MicroRNAs Regulating Renin–Angiotensin–Aldosterone System, Sympathetic Nervous System and Left Ventricular Hypertrophy in Systemic Arterial Hypertension
Abstract
1. Introduction
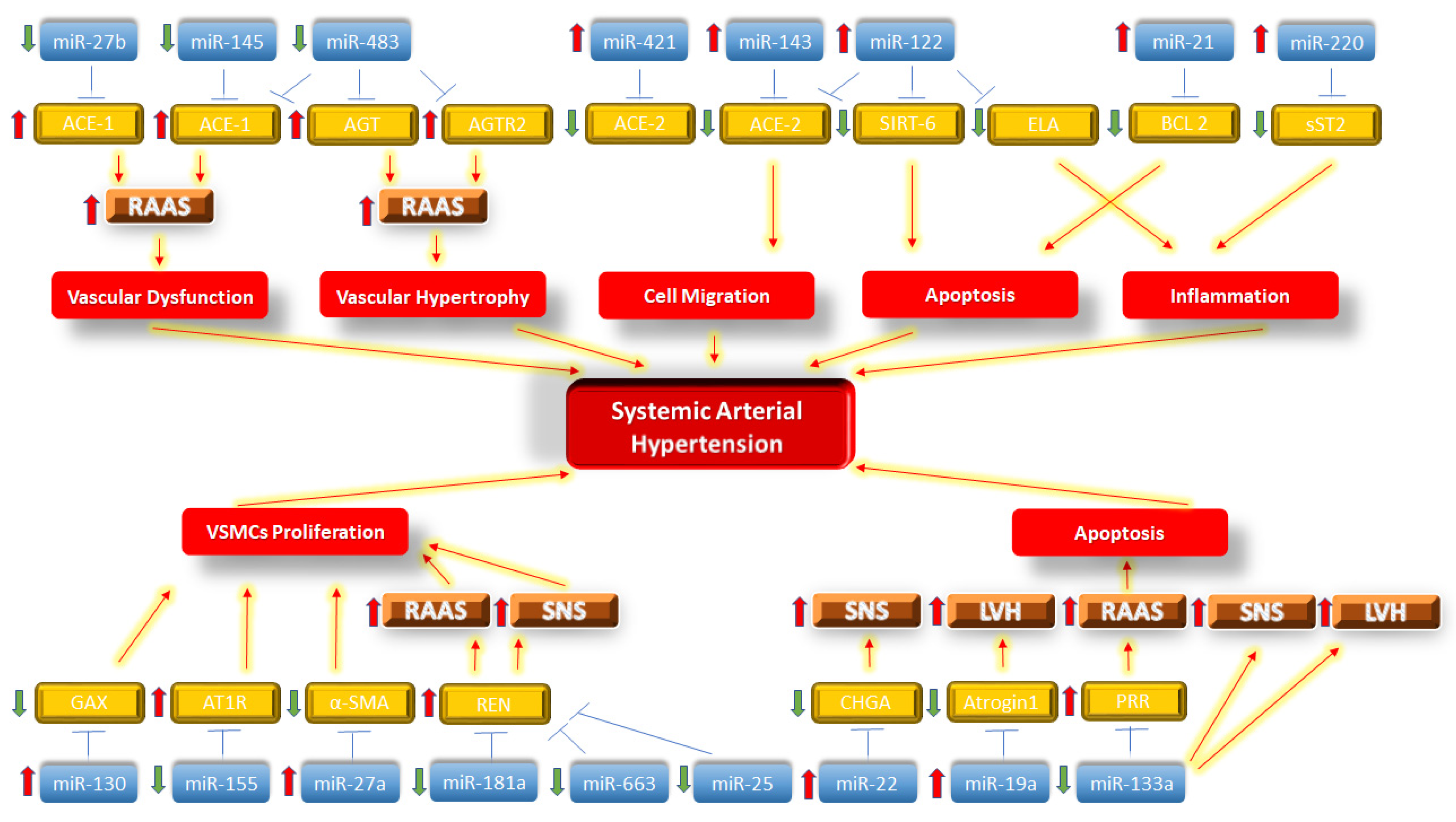
2. MiRNAs and Activation of the Renin–Angiotensin–Aldosterone System in SAH
3. MiRNAs and Sympathetic Nervous System Activation in SAH
4. MiRNAs and Left Ventricular Hypertrophy in SAH
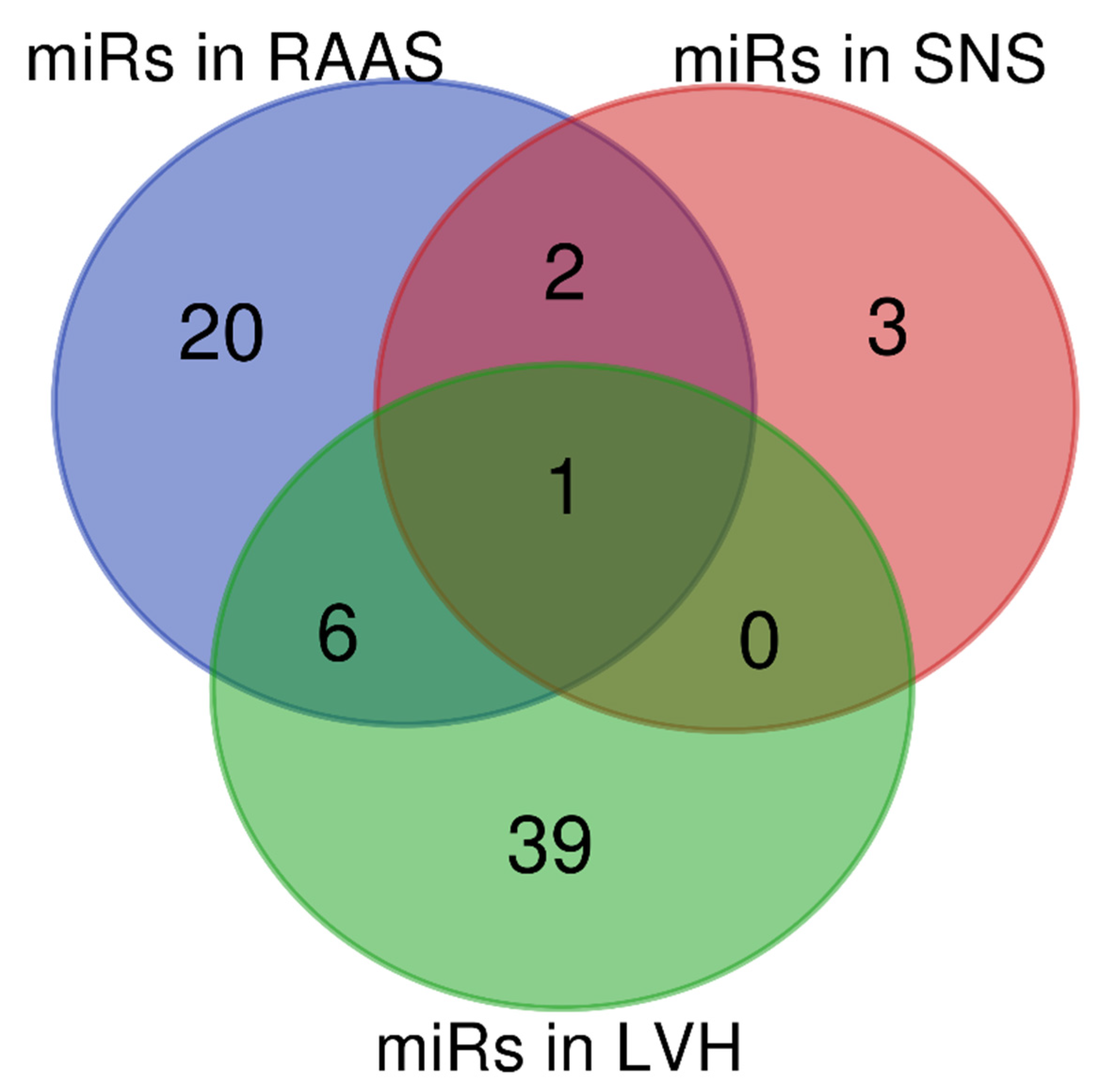
5. Overlapping miRNAs in RAAS, SNS and LVH in SAH
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef]
- Neves, V.J.; Fernandes, T.; Roque, F.R.; Soci, U.P.; Melo, S.F.; de Oliveira, E.M. Exercise training in hypertension: Role of microRNAs. World J. Cardiol. 2014, 6, 713–727. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-based Studies from 90 Countries. Circulation 2016, 134, 441. [Google Scholar] [CrossRef]
- Klimczak, D.; Jazdzewski, K.; Kuch, M. Regulatory mechanisms in arterial hypertension: Role of microRNA in pathophysiology and therapy. Blood Press. 2017, 26, 2–8. [Google Scholar] [CrossRef]
- Frieler, R.A.; Mortensen, R.M. Immune Cell and Other Non-Cardiomyocyte Regulation of Cardiac Hypertrophy and Remodeling. Circulation 2015, 131, 1019. [Google Scholar] [CrossRef]
- Improta-Caria, A.C.; Nonaka, C.K.V.; Cavalcante, B.R.R.; De Sousa, R.A.L.; Júnior, R.A.; de Souza, B.S.F. Modulation of microRNAs as a potential molecular mechanism involved in the beneficial actions of physical exercise in Alzheimer disease. Int. J. Mol. Sci. 2020, 21, 4977. [Google Scholar] [CrossRef]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Improta-Caria, A.C.; Aras, R. Treinamento com Exercício Físico e Doença de Chagas: Função Potencial dos MicroRNAs. Arq. Bras. Cardiol. 2021, 117, 132–141. [Google Scholar] [CrossRef]
- Caria, A.C.I.; Nonaka, C.K.V.; Pereira, C.S.; Soares, M.B.P.; Macambira, S.G.; Souza, B.S.D.F. Exercise training-induced changes in microRNAs: Beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int. J. Mol. Sci. 2018, 19, 3608. [Google Scholar] [CrossRef]
- Watanabe, K.; Narumi, T.; Watanabe, T.; Otaki, Y.; Takahashi, T.; Aono, T.; Goto, J.; Toshima, T.; Sugai, T.; Wanezaki, M.; et al. The association between microRNA-21 and hypertension-induced cardiac remodeling. PLoS ONE 2020, 15, e0226053. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Mirabito Colafella, K.M.; Bovée, D.M.; Danser, A.H.J. The renin-angiotensin-aldosterone system and its therapeutic targets. Exp. Eye Res. 2019, 186, 107680. [Google Scholar] [CrossRef]
- Persson, P.B. Renin: Origin, secretion and synthesis. J. Physiol. 2003, 552, 667. [Google Scholar] [CrossRef]
- Muñoz-Durango, N.; Fuentes, C.A.; Castillo, A.E.; González-Gómez, L.M.; Vecchiola, A.; Fardella, C.E.; Kalergis, A.M. Role of the Renin-Angiotensin-Aldosterone System beyond Blood Pressure Regulation: Molecular and Cellular Mechanisms Involved in End-Organ Damage during Arterial Hypertension. Int. J. Mol. Sci. 2016, 17, 797. [Google Scholar] [CrossRef]
- Chappell, M.C. Biochemical evaluation of the renin-angiotensin system: The good, bad, and absolute? Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H137. [Google Scholar] [CrossRef]
- Gorini, S.; Kim, S.K.; Infante, M.; Mammi, C.; La Vignera, S.; Fabbri, A.; Jaffe, I.Z.; Caprio, M. Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging. Front. Endocrinol. 2019, 10, 584. [Google Scholar] [CrossRef]
- Takahashi, H.; Yoshika, M.; Komiyama, Y.; Nishimura, M. The central mechanism underlying hypertension: A review of the roles of sodium ions, epithelial sodium channels, the renin–angiotensin–aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens. Res. 2011, 34, 1147–1160. [Google Scholar] [CrossRef]
- Hu, B.; Song, J.T.; Qu, H.Y.; Bi, C.L.; Huang, X.Z.; Liu, X.X.; Zhang, M. Mechanical Stretch Suppresses microRNA-145 Expression by Activating Extracellular Signal-Regulated Kinase 1/2 and Upregulating Angiotensin-Converting Enzyme to Alter Vascular Smooth Muscle Cell Phenotype. PLoS ONE 2014, 9, e96338. [Google Scholar] [CrossRef]
