The Deposition of a Lectin from Oreochromis niloticus on the Surface of Titanium Dioxide Nanotubes Improved the Cell Adhesion, Proliferation, and Osteogenic Activity of Osteoblast-like Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Coating of TiO2 with OniL
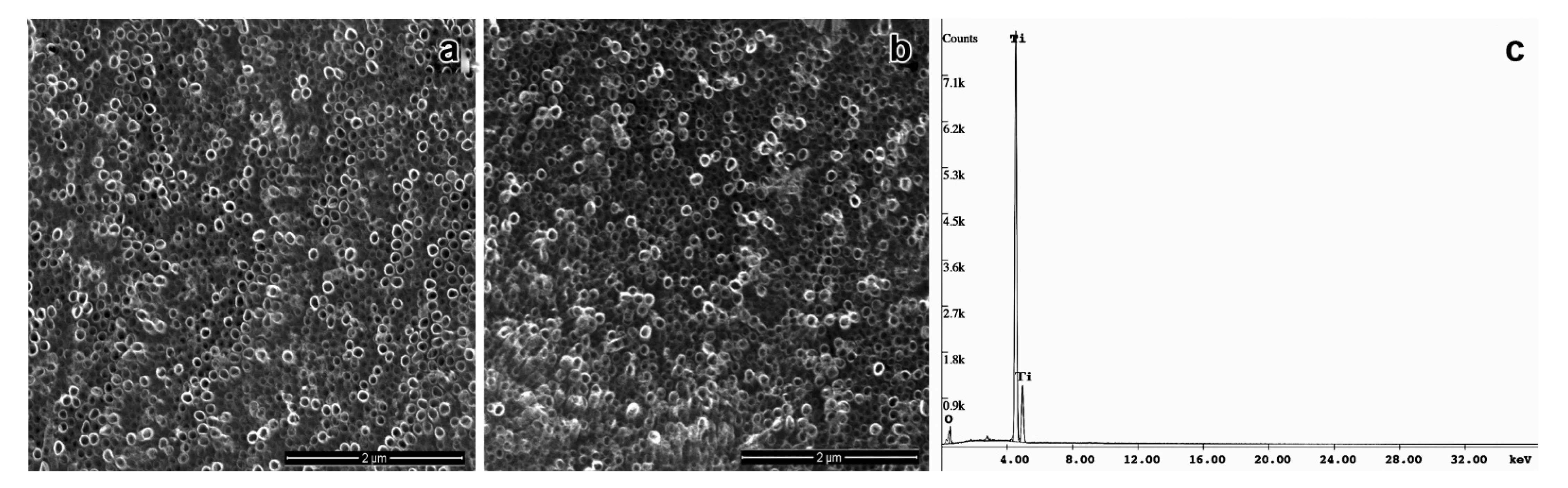
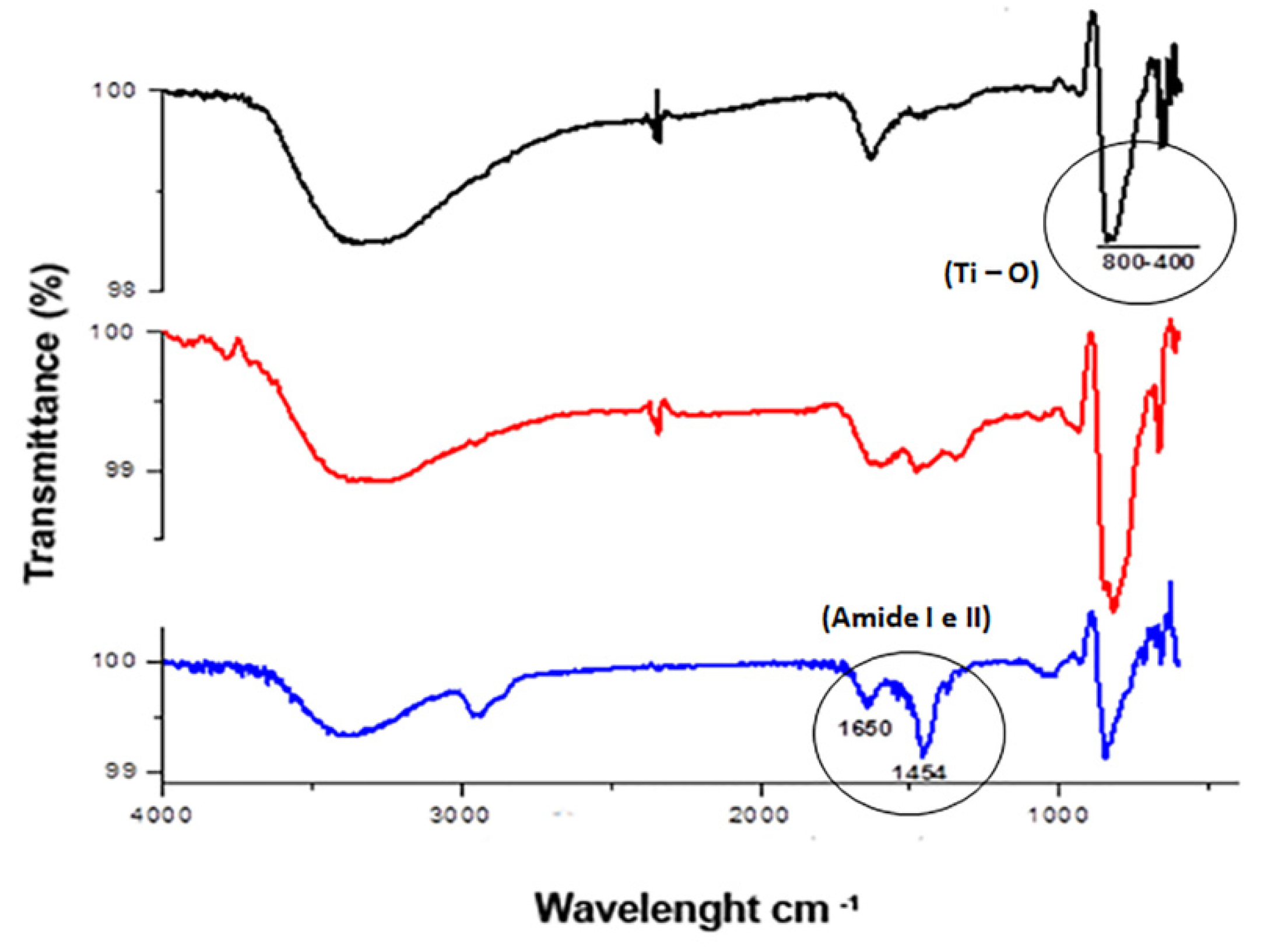
2.4. Morphological Characterization of TiO2NTs
2.5. Cell Culture
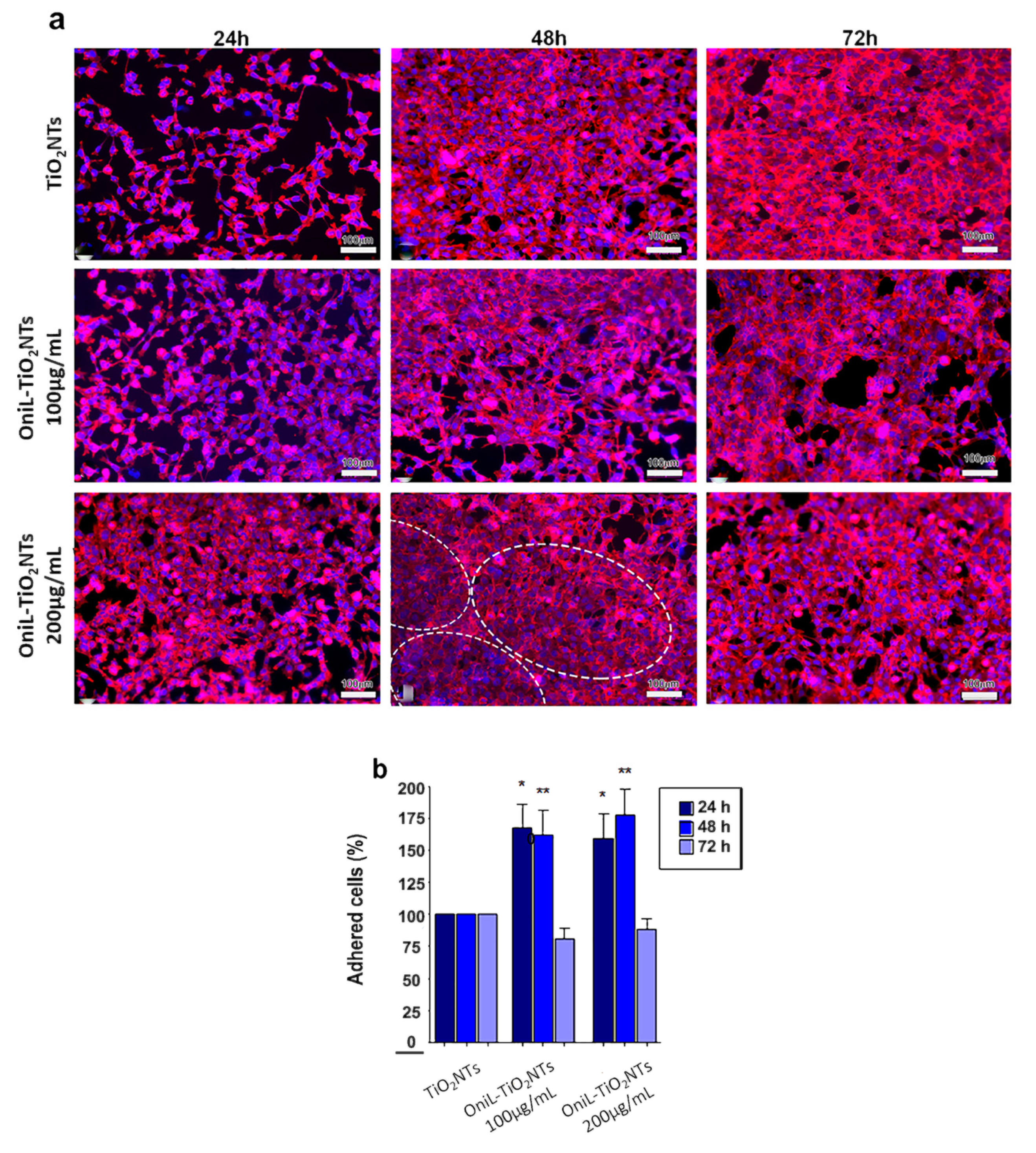
2.6. Cell Adhesion, Proliferation, and Viability Assays
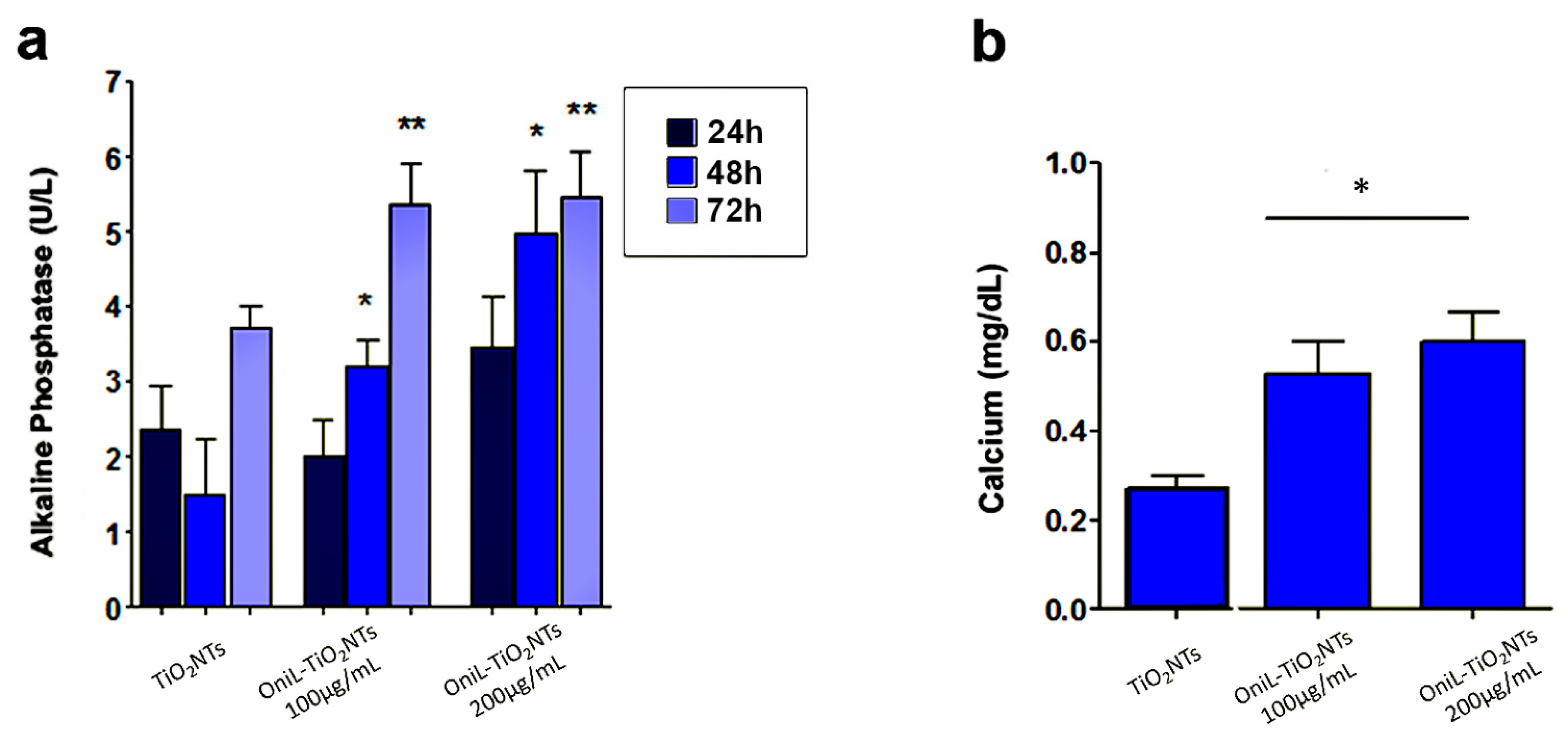
2.7. Osteogenic Potential
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Li, Y. Nanostructured Titanium Dioxide Based on Titanium Alloys: Synthesis and Properties. J. Nanosci. Nanotechnol. 2019, 19, 26–39. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium Nanostructures for Biomedical Applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Mieszkowska, A.; Beaumont, H.; Martocq, L.; Koptyug, A.; Surmeneva, M.A.; Surmenev, R.A.; Naderi, J.; Douglas, T.E.; Gurzawska-Comis, K.A. Phenolic-Enriched Collagen Fibrillar Coatings on Titanium Alloy to Promote Osteogenic Differentiation and Reduce Inflammation. Int. J. Mol. Sci. 2020, 21, 6406. [Google Scholar] [CrossRef]
- Alves, S.A.; Patel, S.B.; Sukotjo, C.; Mathew, M.T.; Filho, P.N.; Celis, J.-P.; Rocha, L.; Shokuhfar, T. Synthesis of Calcium-phosphorous Doped TiO2 Nanotubes by Anodization and Reverse Polarization: A promising strategy for an efficient biofunctional implant surface. Appl. Surf. Sci. 2017, 399, 682–701. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglic, A.; Gongadze, E.; Kabaso, D.; Bauer, S.; Schmuki, P.; Slivnik, T.; van Rienen, U. Adhesion of Osteoblasts to a Nanorough Titanium Implant Surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, W.F.; Arruda, I.R.; Silva, G.M.; Machado, G.; Coelho, L.C.; Correia, M.T. Functionalization of Titanium Dioxide Nanotubes with Biomolecules for Biomedical Applications. Mater. Sci. Eng. C 2017, 81, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Reddy, G.S.; Reddy, B.S.B.; Shekar, P.C.; Sumanthi, J.; Chandra, K.L.P. Biological Role of Lectins: A review. J. Orofac. Sci. 2012, 4, 20. [Google Scholar] [CrossRef]
