Planar Boronic Graphene and Nitrogenized Graphene Heterostructure for Protein Stretch and Confinement
Abstract
:1. Introduction
2. Method
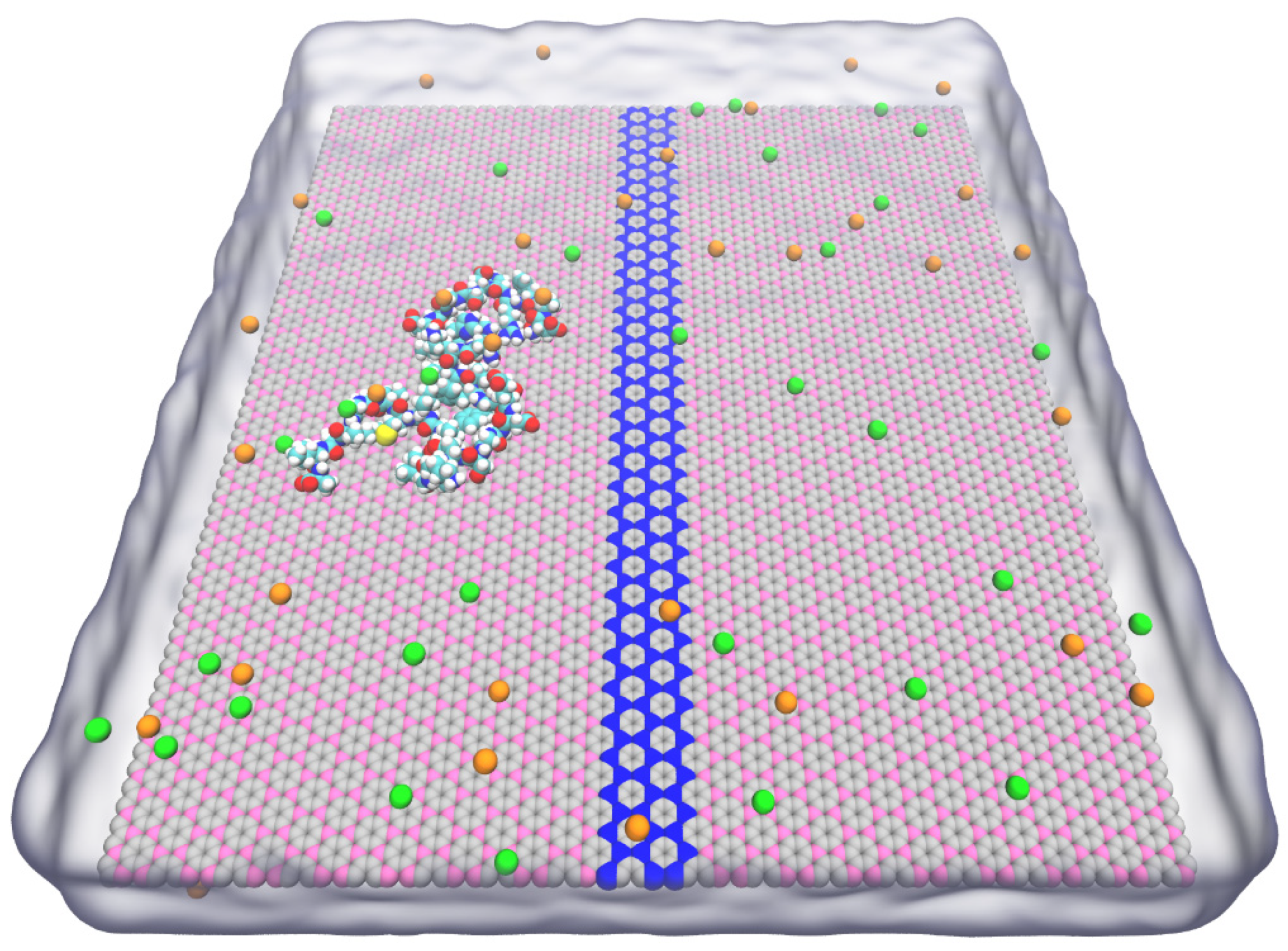
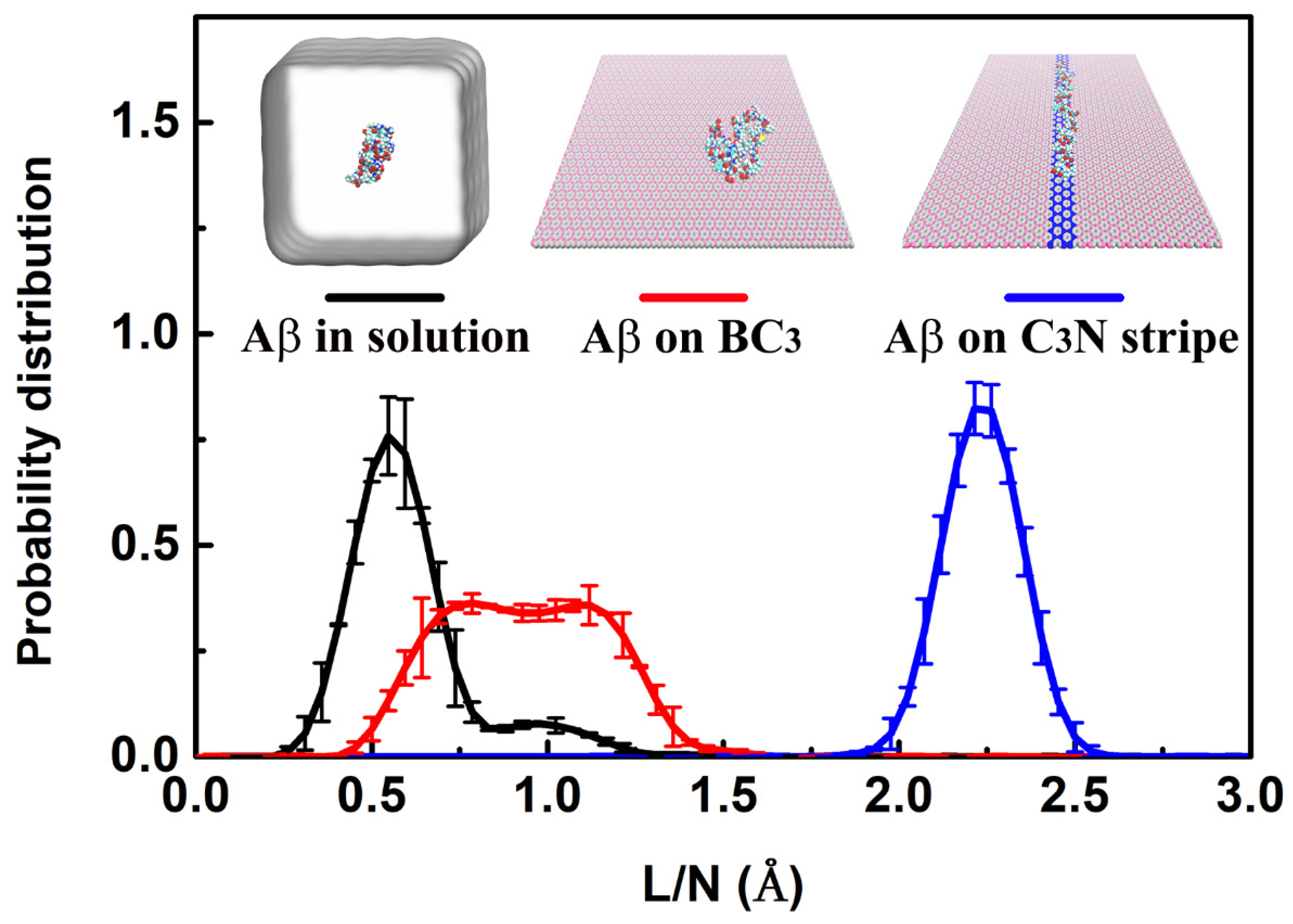
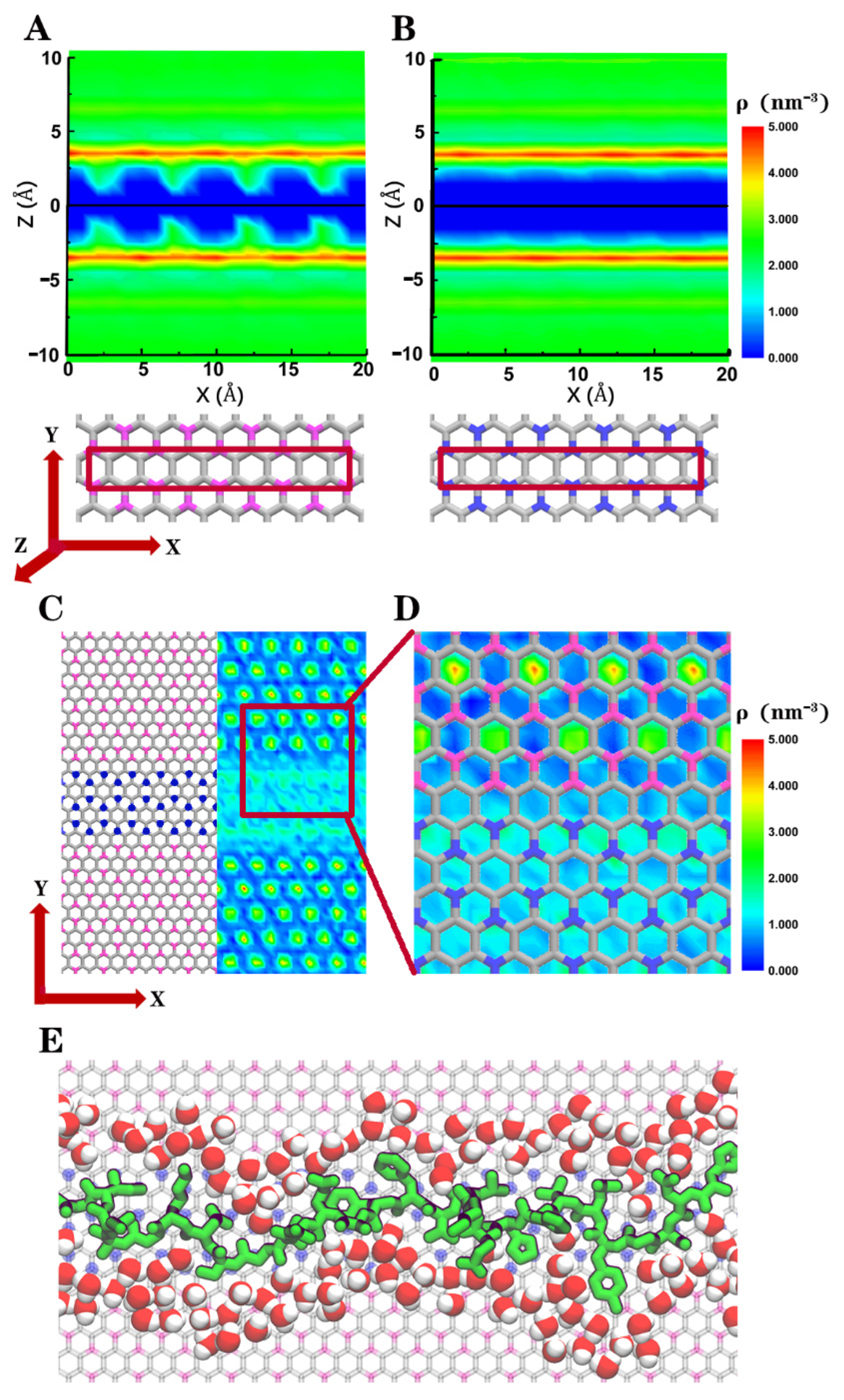
3. Result
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Y.; Docter, M.; Ginkel, J.V.; Ridder, D.D.; Joo, C. Single-molecule protein sequencing through fingerprinting: Computational assessment. Phys. Biol. 2015, 12, 055003. [Google Scholar] [CrossRef] [PubMed]
- Filius, M.; Ginkel, J.V.; Joo, C. Engineering ClpXP for single-molecule protein sequencing. Biophys. J. 2017, 112, 151a. [Google Scholar] [CrossRef]
- Brinkerhoff, H.; Kang, A.S.; Liu, J.; Aksimentiev, A.; Dekker, C. Multiple rereads of single proteins at single–amino acid resolution using nanopores. Science 2021, eabl4381. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, J.A.; Bohländer, P.; Dai, M.; Filius, M.; Howard, C.J.; van Kooten, X.F.; Ohayon, S.; Pomorski, A.; Schmid, S.; Aksimentiev, A. The emerging landscape of single-molecule protein sequencing technologies. Nat. Methods 2021, 18, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Gross, L.; Mohn, F.; Moll, N.; Liljeroth, P.; Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 2009, 325, 1110–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatayer, S.; Albrecht, F.; Zhang, Y.; Urbonas, D.; Pena, D.; Moll, N.; Gross, L. Molecular structure elucidation with charge-state control. Science 2019, 365, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Yokota, K.; Tsutsui, M.; Taniguchi, M. Fast and low-noise tunnelling current measurements for single-molecule detection in an electrolyte solution using insulator-protected nanoelectrodes. Nanoscale 2017, 9, 4076–4081. [Google Scholar] [CrossRef] [PubMed]
- Ventra, M.; Taniguchi, M. Decoding DNA, RNA and peptides with quantum tunnelling. Nat. Nanotechnol. 2016, 11, 117–126. [Google Scholar] [CrossRef]