- Chen, L.J.; Xu, R.; Yu, H.M.; Chang, Q.; Zhong, J.C. The ACE2/apelin signaling, microRNAs, and hypertension. Int. J. Hypertens. 2015, 2015, 896861. [Google Scholar] [CrossRef]
- Lambert, D.W.; Lambert, L.A.; Clarke, N.E.; Hooper, N.M.; Porter, K.E.; Turner, A.J. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin. Sci. 2014, 127, 243–249. [Google Scholar] [CrossRef]
- Kemp, J.R.; Unal, H.; Desnoyer, R.; Yue, H.; Bhatnagar, A.; Karnik, S.S. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system. J. Mol. Cell. Cardiol. 2014, 75, 25–39. [Google Scholar] [CrossRef]
- Boettger, T.; Beetz, N.; Kostin, S.; Schneider, J.; Krüger, M.; Hein, L.; Braun, T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J. Clin. Investig. 2009, 119, 2634–2647. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Zhu, G.; Liu, H.; Guo, R.; Qi, F.; Zou, J. MicroRNA-155 inhibits angiotensin II-induced vascular smooth muscle cell proliferation. J. Renin. Angiotensin. Aldosterone. Syst. 2014, 15, 109–116. [Google Scholar] [CrossRef]
- Wu, W.-H.; Hu, C.-P.; Chen, X.-P.; Zhang, W.-F.; Li, X.-W.; Xiong, X.-M.; Li, Y.-J. MicroRNA-130a mediates proliferation of vascular smooth muscle cells in hypertension. Am. J. Hypertens. 2011, 24, 1087–1093. [Google Scholar] [CrossRef]
- Xu, M.; Deng, H.; Li, H. MicroRNA-27a regulates angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting α-smooth muscle-actin in vitro. Biochem. Biophys. Res. Commun. 2019, 509, 973–977. [Google Scholar] [CrossRef]
- Song, J.; Yang, M.; Liu, Y.; Song, J.; Wang, J.; Chi, H.; Liu, X.; Zuo, K.; Yang, X.; Zhong, J. MicroRNA-122 aggravates angiotensin II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts via the modulation of SIRT6-elabela-ACE2 signaling. Eur. J. Pharmacol. 2020, 883, 173374. [Google Scholar] [CrossRef]
- Liu, B.; Lan, M.; Wei, H.; Zhang, D.; Liu, J.; Teng, J. Downregulated microRNA-133a induces HUVECs injury: Potential role of the (pro) renin receptor in angiotensin II-dependent hypertension. Mol. Med. Rep. 2019, 20, 2796–2804. [Google Scholar] [CrossRef]
- Jiang, X.; Ning, Q.; Wang, J. Angiotensin II induced differentially expressed microRNAs in adult rat cardiac fibroblasts. J. Physiol. Sci. 2013, 63, 31–38. [Google Scholar] [CrossRef]
- Jeppesen, P.L.; Christensen, G.L.; Schneider, M.; Nossent, A.Y.; Jensen, H.B.; Andersen, D.C.; Eskildsen, T.; Gammeltoft, S.; Hansen, J.L.; Sheikh, S.P. Angiotensin II type 1 receptor signalling regulates microRNA differentially in cardiac fibroblasts and myocytes. Br. J. Pharmacol. 2011, 164, 394–404. [Google Scholar] [CrossRef]
- Eskildsen, T.V.; Jeppesen, P.L.; Schneider, M.; Nossent, A.Y.; Sandberg, M.B.; Hansen, P.B.L.; Jensen, C.H.; Hansen, M.L.; Marcussen, N.; Rasmussen, L.M.; et al. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int. J. Mol. Sci. 2013, 14, 11190–11207. [Google Scholar] [CrossRef]
- Jan Van Zonneveld, A.; Au, Y.W.; Stam, W.; Van Gelderen, S.; Rotmans, J.I.; Deen, P.M.T.; Rabelink, T.J.; Bijkerk, R. MicroRNA-132 regulates salt-dependent steady-state renin levels in mice. Commun. Biol. 2020, 3, 238. [Google Scholar] [CrossRef]
- Fernandes, T.; Magalhães, F.C.; Roque, F.R.; Phillips, M.I.; Oliveira, E.M. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: Role of microRNAs-16, -21, and -126. Hypertension 2012, 59, 513–520. [Google Scholar] [CrossRef]
- Shi, L.; Liao, J.; Liu, B.; Zeng, F.; Zhang, L. Mechanisms and therapeutic potential of microRNAs in hypertension. Drug Discov. Today 2015, 20, 1188–1204. [Google Scholar] [CrossRef]
- Chu, H.T.; Li, L.; Jia, M.; Diao, L.L.; Li, Z.B. Correlation between serum microRNA-136 levels and RAAS biochemical markers in patients with essential hypertension. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11752–11760. [Google Scholar] [CrossRef]
- Li, L.; Zhong, D.; Xie, Y.; Yang, X.; Yu, Z.; Zhang, D.; Jiang, X.; Wu, Y.; Wu, F. Blood microRNA 202-3p associates with the risk of essential hypertension by targeting soluble ST2. Biosci. Rep. 2020, 40, BSR20200378. [Google Scholar] [CrossRef]
