Integration and Spatial Organization of Signaling by G Protein-Coupled Receptor Homo- and Heterodimers
Abstract
:1. Introduction
2. The GPCR Signaling ‘Hardware’
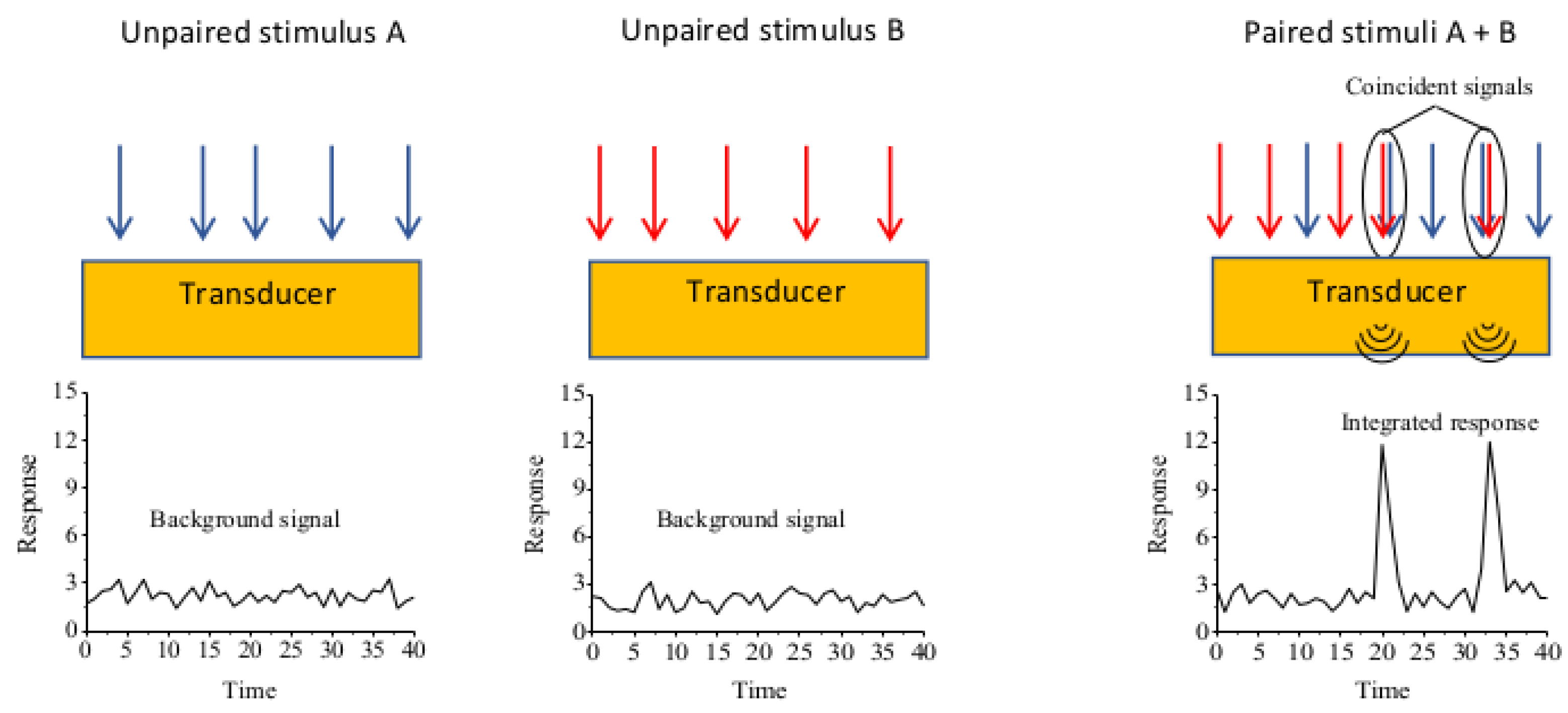
3. Integration and Discrimination of Signaling in Cell Biology
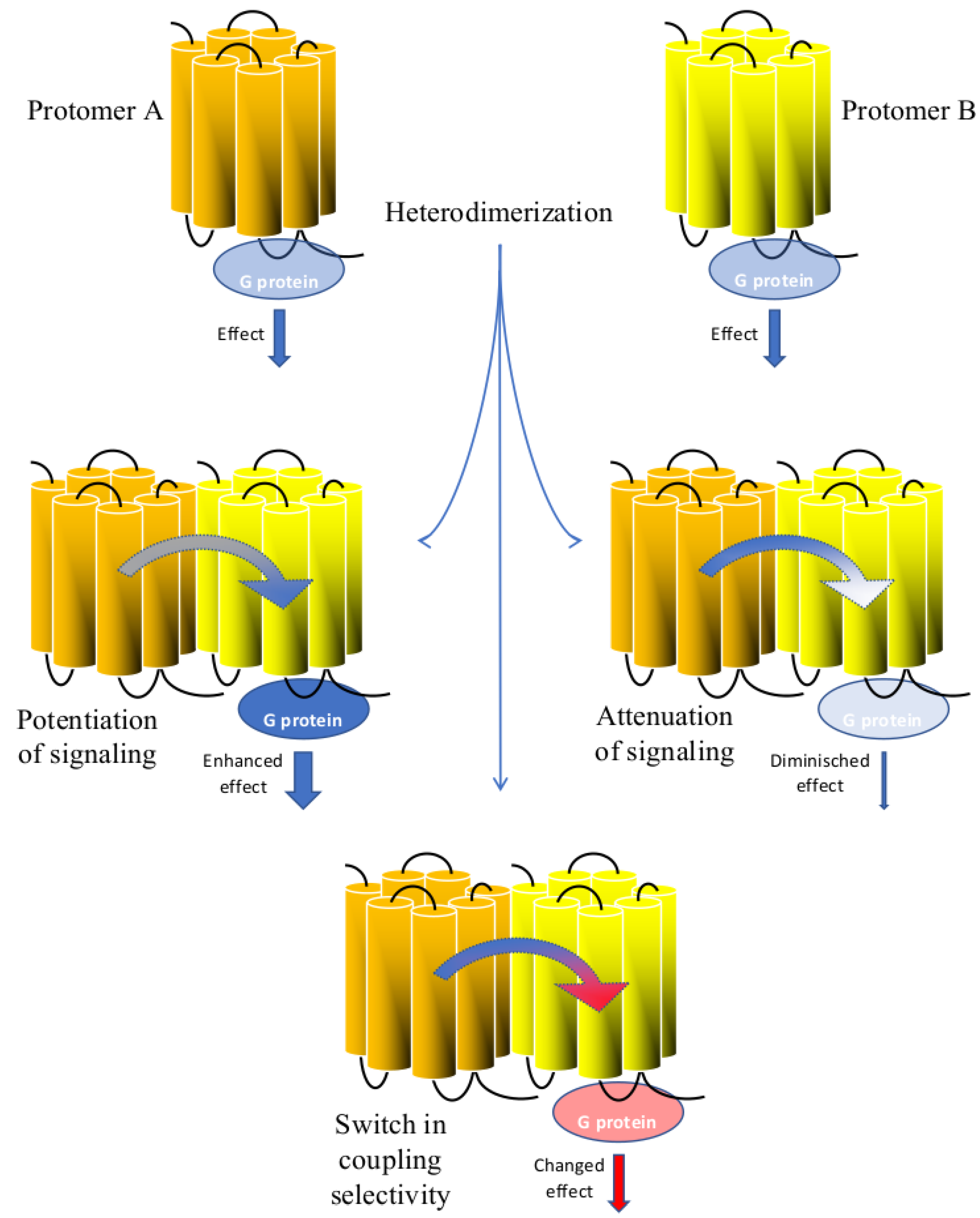
4. Dimerization of GPCRs
5. Signaling Potentiation by GPCR Heterodimers
6. Signaling Attenuation by GPCR Heterodimers
7. Switch in Coupling Selectivity by GPCR Heterodimers
8. Cross Talk between GPCRs and Ionotropic or Tyrosine Kinase Receptors
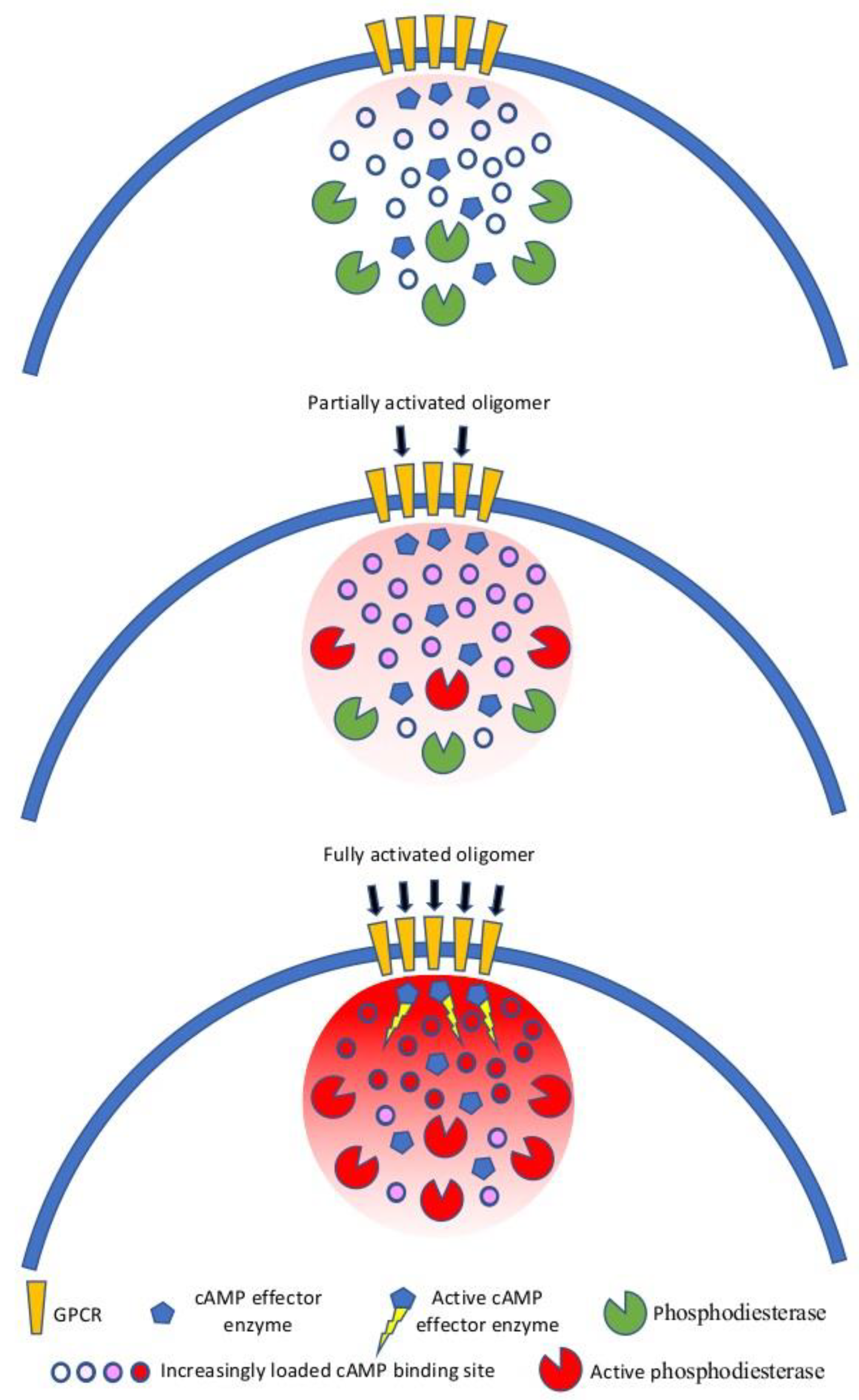
9. GPCR Oligomerization and Spatial Compartmentalization of Signaling
10. Novel Approaches to Study Integration of Signaling by GPCR Homo- and Heterodimers
11. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prazeres, D.M.F.; Martins, S.A.M. G protein-coupled receptors: An overview of signaling mechanisms and screening assays. Methods Mol. Biol. 2015, 1272, 3–19. [Google Scholar] [PubMed]
- Ahtee, L.; Tuominen, R.K. Channel-linked receptors--from subunits to novel drug targets. Trends Pharmacol. Sci. 1999, 20, 391–393. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Strotmann, R.; Schröck, K.; Böselt, I.; Stäubert, C.; Russ, A.; Schöneberg, T. Evolution of GPCR: Change and continuity. Mol. Cell Endocrinol. 2011, 331, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [Green Version]