- SinghBains, J.; Singh, J.; Kamboj, S.S.; Nijjar, K.K.; Agrewala, J.N.; Kumar, V.; Kumar, A.; Saxena, A. Mitogenic and Anti-Proliferative Activity of a Lectin from the Tubers of Voodoo Lily (Sauromatum venosum). Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1723, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.V.; Araújo-Filho, V.S.; Nakazawa, M.; Gomes, Y.M.; Coelho, L.C.; Correia, M.T. Mitogenic Activity of Cratylia Mollis Lectin on Human Lymphocytes. Biologicals 2004, 32, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.D.S.; de Souza, M.A.A.; Vaz, A.F.D.M.; Santana, G.M.D.S.; Gomes, F.S.; Coelho, L.C.B.B.; Paiva, P.M.G.; da Silva, R.M.L.; Silva-Lucca, R.A.; Oliva, M.L.V.; et al. Purification of a Lectin with Antibacterial Activity from Bothrops Leucurus Snake Venom. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2011, 159, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, E.V.M.M.; Bezerra, R.F.; Bezerra, R.D.S.; De Araújo, J.M.; Santos, A.J.G.; Correia, M.T.D.S.; Coelho, L.C.B.B. Detection of the First Lectin with Antimicrobial Activity Present in Serum of the Amazonian Fish Tambaqui Colossoma Macropomum. Fish. Sci. 2012, 78, 879–887. [Google Scholar] [CrossRef]
- De Melo, C.M.L.; Castro, M.C.; De Oliveira, A.P.; Gomes, F.O.S.; Pereira, V.R.A.; Correia, M.T.S.; Coelho, L.C.B.B.; Paiva, P.M.G. Immunomodulatory Response of Cramoll 1,4 lectin on Experimental Lymphocytes. Phytother. Res. 2010, 24, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.D.C.; Coriolano, M.C.; Lino, M.A.D.S.; De Melo, C.M.L.; Bezerra, R.D.S.; De Carvalho, E.V.M.M.; Dos Santos, A.J.G.; Pereira, V.R.A.; Coelho, L.C.B.B. Purification and Characterization of a Mannose Recognition Lectin from Oreochromis niloticus (Tilapia Fish): Cytokine Production in Mice Splenocytes. Appl. Biochem. Biotechnol. 2011, 166, 424–435. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Chen, L.; Guo, Y.; Hu, S.; Cheng, L.; Xiao, W.; Ke, J.; Zhu, Z.; Niu, L. Structure of the Platelet Glycoprotein Ib receptor in Complex with a Novel Antithrombotic Agent. Blood 2021, 137, 844–847. [Google Scholar] [CrossRef]