- Marques, F.Z.; Campain, A.E.; Tomaszewski, M.; Zukowska-Szczechowska, E.; Yang, Y.H.J.; Charchar, F.J.; Morris, B.J. Gene Expression Profiling Reveals Renin mRNA Overexpression in Human Hypertensive Kidneys and a Role for MicroRNAs. Hypertension 2011, 58, 1093–1098. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.; Liu, Y.; Qi, Y.; Tang, C.; Li, X.; Zuo, K.; Sun, D.; Shen, Y.; Pang, D.; et al. Alteration in microRNA-25 expression regulate cardiac function via renin secretion. Exp. Cell Res. 2018, 365, 119–128. [Google Scholar] [CrossRef]
- Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Strapazzon, G.; Ragazzo, F.; Bratti, P.; Fabricio, A.S.; Squarcina, E.; Gion, M.; Palatini, P.; et al. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am. J. Hypertens. 2011, 24, 241–246. [Google Scholar] [CrossRef]
- Sõber, S.; Laan, M.; Annilo, T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem. Biophys. Res. Commun. 2010, 391, 727–732. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Song, D.W.; Ryu, J.Y.; Kim, J.O.; Kwon, E.J.; Kim, D.H. The miR-19a/b family positively regulates cardiomyocyte hypertrophy by targeting atrogin-1 and MuRF-1. Biochem. J. 2014, 457, 151–162. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Chen, Q.; Wu, Q.; Deng, W.; Zhou, H.; Shen, D. Long non-coding RNA cytoskeleton regulator RNA (CYTOR) modulates pathological cardiac hypertrophy through miR-155-mediated IKKi signaling. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 1421–1427. [Google Scholar] [CrossRef]
- Nonaka, C.K.V.; Macêdo, C.T.; Cavalcante, B.R.R.; De Alcântara, A.C.; Silva, D.N.; Bezerra, M.D.R.; Caria, A.C.I.; Tavora, F.R.F.; Neto, J.D.D.S.; Noya-Rabelo, M.M.; et al. Circulating miRNAs as potential biomarkers associated with cardiac remodeling and fibrosis in chagas disease cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 4064. [Google Scholar] [CrossRef]
- DeCicco, D.; Zhu, H.; Brureau, A.; Schwaber, J.S.; Vadigepalli, R. MicroRNA network changes in the brain stem underlie the development of hypertension. Physiol. Genom. 2015, 47, 388–399. [Google Scholar] [CrossRef]
- Zhang, K.; Mir, S.A.; Makena Hightower, C.; Miramontes-Gonzalez, J.P.; Maihofer, A.X.; Chen, Y.; Mahata, S.K.; Nievergelt, C.M.; Schork, N.J.; Freedman, B.I.; et al. Molecular mechanism for hypertensive renal disease: Differential regulation of chromogranin a expression at 3′-untranslated region polymorphism C+87T by MicroRNA-107. J. Am. Soc. Nephrol. 2015, 26, 1816–1825. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, R.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNAs are aberrantly expressed in hypertrophic heart: Do they play a role in cardiac hypertrophy? Am. J. Pathol. 2007, 170, 1831–1840. [Google Scholar] [CrossRef]
- Azibani, F.; Devaux, Y.; Coutance, G.; Schlossarek, S.; Polidano, E.; Fazal, L.; Merval, R.; Carrier, L.; Solal, A.C.; Chatziantoniou, C.; et al. Aldosterone inhibits the fetal program and increases hypertrophy in the heart of hypertensive mice. PLoS ONE 2012, 7, e38197. [Google Scholar] [CrossRef]
- Clark, A.L.; Maruyama, S.; Sano, S.; Accorsi, A.; Girgenrath, M.; Walsh, K.; Naya, F.J. miR-410 and miR-495 Are Dynamically Regulated in Diverse Cardiomyopathies and Their Inhibition Attenuates Pathological Hypertrophy. PLoS ONE 2016, 11, e0151515. [Google Scholar] [CrossRef]
- Jackson, K.L.; Gueguen, C.; Lim, K.; Eikelis, N.; Stevenson, E.R.; Charchar, F.J.; Lambert, G.W.; Burke, S.L.; Paterson, M.R.; Marques, F.Z.; et al. Neural suppression of miRNA-181a in the kidney elevates renin expression and exacerbates hypertension in Schlager mice. Hypertens. Res. 2020, 43, 1152–1164. [Google Scholar] [CrossRef]
- Yang, F.; Li, H.; Du, Y.; Shi, Q.; Zhao, L. Downregulation of microRNA-34b is responsible for the elevation of blood pressure in spontaneously hypertensive rats. Mol. Med. Rep. 2017, 15, 1031. [Google Scholar] [CrossRef]