- Ohshiro, T.; Tsutsui, M.; Yokota, K.; Furuhashi, M.; Taniguchi, M.; Kawai, T. Detection of post-translational modifications in single peptides using electron tunnelling currents. Nat. Nanotechnol. 2014, 9, 835–840. [Google Scholar] [CrossRef]
- Yusko, E.C.; Bruhn, B.R.; Eggenberger, O.M.; Houghtaling, J.; Rollings, R.C.; Walsh, N.C.; Nandivada, S.; Pindrus, M.; Hall, A.R.; Sept, D. Real-time shape approximation and fingerprinting of single proteins using a nanopore. Nat. Nanotechnol. 2017, 12, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Ouldali, H.; Sarthak, K.; Enslen, T.; Piguet, F.; Manivet, P.; Pelta, J.; Behrends, J.C.; Aksimentiev, A.; Oukhaled, A. Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore. Nat. Biotechnol. 2020, 38, 176–181. [Google Scholar] [CrossRef]
- Domon, B.; Aebersold, R. Options and considerations when selecting a quantitative proteomics strategy. Nat. Biotechnol. 2010, 28, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Laura, R.-P.; Chirlmin, J.C.; Dekker, C. Paving the way to single-molecule protein sequencing. Nat. Nanotechnol. 2018, 13, 786–796. [Google Scholar]
- Uversky, V.N. A decade and a half of protein intrinsic disorder: Biology still waits for physics. Protein Sci. 2013, 22, 693–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthukumar, M. Translocation of a confined polymer through a hole. Phys. Rev. Lett. 2001, 86, 3188–3191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Menard, L.D.; Panyukov, S.; Rubinstein, M.; Ramsey, J.M. Enhanced nanochannel translocation and localization of genomic DNA molecules using three-dimensional nanofunnels. Nat. Commun. 2017, 8, 807. [Google Scholar] [CrossRef] [Green Version]
- Luan, B.; Zhou, R. Spontaneous ssDNA stretching on graphene and hexagonal boron nitride in plane heterostructures. Nat. Commun. 2019, 10, 4610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, B. Energetically stretching proteins on patterned two-dimensional nanosheets. Nano Futures 2020, 4, 035001. [Google Scholar] [CrossRef]