- De Melo, C.M.L.; Porto, C.S.; Melo-Júnior, M.R.; Mendes, C.M.; Cavalcanti, C.C.B.; Coelho, L.C.B.B.; Porto, A.L.F.; Leão, A.M.D.A.C.; Correia, M.T.D.S. Healing Activity Induced by Cramoll 1,4 Lectin in Healthy and Immunocompromised Mice. Int. J. Pharm. 2011, 408, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Mu, L.; Yin, X.; Liu, J.; Wu, L.; Bian, X.; Wang, Y.; Ye, J. Identification and Characterization of a Mannose-binding Lectin from Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 67, 244–253. [Google Scholar] [CrossRef]
- Wang, M.; Guo, J.; Zhang, L.; Kuek, V.; Xu, J.; Zou, J. Molecular Structure, Expression, and Functional Role of Clec11a in Skeletal Biology and Cancers. J. Cell. Physiol. 2020, 235, 6357–6365. [Google Scholar] [CrossRef]
- Mu, L.; Yin, X.; Wu, H.; Han, K.; Guo, Z.; Ye, J. MAp34 Regulates the Non-specific Cell Immunity of Monocytes/Macrophages and Inhibits the Lectin Pathway of Complement Activation in a Teleost Fish. Front. Immunol. 2020, 11, 1706. [Google Scholar] [CrossRef]
- Van der Ende, J.; Van Baardewijk, L.; Sier, C.; Schipper, I. Bone Healing and Mannose-Binding Lectin. Int. J. Surg. 2013, 11, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, W.F.; Silva, G.M.; Filho, P.E.C.; Fontes, A.; Oliveira, M.D.; Andrade, C.A.; Silva, M.V.; Coelho, L.C.; Machado, G.; Correia, M.T. Titanium Dioxide Nanotubes Functionalized with Cratylia Mollis Seed Lectin, Cramoll, Enhanced Osteoblast-like Cells Adhesion and Proliferation. Mater. Sci. Eng. C 2018, 90, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem Cell Fate Dictated Solely by Altered Nanotube Dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [Green Version]
- Orava, J.; Kohoutek, T.; Wagner, T. Deposition Techniques for Chalcogenide Thin Films. In Chalcogenide Glasses; Elsevier: Amsterdam, The Netherlands, 2014; pp. 265–309. [Google Scholar]