- Friese, R.S.; Altshuler, A.E.; Zhang, K.; Miramontes-Gonzalez, J.P.; Hightower, C.M.; Jirout, M.L.; Salem, R.M.; Gayen, J.R.; Mahapatra, N.R.; Biswas, N.; et al. MicroRNA-22 and promoter motif polymorphisms at the Chga locus in genetic hypertension: Functional and therapeutic implications for gene expression and the pathogenesis of hypertension. Hum. Mol. Genet. 2013, 22, 3624–3640. [Google Scholar] [CrossRef]
- Carr, G.; Barrese, V.; Stott, J.B.; Povstyan, O.V.; Jepps, T.A.; Figueiredo, H.B.; Zheng, D.; Jamshidi, Y.; Greenwood, I.A. MicroRNA-153 targeting of KCNQ4 contributes to vascular dysfunction in hypertension. Cardiovasc. Res. 2016, 112, 581–589. [Google Scholar] [CrossRef]
- Nossent, A.Y.; Eskildsen, T.V.; Andersen, L.B.; Bie, P.; Brønnum, H.; Schneider, M.; Andersen, D.C.; Welten, S.M.; Jeppesen, P.L.; Hamming, J.F.; et al. The 14q32 microRNA-487b targets the antiapoptotic insulin receptor substrate 1 in hypertension-induced remodeling of the aorta. Ann. Surg. 2013, 258, 743. [Google Scholar] [CrossRef]
- Wei, L.H.; Huang, X.R.; Zhang, Y.; Li, Y.Q.; Chen, H.Y.; Yan, B.P.; Yu, C.-M.; Lan, H.Y. Smad7 inhibits angiotensin II-induced hypertensive cardiac remodelling. Cardiovasc. Res. 2013, 99, 665–673. [Google Scholar] [CrossRef]
- Liu, K.; Hao, Q.; Wei, J.; Li, G.H.; Wu, Y.; Zhao, Y.F. MicroRNA-19a/b-3p protect the heart from hypertension-induced pathological cardiac hypertrophy through PDE5A. J. Hypertens. 2018, 36, 1847–1857. [Google Scholar] [CrossRef]
- Syed, M.; Ball, J.P.; Mathis, K.W.; Hall, M.E.; Ryan, M.J.; Rothenberg, M.E.; Yanes Cardozo, L.L.; Romero, D.G. Microrna-21 ablation exacerbates aldosterone-mediated cardiac injury, remodeling, and dysfunction. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E1154–E1167. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, S.; Ji-yan, C.; Huang, C.; Li, J.; Cai, A.; Feng, Y. Circulating miR-92a expression level in patients with essential hypertension: A potential marker of atherosclerosis. J. Hum. Hypertens. 2017, 31, 200–205. [Google Scholar] [CrossRef]
- Kontaraki, J.E.; Marketou, M.E.; Parthenakis, F.I.; Maragkoudakis, S.; Zacharis, E.A.; Petousis, S.; Kochiadakis, G.E.; Vardas, P.E. Hypertrophic and antihypertrophic microRNA levels in peripheral blood mononuclear cells and their relationship to left ventricular hypertrophy in patients with essential hypertension. J. Am. Soc. Hypertens. 2015, 9, 802–810. [Google Scholar] [CrossRef]
- Yang, Q.; Jia, C.; Wang, P.; Xiong, M.; Cui, J.; Li, L.; Wang, W.; Wu, Q.; Chen, Y.; Zhang, T. MicroRNA-505 identified from patients with essential hypertension impairs endothelial cell migration and tube formation. Int. J. Cardiol. 2014, 177, 925–934. [Google Scholar] [CrossRef]
- Dörr, O.; Liebetrau, C.; Möllmann, H.; Gaede, L.; Troidl, C.; Lankes, S.; Guckel, D.; Boeder, N.; Voss, S.; Bauer, T.; et al. Effect of Renal Sympathetic Denervation on Specific MicroRNAs as an Indicator of Reverse Remodeling Processes in Hypertensive Heart Disease. J. Clin. Hypertens. 2016, 18, 497–502. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, S.; Huang, C.; Chen, J.; Li, J.; Cai, A.; Feng, Y. Circulating miRNA29 family expression levels in patients with essential hypertension as potential markers for left ventricular hypertrophy. Clin. Exp. Hypertens. 2017, 39, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, J.; Zhang, W.; Chen, Y.; Zhang, K.; Popescu, L.M.; Ma, X.; Bond Lau, W.; Rong, R.; Yu, X.; et al. Signature microRNA Expression Profile of Essential Hypertension and Its Novel Link to Human Cytomegalovirus Infection. Circulation 2011, 124, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kontaraki, J.E.; Marketou, M.E.; Zacharis, E.A.; Parthenakis, F.I.; Vardas, P.E. Differential expression of vascular smooth muscle-modulating microRNAs in human peripheral blood mononuclear cells: Novel targets in essential hypertension. J. Hum. Hypertens. 2014, 28, 510–516. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Huang, C.; Chen, J.Y.; Li, J.; Feng, Y.Q. The association of circulating miR-30a, miR-29 and miR-133 with white-coat hypertension. Biomark. Med. 2016, 10, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Huang, C.; Zhang, B.; Feng, Y.Q. Association of circulating miR-155 expression level and inflammatory markers with white coat hypertension. J. Hum. Hypertens. 2020, 34, 397–403. [Google Scholar] [CrossRef] [PubMed]