- Takesono, A.; Cismowski, M.J.; Ribas, C.; Bernard, M.; Chung, P.; Hazard, S., 3rd; Duzic, E.; Lanier, S.M. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J. Biol. Chem. 1999, 274, 33202–33205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumer, J.B.; Smrcka, A.V.; Lanier, S.M. Mechanistic pathways and biological roles for receptor-independent activators of G-protein signaling. Pharmacol. Ther. 2007, 113, 488–506. [Google Scholar] [CrossRef] [Green Version]
- Heuss, C.; Gerber, U. G-protein-independent signaling by G-protein-coupled receptors. Trends Neurosci. 2000, 23, 469–475. [Google Scholar] [CrossRef]
- Sokolina, K.; Kittanakom, S.; Snider, J.; Kotlyar, M.; Maurice, P.; Gandía, J.; Benleulmi-Chaachoua, A.; Tadagaki, K.; Oishi, A.; Wong, V.; et al. Systematic protein-protein interaction mapping for clinically relevant human GPCRs. Mol. Syst. Biol. 2017, 13, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenakin, T. The evolution of receptors: From on–off switches to microprocessors. In GPCR Molecular Pharmacology and Drug Targeting: Shifting Paradigms and New Directions; Gilchrist, A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–26. [Google Scholar]