- He, Z.; Zhou, R. Exploring in-plane graphene and hexagonal boron nitride array for separation of single nucleotides. ACS Nano 2021, 15, 11704–11710. [Google Scholar] [CrossRef]
- Duan, X.; Chen, W.; Shaw, J.C.; Rui, C.; Chen, Y.; Li, H.; Wu, X.; Tang, Y.; Zhang, Q.; Pan, A. Lateral epitaxial growth of two-dimensional layered semiconductor heterojunctions. Nat. Nanotechnol. 2014, 9, 1024–1030. [Google Scholar] [CrossRef]
- Huang, C.; Wu, S.; Sanchez, A.M.; Peters, J.; Beanland, R.; Rivera, P.; Yao, W.; Cobden, D.H.; Xu, X. Lateral heterojunctions within monolayer semiconductors. Nat. Mater. 2014, 13, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, M.-Y.T. Strain distributions and their influence on electronic structures of WSe2-MoS2 laterally strained heterojunctions. Nat. Nanotechnol. 2018, 13, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Chen, L.; Elibol, K.; He, L.; Wang, H.; Chen, C.; Jiang, C.; Li, C.; Wu, T.; Cong, C.X. Towards chirality control of graphene nanoribbons embedded in hexagonal boron nitride. Nat. Mater. 2021, 20, 202–207. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhou, R. Planar graphene/h-BN/graphene heterostructures for protein stretching and confinement. Nanoscale 2020, 12, 13822–13828. [Google Scholar] [CrossRef]
- Bohayra, M. Ultra-high stiffness and thermal conductivity of graphene-like C3N. Carbon 2017, 118, 25–34. [Google Scholar]
- King, T.C.; Matthews, P.D.; Glass, H.; Cormack, J.A.; Holgado, J.P.; Leskes, M.; Griffin, J.M.; Scherman, O.A.; Barker, P.D.; Grey, C.P. Theory and Practice: Bulk synthesis of C3B and its H2- and Li-storage capacity. Angew. Chem. Int. Ed. 2015, 127, 6017–6021. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, F.; Liu, Y.; Yang, Y.; Li, W. Orientational binding and directional transport of DNA on nanomaterial heterojunctions. Nanoscale 2020, 12, 5217–5226. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Sun, B.; Liu, S.; Liu, H. Two-dimensional nanomaterials for cancer nano-theranostics. Small 2017, 13, 1603446. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Ahmed, R.; Omidian, Z.; Giwa, A.; Cornwell, B.; Majety, N.D.R.; Lee, S.; Zhang, H.; Michels, A.; Desiderio, S. A public BCR present in a unique dual-receptor-expressing lymphocyte from type 1 diabetes patients encodes a potent T cell autoantigen. Cell 2019, 177, 1583–1599. [Google Scholar] [CrossRef] [PubMed]
- Binquan, L.; Ruhong, Z. Atomic-scale fluidic diodes based on triangular nanopores in bilayer hexagonal boron nitride. Nano Lett. 2019, 19, 977–982. [Google Scholar]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; Van Der Spoel, D. Gromacs 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. Vmd: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Jamsawang, P. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 2018, 79, 926–935. [Google Scholar]
- Foloppe, N.; Mackerell, A.D. All-atom empirical force field for nucleic acids: I. Parameter optimization based on small molecule and condensed phase macromolecular target data. J. Comput. Chem. 2000, 21, 86–104. [Google Scholar] [CrossRef]
- Giampaga, M.; Zhestkov, Y.; Pitman, M.; Suits, F.; Grossfield, A.; Zhestkov, Y.; Pitman, M.C.; Suits, F.; Grossfield, A.; Pitera, J. Blue matter: Strong scaling of molecular dynamics on blue gene/L. In International Conference on Computational Science; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Kaminski, G.A.; Friesner, R.A.; Zhou, R. A computationally inexpensive modification of the point dipole electrostatic polarization model for molecular simulations. J. Comput. Chem. 2003, 24, 267. [Google Scholar] [CrossRef]
- Zhou, R. Hydrophobic collapse in multidomain protein folding. Science 2004, 305, 1605–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, N.; Weber, J.K.; Wang, S.; Luan, B.; Yue, H.; Xi, X.; Du, J.; Yang, Z.; Wei, W.; Zhou, R. Pegylated graphene oxide elicits strong immunological responses despite surface passivation. Nat. Commun. 2017, 8, 14537. [Google Scholar] [CrossRef] [Green Version]
