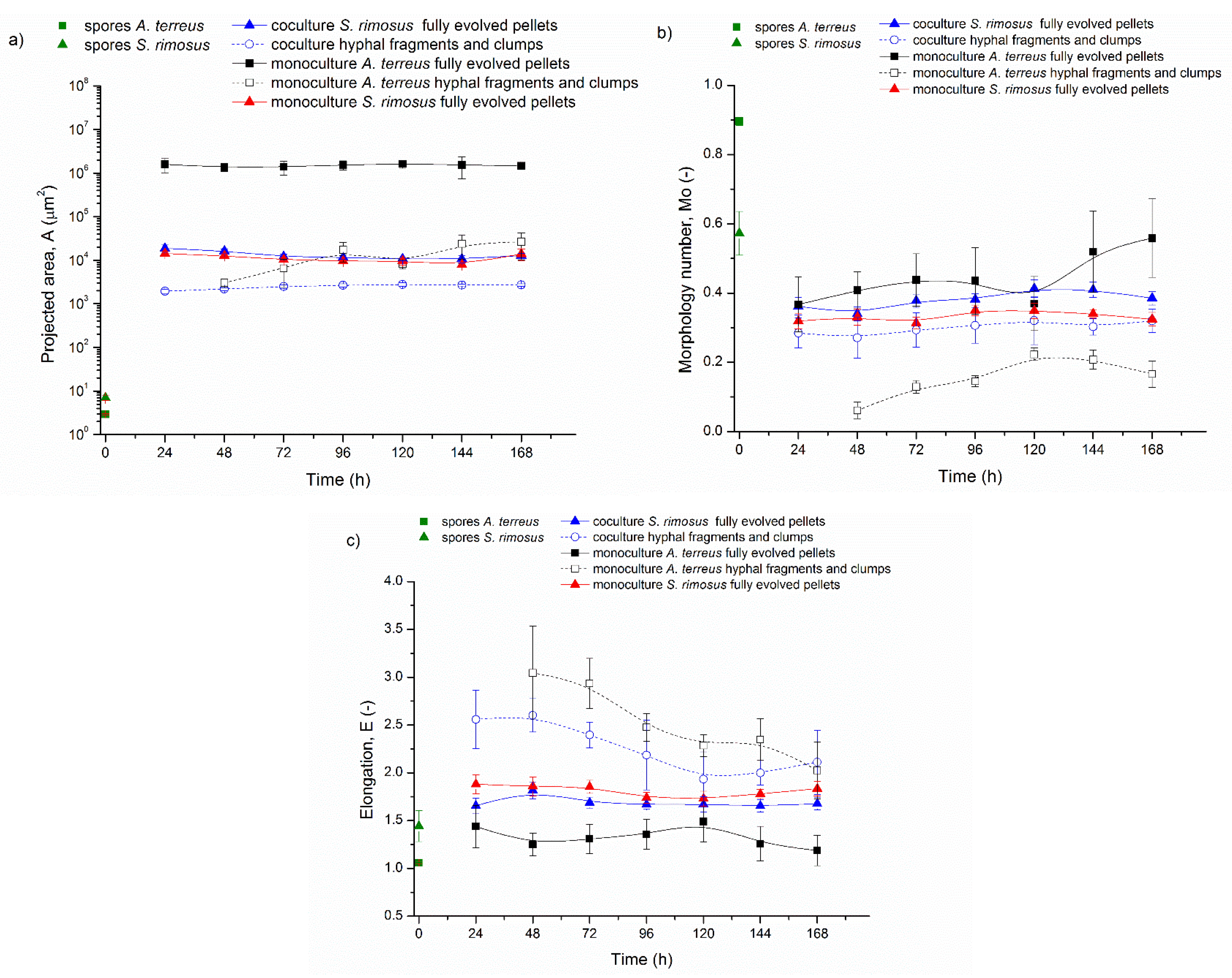
3.1. Bioreactor Cocultures Initiated by Simultaneous Introduction of A. terreus and S. rimosus Precultures
3.2. Delayed Introduction of S. rimosus to Bioreactor Coculture
3.3. Bioreactor Cocultures Initiated by Simultaneous Introduction of A. terreus and S. rimosus Spores
3.4. Air Flowrate and Stirring Speed Influence on Microbial Morphology in Cocultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef] [PubMed]
- Wurster, S.; Sass, G.; Albert, N.D.; Nazik, H.; Déziel, E.; Stevens, D.A.; Kontoyiannis, D.P. Live imaging and quantitative analysis of Aspergillus fumigatus growth and morphology during inter-microbial interaction with Pseudomonas aeruginosa. Virulence 2020, 11, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.-W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Z.; Salim, A.; Capon, R.J. Chaunopyran A: Co-Cultivation of Marine Mollusk-Derived Fungi Activates a Rare Class of 2-Alkenyl-Tetrahydropyran. J. Nat. Prod. 2017, 80, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Harwani, D.; Begani, J.; Lakhani, J. Co-Cultivation Strategies to Induce De Novo Synthesis of Novel Chemical Scaffolds from Cryptic Secondary Metabolite Gene Clusters. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2017; pp. 617–631. [Google Scholar] [CrossRef]
- Nai, C.; Meyer, V. From Axenic to Mixed Cultures: Technological Advances Accelerating a Paradigm Shift in Microbiology. Trends Microbiol. 2018, 26, 538–554. [Google Scholar] [CrossRef] [Green Version]
- Tshikantwa, T.S.; Ullah, M.W.; He, F.; Yang, G. Current Trends and Potential Applications of Microbial Interactions for Human Welfare. Front. Microbiol. 2018, 9, 1156. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Chen, L.; Sui, Y.; Chen, C.; Zhang, W.; Zhou, J.; Dong, W.; Jiang, M.; Xin, F.; Ochsenreither, K. Biotechnological potential and applications of microbial consortia. Biotechnol. Adv. 2020, 40, 107500. [Google Scholar] [CrossRef]
- Tan, Z.Q.; Leow, H.Y.; Lee, D.C.W.; Karisnan, K.; Song, A.A.L.; Mai, C.W.; Yap, W.S.; Lim, E.; Lai, K.S. Co-Culture Systems for the Production of Secondary Metabolites: Current and Future Prospects. Open Biotechnol. J. 2019, 13, 18–26. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, G.; Chen, X.; Huang, M.; Wan, X. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi. Chem. Nat. Compd. 2011, 47, 767–769. [Google Scholar] [CrossRef]
- Nicault, M.; Zaiter, A.; Dumarcay, S.; Chaimbault, P.; Gelhaye, E.; Leblond, P.; Bontemps, C. Elicitation of Antimicrobial Active Compounds by Streptomyces-Fungus Co-Cultures. Microorganisms 2021, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Zhang, H. Utilizing cross-species co-cultures for discovery of novel natural products. Curr. Opin. Biotechnol. 