- DeLalio, L.J.; Sved, A.F.; Stocker, S.D. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can. J. Cardiol. 2020, 36, 712. [Google Scholar] [CrossRef]
- Grassi, G.; Ram, V.S. Evidence for a critical role of the sympathetic nervous system in hypertension. J. Am. Soc. Hypertens. 2016, 10, 457–466. [Google Scholar] [CrossRef]
- Seravalle, G.; Mancia, G.; Grassi, G. Role of the Sympathetic Nervous System in Hypertension and Hypertension-Related Cardiovascular Disease. High Blood Press. Cardiovasc. Prev. 2014, 21, 89–105. [Google Scholar] [CrossRef]
- Wang, G.; Kwan, B.C.-H.; Lai, F.M.-M.; Choi, P.C.-L.; Chow, K.-M.; Li, P.K.-T.; Szeto, C.-C. Intrarenal Expression of miRNAs in Patients with Hypertensive Nephrosclerosis. Am. J. Hypertens. 2010, 23, 78–84. [Google Scholar] [CrossRef]
- Stansfield, W.E.; Ranek, M.; Pendse, A.; Schisler, J.C.; Wang, S.; Pulinilkunnil, T.; Willis, M.S. The Pathophysiology of Cardiac Hypertrophy and Heart Failure. Cell. Mol. Pathobiol. Cardiovasc. Dis. 2014, 51–78. [Google Scholar] [CrossRef]
- Stevens, S.M.; Reinier, K.; Chugh, S.S. Increased Left Ventricular Mass as a Predictor of Sudden Cardiac Death: Is it Time to put it to the Test? Circ. Arrhythm. Electrophysiol. 2013, 6, 212. [Google Scholar] [CrossRef]
- Nadruz, W. Myocardial remodeling in hypertension. J. Hum. Hypertens. 2014, 29, 1–6. [Google Scholar] [CrossRef]
- Shenasa, M.; Shenasa, H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int. J. Cardiol. 2017, 237, 60–63. [Google Scholar] [CrossRef]
- Cacciapuoti, F. Molecular mechanisms of left ventricular hypertrophy (LVH) in systemic hypertension (SH)—possible therapeutic perspectives. J. Am. Soc. Hypertens. 2011, 5, 449–455. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Matkovich, S.J.; Wang, W.; Tu, Y.; Eschenbacher, W.H.; Dorn, L.E.; Condorelli, G.; Diwan, A.; Nerbonne, J.M.; Dorn, G.W. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ. Res. 2010, 106, 166–175. [Google Scholar] [CrossRef]
- Yao, S.Y.; Liu, J.; Li, Y.; Wang, M.; Wang, C.; Xue, H. Association between plasma microRNA-29a and left ventricular hypertrophy in patients with hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2019, 47, 215–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, S.; Ding, X.; Lu, C.; Wu, R.; Wu, H.; Shang, Y.; Pang, M. MicroRNA-30a-5p silencing polarizes macrophages toward M2 phenotype to alleviate cardiac injury following viral myocarditis by targeting SOCS1. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1348–H1360. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019, 28, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Diabetes Mellitus and Cardiovascular Risk Assessment in Mothers with a History of Gestational Diabetes Mellitus Based on Postpartal Expression Profile of MicroRNAs Associated with Diabetes Mellitus and Cardiovascular and Cerebrovascular Diseases. Int. J. Mol. Sci. 2020, 21, 2437. [Google Scholar] [CrossRef]
- Renaud, L.; Harris, L.G.; Mani, S.K.; Kasiganesan, H.; Chou, J.C.; Baicu, C.F.; Van Laer, A.; Akerman, A.W.; Stroud, R.E.; Jones, J.A.; et al. HDACs Regulate miR-133a Expression in Pressure Overload Induced Cardiac Fibrosis. Circ. Heart Fail. 2015, 8, 1094. [Google Scholar] [CrossRef]
- Akerman, A.W.; Blanding, W.M.; Stroud, R.E.; Nadeau, E.K.; Mukherjee, R.; Ruddy, J.M.; Zile, M.R.; Ikonomidis, J.S.; Jones, J.A. Elevated Wall Tension Leads to Reduced miR-133a in the Thoracic Aorta by Exosome Release. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2019, 8, e010332. [Google Scholar] [CrossRef]