- Chowell, D.; Morris, L.G.; Grigg, C.M.; Weber, J.K.; Samstein, R.M.; Makarov, V.; Kuo, F.; Kendall, S.M.; Requena, D.; Riaz, N. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018, 359, 582–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darden, T.; York, D.; Pedersen, L. Particle mesh ewald: An N·log(N) method for ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182. [Google Scholar]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; He, J.; Chang, S.; Zhang, P.; Liang, F.; Li, S.; Tuchband, M.; Fuhrmann, A.; Ros, R.; Lindsay, S. Identifying single bases in a DNA oligomer with electron tunnelling. Nat. Nanotechnol. 2010, 5, 868–873. [Google Scholar] [CrossRef]
- Tanaka, H.; Kawai, T. Partial sequencing of a single DNA molecule with a scanning tunneling microscope. Nat. Nanotechnol. 2009, 4, 518–522. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; He, Z.; Meng, L.; Liang, H.; Zhou, R. Planar Boronic Graphene and Nitrogenized Graphene Heterostructure for Protein Stretch and Confinement. Biomolecules 2021, 11, 1756. https://doi.org/10.3390/biom11121756
Su X, He Z, Meng L, Liang H, Zhou R. Planar Boronic Graphene and Nitrogenized Graphene Heterostructure for Protein Stretch and Confinement. Biomolecules. 2021; 11(12):1756. https://doi.org/10.3390/biom11121756
Chicago/Turabian StyleSu, Xuchang, Zhi He, Lijun Meng, Hong Liang, and Ruhong Zhou. 2021. "Planar Boronic Graphene and Nitrogenized Graphene Heterostructure for Protein Stretch and Confinement" Biomolecules 11, no. 12: 1756. https://doi.org/10.3390/biom11121756
APA StyleSu, X., He, Z., Meng, L., Liang, H., & Zhou, R. (2021). Planar Boronic Graphene and Nitrogenized Graphene Heterostructure for Protein Stretch and Confinement. Biomolecules, 11(12), 1756. https://doi.org/10.3390/biom11121756