2021, 69, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Siemieniewicz, K.W.; Schrempf, H. Concerted responses between the chitin-binding protein secreting Streptomyces olivaceoviridis and Aspergillus proliferans. Microbiology 2007, 153, 593–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caceres, I.; Snini, S.P.; Puel, O.; Mathieu, F. Streptomyces roseolus, A Promising Biocontrol Agent Against Aspergillus flavus, the Main Aflatoxin B1 Producer. Toxins 2018, 10, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Ding, W.; Ma, Z. Induced production of cytochalasans in co-culture of marine fungus Aspergillus flavipes and actinomycete Streptomyces sp. Nat. Prod. Res. 2016, 30, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- König, C.C.; Scherlach, K.; Schroeckh, V.; Horn, F.; Nietzsche, S.; Brakhage, A.A.; Hertweck, C. Bacterium Induces Cryptic Meroterpenoid Pathway in the Pathogenic Fungus Aspergillus Fumigatus. ChemBioChem 2013, 14, 938–942. [Google Scholar] [CrossRef]
- Wu, C.; Zacchetti, B.; Ram, A.F.; Van Wezel, G.P.; Claessen, D.; Choi, Y.H. Expanding the chemical space for natural products by Aspergillus-Streptomyces co-cultivation and biotransformation. Sci. Rep. 2015, 5, 10868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroe, M.C.; Netzker, T.; Scherlach, K.; Krüger, T.; Hertweck, C.; Valiante, V.; A Brakhage, A. Targeted induction of a silent fungal gene cluster encoding the bacteria-specific germination inhibitor fumigermin. eLife 2020, 9, 52541. [Google Scholar] [CrossRef] [PubMed]
- Gonciarz, J.; Kowalska, A.; Bizukojc, M. Application of microparticle-enhanced cultivation to increase the access of oxygen to Aspergillus terreus ATCC 20542 mycelium and intensify lovastatin biosynthesis in batch and continuous fed-batch stirred tank bioreactors. Biochem. Eng. J. 2016, 109, 178–188. [Google Scholar] [CrossRef]
- Baptista, P.; de Pinho, P.G.; Moreira, N.; Malheiro, R.; Reis, F.; Padrão, J.; Tavares, R.; Lino-Neto, T. In vitro interactions between the ectomycorrhizal Pisolithus tinctorius and the saprotroph Hypholoma fasciculare fungi: Morphological aspects and volatile production. Mycology 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wurster, S.; Kumaresan, P.R.; Albert, N.D.; Hauser, P.J.; Lewis, R.E.; Kontoyiannis, D.P. Live Monitoring and Analysis of Fungal Growth, Viability, and Mycelial Morphology Using the IncuCyte NeuroTrack Processing Module. mBio 2019, 10, e00673-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boruta, T.; Milczarek, I.; Bizukojc, M. Evaluating the outcomes of submerged co-cultivation: Production of lovastatin and other secondary metabolites by Aspergillus terreus in fungal co-cultures. Appl. Microbiol. Biotechnol. 2019, 103, 5593–5605. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-H.; Park, S.J.; Ho, L.K.; Park, J.Y. Proteus vulgaris and Proteus mirabilis Decrease Candida albicans Biofilm Formation by Suppressing Morphological Transition to Its Hyphal Form. Yonsei Med. J. 2017, 58, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Bor, B.; Cen, L.; Agnello, M.; Shi, W.; He, X. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci. Rep. 2016, 6, 27956. [Google Scholar] [CrossRef] [PubMed]
- TAspray, T.J.; Jones, E.E.; Davies, M.W.; Shipman, M.; Bending, G.D. Increased hyphal branching and growth of ectomycorrhizal fungus Lactarius rufus by the helper bacterium Paenibacillus sp. Mycorrhiza 2013, 23, 403–410. [Google Scholar] [CrossRef]