- Lan, M.-Y.; Liu, C.-P.; Huang, H.-H.; Chang, J.-K.; Lee, S.-W. Diameter-Sensitive Biocompatibility of Anodic TiO2 Nanotubes Treated with Supercritical CO2 Fluid. Nanoscale Res. Lett. 2013, 8, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voltrova, B.; Hybasek, V.; Blahnova, V.; Sepitka, J.; Lukasova, V.; Vocetkova, K.; Sovkova, V.; Matejka, R.; Fojt, J.; Joska, L.; et al. Different Diameters of Titanium Dioxide Nanotubes Modulate Saos-2 Osteoblast-like Cell Adhesion and Osteogenic Differentiation and Nanomechanical Properties of the Surface. RSC Adv. 2019, 9, 11341–11355. [Google Scholar] [CrossRef] [Green Version]
- Khaw, J.S.; Bowen, C.R.; Cartmell, S.H. Effect of TiO2 Nanotube Pore Diameter on Human Mesenchymal Stem Cells and Human Osteoblasts. Nanomaterials 2020, 10, 2117. [Google Scholar] [CrossRef]
- Chang, Y.; Shao, Y.; Liu, Y.; Xia, R.; Tong, Z.; Zhang, J.; Zhai, Z.; Cheng, W.; Li, H. Mechanical Strain Promotes Osteogenic Differentiation of Mesenchymal Stem Cells on TiO2 Nanotubes Substrate. Biochem. Biophys. Res. Commun. 2019, 511, 840–846. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Bauer, S.; Roedl, S.; Neukam, F.W.; Schmuki, P.; Schlegel, K.A. The Diameter of Anodic TiO2 Nanotubes affects Bone Formation and Correlates with the Bone Morphogenetic Protein-2 Expression In Vivo. Clin. Oral Implant. Res. 2011, 23, 359–366. [Google Scholar] [CrossRef]
- Khrunyk, Y.Y.; Belikov, S.V.; Tsurkan, M.V.; Vyalykh, I.V.; Markaryan, A.; Karabanalov, M.S.; Popov, A.A.; Wysokowski, M. Surface-Dependent Osteoblasts Response to TiO2 Nanotubes of Different Crystallinity. Nanomaterials 2020, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; Van Der Heyde, H.; Jin, S. Improved Bone-forming Functionality on Diameter-controlled TiO2 Nanotube Surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef]
- Lan, M.-Y.; Liu, C.-P.; Huang, H.-H.; Lee, S.-W. Both Enhanced Biocompatibility and Antibacterial Activity in Ag-Decorated TiO2 Nanotubes. PLoS ONE 2013, 8, e75364. [Google Scholar] [CrossRef] [Green Version]