- Eckhouse, S.R.; Akerman, A.W.; Logdon, C.B.; Oelsen, J.M.; O’Quinn, E.C.; Nadeau, E.K.; Stroud, R.E.; Mukherjee, R.; Jones, J.A.; Spinale, F.G. Differential membrane type 1 matrix metalloproteinase substrate processing with ischemia–reperfusion: Relationship to interstitial microRNA dynamics and myocardial function. J. Thorac. Cardiovasc. Surg. 2013, 145, 267. [Google Scholar] [CrossRef][Green Version]
- Castoldi, G.; di Gioia, C.R.T.; Bombardi, C.; Catalucci, D.; Corradi, B.; Gualazzi, M.G.; Leopizzi, M.; Mancini, M.; Zerbini, G.; Condorelli, G.; et al. MiR-133a regulates collagen 1A1: Potential role of miR-133a in myocardial fibrosis in angiotensin II-dependent hypertension. J. Cell. Physiol. 2012, 227, 850–856. [Google Scholar] [CrossRef] [PubMed]
| MicroRNAs in RAAS, SNS and LVH | ||||
|---|---|---|---|---|
| In Vitro Studies | ||||
| MicroRNAs | Study Model | Findings | miRNA Targets | Reference |
| ↓ miR-143/145 | Vascular smooth muscle cells (VSMCs) obtained from miR143/145-/- mice | miR-143/145 deficiency reduces the contractility of vascular smooth muscle cells. | ACE | [22] |
| ↓ miR-483-3p | VSMCs—human and rat aortic smooth muscle cells | miR483-3p is reduced after in vitro stimulation with angiotensin II, which activates the renin angiotensin aldosterone system (RAAS). | Multiple components of the RAS: ACE1, ACE2, AGTR2 | [21] |
| ↑ miR-130a | VSMCs were prepared from the thoracic aorta of Sprague–Dawley rats | miR-130a induces the proliferation of VSMCs, by targeting GAX, which has inhibitory actions on VSMCs proliferation. | GAX | [24] |
| ↑ miR-124, miR-135a | HeLa cells | Mineralocorticoid receptor NR3C2 is a target of miR-124 and miR-135a, which can be involved in the regulation of RAAS. | NR3C2 | [39] |
| ↑ miR-29b, miR-129, miR-132, miR-212 | Cardiac fibroblasts and HEK293N cells | Overexpressed miRNAs activate Gaq/11, ERK-1/2 and AT1R. | AT1R | [29] |
| ↑ miR-132, miR-212 | H9c2 cells and primary cardiomyocytes | MiRNAs 132 and 212 were overexpressed regulating FoxO3 inducing LVH. | FoxO3 | [40] |
| ↑ miR-125b, miR-132, miR-146b ↓ miR-181b, miR-204, miR-300 | Cardiac fibroblasts treated by AngII |
A group of dysregulated miRNAs when treated with AngII, demonstrating important roles in hypertension and cardiac fibrosis. | MMP9, MMP16, TIMP3 | [28] |
| ↓ miR-155 | Primary VSMCs from the aorta of C57/BL6 mice | Angiotensin II stimulation decreases expression of miR-155, inducing cell proliferation and survival. | AT1R | [23] |
| ↑ miR-19a, miR-19b | Cardiomyocytes derived from neonatal rats stimulated with ET-1 | MiR-19a and miR-19 promoted cardiomyocyte hypertrophy by regulating atrogin-1 and MURF-1. | Atrogin-1, MURF-1 | [41] |
| ↑ miR-27a | VSMCs stimulated with Ang-II | MiR-27a was overexpressed generating proliferation, migration and vascular dysfunction. | aSMA | [25] |
| ↓ miR-133a | HUVECs stimulated with Ang-II | MiR-133a was downregulated, increasing PRR expression, which will exacerbate the signaling pathway of the RAAS, promoting apoptosis. | PRR | [27] |
| ↑ miR-155 | Cardiomyocytes stimulated with Ang-II | MiR-155 was overexpressed reducing IKBKE promoting inflammation and cardiomyocyte hypertrophy. | IKBKE | [42] |
| ↑ miR-19a, miR-21, miR-29b, miR-199b | Cardiac fibroblasts and ipsc-derived cardiomyocytes stimulated with ET-1 | MiR-21 was overexpressed, leading to cardiac hypertrophy and fibrosis. | SPRY1 | [43] |
| ↑ miR-122 | Rat aortic adventitial fibroblasts | MiR-122 was overexpressed, promoting reduced autophagic flux and increased cell migration, oxidative stress, inflammation and apoptosis. | SIRT-6, ELA, ACE2 | [26] |
| In Vivo Studies | ||||
| ↑ miR-135a, miR-376a | Spontaneous hypertensive rats | Downregulation of Agtrap transcript by miR-135a and miR-376a; disinhibition of AT1R signaling; miR-135a downregulates Ptgr1 to increase the levels of LTB4, leading to the development of hypertension. | PTGR1, AGTRAP | [44] |
| ↑ miR-107 | Hypertensive mouse model | A polymorphism in the CHGA 3’-untranslated region known as C+87T (rs7610), promotes increased inhibition of CHGA by miR-107, leading to increased sympathetic nerve activity. | CHGA | [45] |