- Lőrincz, Z.; Preininger, É.; Kósa, A.; Pónyi, T.; Nyitrai, P.; Sarkadi, L.; Kovács, G.M.; Böddi, B.; Gyurján, I. Artificial tripartite symbiosis involving a green alga (Chlamydomonas), a bacterium (Azotobacter) and a fungus (Alternaria): Morphological and physiological characterization. Folia Microbiol. 2010, 55, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Anzai, M.; Fischman, O. Bacillus subtilis induces morphological changes in Fonsecaea pedrosoi in vitro resulting in more resistant fungal forms in vivo. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Hogan, D.A.; Åshild, V.; Kolter, R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 2004, 54, 1212–1223. [Google Scholar] [CrossRef]
- Treloar, N.J.; Fedorec, A.J.H.; Ingalls, B.; Barnes, C.P. Deep reinforcement learning for the control of microbial co-cultures in bioreactors. PLoS Comput. Biol. 2020, 16, e1007783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boruta, T.; Ścigaczewska, A.; Bizukojć, M. “Microbial Wars” in a Stirred Tank Bioreactor: Investigating the Co-Cultures of Streptomyces rimosus and Aspergillus terreus, Filamentous Microorganisms Equipped with a Rich Arsenal of Secondary Metabolites. Front. Bioeng. Biotechnol. 2021, 9, 713639. [Google Scholar] [CrossRef] [PubMed]
- Zuck, K.M.; Shipley, S.; Newman, D.J. Induced Production of N-Formyl Alkaloids from Aspergillus fumigatus by Co-culture with Streptomyces peucetius. J. Nat. Prod. 2011, 74, 1653–1657. [Google Scholar] [CrossRef]
- Rateb, M.E.; Hallyburton, I.; Houssen, W.E.; Bull, A.T.; Goodfellow, M.; Santhanam, R.; Jaspars, M.; Ebel, R. Induction of diverse secondary metabolites in Aspergillus fumigatus by microbial co-culture. RSC Adv. 2013, 3, 14444–14450. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Ichinose, K.; Choi, Y.H.; Van Wezel, G.P. Aromatic Polyketide GTRI-02 is a Previously Unidentified Product of theactGene Cluster inStreptomyces coelicolor A3(2). ChemBioChem 2017, 18, 1428–1434. [Google Scholar] [CrossRef]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual Induction of New Microbial Secondary Metabolites by Fungal Bacterial Co-cultivation. Front. Microbiol. 2017, 8, 1284. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Razek, A.S.; Hamed, A.; Frese, M.; Sewald, N.; Shaaban, M. Penicisteroid C: New polyoxygenated steroid produced by co-culturing of Streptomyces piomogenus with Aspergillus niger. Steroids 2018, 138, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Z.G.; Cruz-Morales, P.; Licona-Cassani, C.; Marcellin, E.; Capon, R.J. Inter-Kingdom beach warfare: Microbial chemical communication activates natural chemical defences. ISME J. 2018, 13, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, T.; Hestler, T.; Krull, R. Morphology engineering—Osmolality and its effect on Aspergillus niger morphology and productivity. Microb. Cell Factories 2011, 10, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, A.; Boruta, T.; Bizukojc, M. Morphological evolution of various fungal species in the presence and absence of aluminum oxide microparticles: Comparative and quantitative insights into microparticle-enhanced cultivation (MPEC). Microbiol. 2018, 7, e00603. [Google Scholar] [CrossRef]
- Kowalska, A.; Boruta, T.; Bizukojć, M. Performance of fungal microparticle-enhanced cultivations in stirred tank bioreactors depends on species and number of process stages. Biochem. Eng. J. 2020, 161, 107696. [Google Scholar] [CrossRef]