- Minagar, S.; Wang, J.; Berndt, C.; Ivanova, E.; Wen, C. Cell Response of Anodized Nanotubes on Titanium and Titanium Alloys. J. Biomed. Mater. Res. Part A 2013, 101, 2726–2739. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Bouropoulos, N.; Fatouros, D.G.; Kontopoulou, I.; Roldo, M. Synthesis of Carbon Nanotubes Loaded Hydroxyapatite: Potential for Controlled Drug Release from Bone Implants. J. Adv. Ceram. 2016, 5, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Minhas, B.; Dino, S.; Zuo, Y.; Qian, H.; Zhao, X. Improvement of Corrosion Resistance of TiO2 Layers in Strong Acidic Solutions by Anodizing and Thermal Oxidation Treatment. Materials 2021, 14, 1188. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kim, H.-M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural Dependence of Apatite Formation on Titania Gels in a Simulated Body Fluid. J. Biomed. Mater. Res. 2003, 64A, 164–170. [Google Scholar] [CrossRef]
- Saha, S.; Pramanik, K.; Biswas, A. Silk fibroin coated TiO2 nanotubes for Improved Osteogenic Property of Ti6Al4V Bone Implants. Mater. Sci. Eng. C 2019, 105, 109982. [Google Scholar] [CrossRef]
- Jin, Z.; Yan, X.; Liu, G.; Lai, M. Fibronectin modified TiO2 Nanotubes Modulate Endothelial Cell Behavior. J. Biomater. Appl. 2018, 33, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Awad, N.K.; Edwards, S.L.; Morsi, Y. A review of TiO2 NTs on Ti metal: Electrochemical Synthesis, Functionalization and Potential Use as Bone Implants. Mater. Sci. Eng. C 2017, 76, 1401–1412. [Google Scholar] [CrossRef]
- Maheswari, P.; Harish, S.; Navaneethan, M.; Muthamizhchelvan, C.; Ponnusamy, S.; Hayakawa, Y. Bio-modified TiO2 nanoparticles with Withania somnifera, Eclipta prostrata and Glycyrrhiza glabra for Anticancer and Antibacterial Applications. Mater. Sci. Eng. C 2020, 108, 110457. [Google Scholar] [CrossRef]
- Takahashi, K.; Chang, W.-C.; Takahashi, M.; Pavlov, V.; Ishida, Y.; La Bonte, L.; Shi, L.; Fujita, T.; Stahl, G.L.; Van Cott, E.M. Mannose-binding Lectin and its Associated Proteases (MASPs) Mediate Coagulation and its Deficiency is a Risk Factor in Developing Complications from Infection, Including Disseminated Intravascular Coagulation. Immunology 2011, 216, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Bavykin, D.; Milsom, E.; Marken, F.; Kim, D.; Marsh, D.; Riley, D.; Walsh, F.; El-Abiary, K.; Lapkin, A. A novel Cation-binding TiO2 Nanotube Substrate for Electro- and Bioelectro-catalysis. Electrochem. Commun. 