| ↑ miR-21, miR-126, miR-146 ↓ miR-29b, miR-133a, miR-133b, miR-149, miR-150, miR-185 | Cardiac hypertrophy C576BJ mice model | MicroRNAs were deregulated after aortic banding generating cardiac hypertrophy. | ANF, BNF, β-MHC | [46] |
| ↑ miR-208a, miR-208b |
Cardiac hyperaldosteronism (AS mice) and systemic hypertension (Ren) | Aldosterone and renin overexpression increases the expression of miR-208a and miR-208b inhibiting Sox6 and increasing cardiac hypertrophy. | Sox6 | [47] |
| ↑ miR-16, miR-21 ↓ miR-126 | Spontaneous hypertensive rats | MiR-16 is overexpressed, reducing VEGF expression, promoting decreased angiogenesis and miR-21 is also highly expressed, attenuating Bcl2 expression, inducing apoptosis. miR-126 is downregulated increasing PI3KR2 expression by inhibiting the VEGFR pathway. | VEGF, Bcl2, PI3KR2 | [32] |
| ↑ miR-132, miR-212 | Transaortic constriction mice (TAC) | MiRNAs 132 and 212 were overexpressed regulating FoxO3 inducing LVH. | FoxO3 | [40] |
| ↑ miR-132, miR-212 | Angiotensin II-induced hypertensive rats | MiR-132/212 are increased in heart, kidney, aorta and plasma of angiotensin II-induced hypertensive rats. | PTEN, ERK/MAPK | [30] |
| ↑ miR-410, miR-495 |
Ang-II stimulated rat model promoting cardiac hypertrophy | MiR-410 and miR-495 are increased in this cardiac hypertrophy model. | Nppa, Nppb | [48] |
| ↓ miR-181a | Genetically hypertensive mice (BPH/2J) | miR-181a was downregulated by increasing REN expression, increasing sympathetic nervous system activity. | REN1 | [49] |
| ↓ miR-34b | Spontaneous hypertensive rats | miR-34b was found downregulated in spontaneous hypertensive rats, increasing the levels of CDK6, leading to increased proliferation of VSMCs. | CDK6 | [50] |
| ↑ miR-22 | Spontaneous hypertensives rats | miR-22 associated with dysregulation of Chga in brainstem cardiovascular control nuclei contributing to the pathogenesis of hypertension in SHR. | Chga | [51] |
| ↑ miR-153 | Spontaneous hypertensives rats | miR-153 upregulation leads to reduced Kv7.4 channel expression, vasoconstriction, and vascular wall thickening. | KCNQ4, Kv7.4 | [52] |
| ↑ miR-487b | Rat model of angiotensin II-induced hypertension | MiR-487b is upregulated by AngII and targets the vasoactive molecule IRS1, causing loss of adventitial and medial integrity. | IRS1 | [53] |
| ↓ miR-29b | Mouse model of Ang II-induced hypertension | MiR-29b is downregulated in mouse model of Ang-II-induced hypertension, promoting LVH. | COL-I, TGFb | [54] |
| ↓ miR-19a, miR-19b | Ang-II-induced cardiac hypertrophy mouse model | Ang-II-induced pressure overload in rats reduced the expression of miR-19a and miR-19b, increasing the expression of PDE5A, generating LVH. | PDE5A | [55] |
| ↑ miR-21 | ALDO/SALT Hypertensives Mice | This study showed that miR-21 is upregulated by excess ALDO in the LV. | Spry1, Spry2, PTEN, PDCD4, Bcl2 | [56] |
| Clinical Studies | ||||
| ↑ miR-92a | Hypertensive patients (n = 240) | Plasma levels of miR-92a are increased in hypertensive patients and correlate with 24 h mean systolic and diastolic blood pressure, 24 h mean pulse pressure, 24 h daytime and nighttime pulse pressure, increased carotid intima–media thickness and carotid-femoral pulse wave velocity. | KLF2, KLF4, eNOS | [57] |
| ↑ miR-1, miR-208b, miR-499, miR-21 ↓ miR-133a, miR-26b | Hypertensive patients (n = 132) | Analysis of expression in PBMCs: miR-1, miR-133a, miR-26b, miR-208b, miR-499, and miR-21 show distinct expression profiles in hypertensive patients compared to healthy subjects; association with LVH. | MEF2a, BMPR2, PDCD4, PTEN | [58] |
| ↑ miR-505 | Hypertensive patients (n = 192) | Plasma levels of miR-505 are increased in hypertensive patients compared to healthy subjects and is positively correlated with systolic blood pressure; impaired endothelial migration and tube formation in culture by direct regulation of FGF18 and indirect regulation of HMGB1. | FGF18 | [59] |