- Cairns, T.C.; Feurstein, C.; Zheng, X.; Zheng, P.; Sun, J.; Meyer, V. A quantitative image analysis pipeline for the characterization of filamentous fungal morphologies as a tool to uncover targets for morphology engineering: A case study using aplD in Aspergillus niger. Biotechnol. Biofuels 2019, 12, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Zeng, J.; Yu, X.; Dong, T.; Chen, S. Improved lipid accumulation by morphology engineering of oleaginous fungus Mortierella isabellina. Biotechnol. Bioeng. 2014, 111, 1758–1766. [Google Scholar] [CrossRef]
- Krull, R.; Wucherpfennig, T.; Esfandabadi, M.E.; Walisko, R.; Melzer, G.; Hempel, D.C.; Kampen, I.; Kwade, A.; Wittmann, C. Characterization and control of fungal morphology for improved production performance in biotechnology. J. Biotechnol. 2013, 163, 112–123. [Google Scholar] [CrossRef] [PubMed]





| Reported Results of the Cocultures | Cocultured Strains | Cultivation Method | References | |
|---|---|---|---|---|
| 1 | Mixture of A. proliferans and S. olivaceoviridis spores in the coculture provoked germination and growth of the cultivated microorganisms in medium without carbon source | Aspergillus proliferans Streptomyces olivaceoviridis | Flasks without shaking | Siemieniewicz and Schrempf [13] |
| 2 | Lecanoric acid, F-9775A, F-9775B | Aspergillus nidulans Streptomyces hygroscopicus | Shaking flasks | Schroeckh et al. [3] |
| 3 | Fumiformamide, N,N′-((1Z,3Z)-1,4-bis(4-methoxyphenyl)buta-1,3-diene-2,3-diyl)diformamide | Aspergillus fumigatus Streptomyces peucetius | Shaking flasks | Zuck et al. [31] |
| 4 | Fumicycline A (activation of a silent polyketide synthase gene cluster) | Aspergillus fumigatus Streptomyces rapamycinicus | Petri dishes | König et al. [16] |
| 5 | Ergosterol, brevianamide F, spirotryprostatin A, 6-methoxy spirotryprostatin B, fumitremorgin C and its 12,13-dihydroxy derivative, fumitremorgin B, verruculogen, 11-O-methylpseurotin A and its new isomer 11-O-methylpseurotin A2 | Aspergillus fumigatus MBC-F1-10 Streptomyces bullii | Shaking flasks | Rateb et al. [32] |
| 6 | Cyclic dipeptide cyclo(Phe-Phe), 2-hydroxyphenylacetic acid, (E)-2-(3-hydroxyprop-1-en-1-yl)-phenol, (2E,4E)-3-(2-carboxy-1-hydroxyethyl)-2,4-hexadienedioxic acid | Aspergillus niger Streptomyces coelicolor | Shaking flasks | Wu et al. [17] |
| 7 | Rosellichalasin, aspochalasin E, aspochalasin P, aspochalasin H, aspochalasin M, 19,20-dihydro-aspochalasin D | Aspergillus flavipes Streptomyces sp. | Shaking flasks | Yu et al. [15] |
| 8 | Poliketide GTRI-02 | Aspergillus niger N402 Streptomyces coelicolor A3(2) | Shaking flasks | Wu et al. [33] |
| 9 | Luteoride D, luteoride derivative, pseurotin G, pseurotin derivative, terezine D, 11-O-methylpseurotin A, lasso peptide chaxapeptin, the titre of chaxapeptin was doubled, pentalenic acid, brevianamide X. | Aspergillus fumigatus MR2012 Streptomyces leeuwenhoekii strain C34 and strain C58 | Shaking flasks | Wakefield et al. [34] |
| 10 | Penicisteroid C | Aspergillus niger Streptomyces piomogenus | Shaking flasks | Abdel-Razek et al. [35] |