2005, 7, 1050–1058. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Bai, H. Photocatalytic Removal of NO and NO2 Using Titania Nanotubes Synthesized by Hydrothermal Method. J. Environ. Sci. 2014, 26, 1180–1187. [Google Scholar] [CrossRef]
- Nguyen, A.N.; Solard, J.; Nong, H.T.T.; Ben Osman, C.; Gomez, A.; Bockelée, V.; Tencé-Girault, S.; Schoenstein, F.; Simón-Sorbed, M.; Carrillo, A.E.; et al. Spin Coating and Micro-Patterning Optimization of Composite Thin Films Based on PVDF. Materials 2020, 13, 1342. [Google Scholar] [CrossRef] [Green Version]
- Mohedano, M.; Matykina, E.; Arrabal, R.; Pardo, A.; Merino, M. Metal Release from Ceramic Coatings for Dental Implants. Dent. Mater. 2014, 30, e28–e40. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Li, B.; Liu, B.; Wu, G.; Ibrahim, M.; Xie, G.; Li, H.; Sun, G. Differentiation in MALDI-TOF MS and FTIR Spectra Between Two Closely Related Species Acidovorax oryzae and Acidovorax citrulli. BMC Microbiol. 2012, 12, 182. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.; Lewis, A.; Lewis, P. Prediction of Glycoprotein Secondary Structure Using ATR-FTIR. Vib. Spectrosc. 2013, 69, 21–29. [Google Scholar] [CrossRef]
- Tian, A.; Qin, X.; Wu, A.; Zhang, H.; Xu, Q.; Xing, D.; Yang, H.; Qiu, B.; Xue, X.; Zhang, N.; et al. Nanoscale TiO2 Nanotubes Govern the Biological Behavior of Human Glioma and Osteosarcoma Cells. Int. J. Nanomed. 2015, 10, 2423–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, E.; Adachi, T.; Boschetto, F.; Zanocco, M.; Rondinella, A.; Zhu, W.; Bock, R.; McEntire, B.; Bal, S.B.; Pezzotti, G. Biological Response of Human Osteosarcoma Cells to Si3N4-doped Bioglasses. Mater. Des. 2018, 159, 79–89. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Hauck, C.R.; Sieg, D.J. Signaling through Focal Adhesion Kinase. Prog. Biophys. Mol. Biol. 1999, 71, 435–478. [Google Scholar] [CrossRef] [Green Version]