| ↓ miR-133a | Hypertensive patients (n = 90) | Increased renal sympathetic nervous system induces downregulation of miR-133a. | PRR | [60] |
| ↑ miR-202 | Hypertensive patients (n = 182) | miR-202-3p exerts a protective role against EH by antagonizing the induction of sST2 by Ang-II. | ST2 | [35] |
| ↑ miR-29a, miR-29b, miR-29c | Hypertensive patients (n = 84) | Plasma levels of mir-29a, b and c were increased in patients with hypertension, with positive correlations with office systolic and diastolic blood pressure, office pulse pressure, 24 h mean systolic and diastolic blood pressure, 24 h mean pulse pressure and left ventricular hypertrophy. | COL1A1, COL1A2, COL3A1, VEGFA, TGF-β | [61] |
| ↓ miR-136 | Hypertensive patients (n = 110) | miR-136 is downregulated in patients with hypertension and is associated with elevated levels of RAAS biochemical markers. | Wnt, Notch3 | [34] |
| ↑ miR-516b, miR-600, miR-605, miR-623, let-7e ↓ miR-18b, miR-30d, miR-296-5p, miR-324-3p, miR-486-5p, miR-518b, miR-1236, miR-1227 | Hypertensive patients (n = 194) | Plasma levels of miRNAs were distinct plasma miRNA expression pattern in hypertensive patients, compared with healthy subjects. | MAPK10, RICTOR, NFAT5, MAP3K9, MAP3K1, STAT3 | [62] |
| ↑ miR-132, miR-212 | Hypertensive patients (n = 64) | miR-132/212 are increased in the arteries of hypertensive patients. | PTEN, ERK/MAPK | [30] |
| ↓ miR-126 | Hypertensive patients (n = 89) | Hypertensive patients showed significantly lower miR-126 expression levels in PBMCs, and positive correlation with 24 h mean pulse pressure. | SPRED-1, VEGF, PI3KR2 | [63] |
| ↓ miR-155 | Hypertensive patients (n = 64) | AT1R protein expression was positively correlated with systolic and diastolic blood pressure and negatively correlated with miR-155 expression level in PBMCs. | AT1R | [38] |
| ↑ miR-21, miR-126, miR-196a, miR-451 ↓ miR-181a, miR-638, miR-663 | Hypertensive patients (n = 14) |
MiR-663 can regulate REN and APOE mRNA levels, whereas miR-181a regulated REN and AIFM1 mRNA. | REN, APOE, AIFM1 | [36] |
| ↑ miR-29, miR-30a ↓ miR-133 | Hypertensive patients | MiRNAs are dysregulated in the plasma of hypertensive patients, associated with cardiomyocyte hypertrophy. | TGF-β1, Sp-1 | [64] |
| ↓ miR-25 | Hypertensive heart disease patients | miR-25 is downregulated in the serum of hypertensive patients, elevating the renin expression, promoting RAAS activation. | REN | [37] |
| ↑ miR-155 | Hypertensive patients | MiR-155 is overexpressed and associated with inflammatory markers. | TGF-β1 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Improta-Caria, A.C.; Aras, M.G.; Nascimento, L.; De Sousa, R.A.L.; Aras-Júnior, R.; Souza, B.S.d.F. MicroRNAs Regulating Renin–Angiotensin–Aldosterone System, Sympathetic Nervous System and Left Ventricular Hypertrophy in Systemic Arterial Hypertension. Biomolecules 2021, 11, 1771. https://doi.org/10.3390/biom11121771
Improta-Caria AC, Aras MG, Nascimento L, De Sousa RAL, Aras-Júnior R, Souza BSdF. MicroRNAs Regulating Renin–Angiotensin–Aldosterone System, Sympathetic Nervous System and Left Ventricular Hypertrophy in Systemic Arterial Hypertension. Biomolecules. 2021; 11(12):1771. https://doi.org/10.3390/biom11121771
Chicago/Turabian StyleImprota-Caria, Alex Cleber, Marcela Gordilho Aras, Luca Nascimento, Ricardo Augusto Leoni De Sousa, Roque Aras-Júnior, and Bruno Solano de Freitas Souza. 2021. "MicroRNAs Regulating Renin–Angiotensin–Aldosterone System, Sympathetic Nervous System and Left Ventricular Hypertrophy in Systemic Arterial Hypertension" Biomolecules 11, no. 12: 1771. https://doi.org/10.3390/biom11121771
APA StyleImprota-Caria, A. C., Aras, M. G., Nascimento, L., De Sousa, R. A. L., Aras-Júnior, R., & Souza, B. S. d. F. (2021). MicroRNAs Regulating Renin–Angiotensin–Aldosterone System, Sympathetic Nervous System and Left Ventricular Hypertrophy in Systemic Arterial Hypertension. Biomolecules, 11(12), 1771. https://doi.org/10.3390/biom11121771