| 11 | The aflatoxin B1 concentration reduction by S. roseolus, A. flavius morphological changes | Aspergillus flavus Streptomyces roseolus | Petri dishes | Caceres et al. [14] |
| 12 | Heronapyrrole B | Aspergillus sp. CMB-AsM0423 Streptomyces sp.CMB-M0423 | Microbioreactor system | Khalil et al. [36] |
| 13 | Fumigermin | Aspergillus fumigatus: Af293, A1163, ATCC 46645, IBT 16806,ΔfgnA, tetOn-fgnA, Aspergillus nidulas: RMS011, fgnA_ATCC, fgnA_Af293, fgnA_A1163_repaired, fgn_cluster, Streptomyces iranensis HM35, Streptomyces lividans PM02, Streptomyces coelicolor A(3)2, Streptomyces rapamycinicus ATCC 29253 | Shaking flasks | Stroe et al. [18] |
| ATSR2 | ATSR3 | ATSR4 | ATSR5 | ATSR6 | ATSR7 | ATSR8 | ATSR9 | |
| g·L−1 | ||||||||
| lactose | 0 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| glucose | 20 | 20 | 20 | 20 | 0 | 0 | 20 | 0 |
| yeast extract | 5 | 2 | 4 | 4 | 4 | 4 | 4 | 4 |
| (NH4)2SO4 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| KH2PO4 | 1.51 | 1.51 | 1.51 | 1.51 | 1.51 | 1.51 | 1.51 | 1.51 |
| pH | 7—value controlled during experiment | 7—value controlled during experiment | 7—value controlled during experiment | initial value 6.5; without pH control | initial value 6.5; without pH control | initial value 6.5; without pH control | initial value 6.5; without pH control | initial value 6.5; without pH control |
| Other components added to each culture | Salt components: 1.51 g·L−1 KH2PO4, 0.51 g·L−1 MgSO4·7H2O, 0.4 g·L−1 NaCl, 1 mg·L−1 ZnSO4·7 H2O, 2 mg·L−1 Fe(NO3)3·9H2O 0.04 mg·L−1 biotin A 1 mL l−1 of the trace elements solution was added to each culture. It contained: 100 mg·L−1 H3BO3, 50 mg·L−1 MnSO4, 250 mg·L−1 CuSO4·5H2O, 50 mg·L−1 Na2MoO4·2H2O. | |||||||
| 24 h | E of Fully Evolved A. terreus Pellets in Coculture | E of Fully Evolved A. terreus Pellets in Monoculture | p Value |
|---|---|---|---|
| ATSR2 | 1.56 | 1.08 | p< 0.001 |
| ATSR3 | 1.61 | 1.19 | p< 0.01 |
| ATSR4 | 1.68 | 1.16 | p = 0.05 |
| ATSR5 | 1.50 | 1.14 | p< 0.05 |
| ATSR6 | 1.40 | 1.16 | p< 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścigaczewska, A.; Boruta, T.; Bizukojć, M. Quantitative Morphological Analysis of Filamentous Microorganisms in Cocultures and Monocultures: Aspergillus terreus and Streptomyces rimosus Warfare in Bioreactors. Biomolecules 2021, 11, 1740. https://doi.org/10.3390/biom11111740
Ścigaczewska A, Boruta T, Bizukojć M. Quantitative Morphological Analysis of Filamentous Microorganisms in Cocultures and Monocultures: Aspergillus terreus and Streptomyces rimosus Warfare in Bioreactors. Biomolecules. 2021; 11(11):1740. https://doi.org/10.3390/biom11111740
Chicago/Turabian StyleŚcigaczewska, Anna, Tomasz Boruta, and Marcin Bizukojć. 2021. "Quantitative Morphological Analysis of Filamentous Microorganisms in Cocultures and Monocultures: Aspergillus terreus and Streptomyces rimosus Warfare in Bioreactors" Biomolecules 11, no. 11: 1740. https://doi.org/10.3390/biom11111740
APA StyleŚcigaczewska, A., Boruta, T., & Bizukojć, M. (2021). Quantitative Morphological Analysis of Filamentous Microorganisms in Cocultures and Monocultures: Aspergillus terreus and Streptomyces rimosus Warfare in Bioreactors. Biomolecules, 11(11), 1740. https://doi.org/10.3390/biom11111740