- Violin, K.B.; Ribeiro, C.; Goia, T.S.; Bressiani, J.C.; Bressiani, A.H.D.A. Lectinhistochemistry Evaluation of Rabbits Tibia Implanted with Macroporous Biphasic Ceramic Implants. Key Eng. Mater. 2012, 529–530, 331–336. [Google Scholar] [CrossRef]
- Ikeda, T.; Futaesaky, Y.; Tshchida, N. In Vitro Differentiation of the Human Osteosarcoma Cell Lines, HOS, KHOS. Virchows Arch. B 1992, 62, 199–206. [Google Scholar] [CrossRef]
- Min, S.-K.; Kang, H.K.; Jang, D.H.; Jung, S.Y.; Kim, O.B.; Min, B.-M.; Yeo, I.-S. Titanium Surface Coating with a Laminin-Derived Functional Peptide Promotes Bone Cell Adhesion. BioMed Res. Int. 2013, 2013, 638348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalby, M.; Childs, S.; Riehle, M.; Johnstone, H.; Affrossman, S.; Curtis, A. Fibroblast Reaction to Island Topography: Changes in Cytoskeleton and Morphology with Time. Biomaterials 2003, 24, 927–935. [Google Scholar] [CrossRef]
- Doe, Y.; Ida, H.; Seiryu, M.; Deguchi, T.; Takeshita, N.; Sasaki, S.; Sasaki, S.; Irie, D.; Tsuru, K.; Ishikawa, K.; et al. Titanium Surface Treatment by Calcium Modification with Acid-etching Promotes Osteogenic activity and Stability of Dental Implants. Materialia 2020, 12, 100801. [Google Scholar] [CrossRef]
- Stan, M.-S.; Memet, I.; Fratila, C.; Krasicka-Cydzik, E.; Roman, I.; Dinischiotu, A. Effects of Titanium-based Nanotube Films on Osteoblast Behaviorin Vitro. J. Biomed. Mater. Res. Part A 2015, 103, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B.; Ooi, V.E.; Liu, W.K. Effects of lectins with different carbohydrate-binding specificities on hepatoma, choriocarcinoma, melanoma and osteosarcoma cell lines. Int. J. Biochem. Cell Biol. 2000, 32, 365–372. [Google Scholar] [CrossRef]
- Da Silva, C.D.C.; de Melo, C.M.L.; Carvalho, E.V.M.M.; Bezerra, F.; dos Santos, A.J.G.; Pereira, V.R.A.; Coelho, L.C.B.B. Pro-liferative Effect of Tilapia Fish (Oreochromis niloticus) Lectin on BALB/c Mice Splenocytes. Protein Peptide Lett. 2019, 26, 887–892. [Google Scholar] [CrossRef]
- Detraves, C.; Francis, F.; Baricault, L.; Fournier, D.; Paquereau, L. Inhibitory Action of a New Lectin from Xerocomus Chrysenteron on Cell-substrate Adhesion. Mol. Cell. Biochem. 2004, 258, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.C.; de Paula, C.A.; Ferreira, J.G.; Gamero, E.J.P.; Vaz, A.M.; Sampaio, M.U.; Correia, M.T.S.; Oliva, M.L.V. Bauhinia Forficata Lectin (BfL) Induces Cell Death and Inhibits Integrin-mediated Adhesion on MCF7 Human Breast Cancer Cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2262–2271. [Google Scholar] [CrossRef] [PubMed]

| TiO2NTs Treatment | Cdl (µF) | Rct (kΩ) |
|---|---|---|
| TiO2NTs | 38.89 | 1.71 |
| Neg-TiO2NTs | 35.33 | 3.30 |
| OniL-TiO2NTs | 6.89 | 24.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Anjos, K.F.L.; da Silva, C.D.C.; de Souza, M.A.A.; de Mattos, A.B.; Coelho, L.C.B.B.; Machado, G.; de Melo, J.V.; de Figueiredo, R.C.B.Q. The Deposition of a Lectin from Oreochromis niloticus on the Surface of Titanium Dioxide Nanotubes Improved the Cell Adhesion, Proliferation, and Osteogenic Activity of Osteoblast-like Cells. Biomolecules 2021, 11, 1748. https://doi.org/10.3390/biom11121748
dos Anjos KFL, da Silva CDC, de Souza MAA, de Mattos AB, Coelho LCBB, Machado G, de Melo JV, de Figueiredo RCBQ. The Deposition of a Lectin from Oreochromis niloticus on the Surface of Titanium Dioxide Nanotubes Improved the Cell Adhesion, Proliferation, and Osteogenic Activity of Osteoblast-like Cells. Biomolecules. 2021; 11(12):1748. https://doi.org/10.3390/biom11121748
Chicago/Turabian Styledos Anjos, Keicyanne Fernanda Lessa, Cynarha Daysy Cardoso da Silva, Mary Angela Aranda de Souza, Alessandra Batista de Mattos, Luana Cassandra Breitenbach Barroso Coelho, Giovanna Machado, Janaina Viana de Melo, and Regina Celia Bressan Queiroz de Figueiredo. 2021. "The Deposition of a Lectin from Oreochromis niloticus on the Surface of Titanium Dioxide Nanotubes Improved the Cell Adhesion, Proliferation, and Osteogenic Activity of Osteoblast-like Cells" Biomolecules 11, no. 12: 1748. https://doi.org/10.3390/biom11121748