- Sadana, R.; Dessauer, C.W. Physiological roles for G protein-regulated adenylyl cyclase isoforms: Insights from knockout and overexpression studies. Neurosignals 2009, 17, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Cockcroft, S. Phospholipase C families: Common themes and versatility in physiology and pathology. Prog. Lipid Res. 2020, 80, 101065. [Google Scholar] [CrossRef]
- Ferré, S. The GPCR heterotetramer: Challenging classical pharmacology. Trends Pharmacol. Sci. 2015, 36, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Mons, N.; Guillow, J.L.; Jaffard, R. The role of Ca2+/calmodulin–stimulated adenylyl cyclase as molecular coincidence detectors in memory formation. Cell. Mol. Life Sci. 1999, 55, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Abrams, T.W.; Karl, K.A.; Kandel, E.R. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: Dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J. Neurosci. 1991, 11, 2655–2666. [Google Scholar] [CrossRef]
- Schacher, S.; Castellucci, V.F.; Kandel, E.R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science 1988, 240, 1667–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werry, T.D.; Wilkinson, G.F.; Willars, G.B. Cross talk between P2Y2 nucleotide receptors and CXC chemokine receptor 2 resulting in enhanced Ca2+ signaling involves enhancement of phospholipase C activity and is enabled by incremental Ca2+ release in human embryonic kidney cells. J. Pharmacol. Exp. Ther. 2003, 307, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Selbie, L.A.; Hill, S.J. G protein-coupled receptor cross-talk: The fine-tuning of multiple receptor-signaling pathways. Trends Pharmacol. Sci. 1998, 19, 87–93. [Google Scholar] [CrossRef]
- Toms, N.J.; Roberts, P.J. Group 1 mGlu receptors elevate [Ca2+]i in rat cultured cortical type 2 astrocytes: [Ca2+]i synergy with adenosine A1 receptors. Neuropharmacology 1999, 38, 1511–1517. [Google Scholar] [CrossRef]
- Bock, A.; Annibale, P.; Konrad, C.; Hannawacker, A.; Anton, S.E.; Maiellaro, I.; Zabel, U.; Sivaramakrishnan, S.; Falcke, M.; Lohse, M.J. Optical Mapping of cAMP Signaling at the Nanometer Scale. Cell 2020, 182, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Cordomí, A.; Casadó-Anguera, V.; Moreno, E.; Cai, N.S.; Cortés, A.; Canela, E.I.; Dessauer, C.W.; Casadó, V.; Pardo, L.; et al. Evidence for functional pre-coupled complexes of receptor heteromers and adenylyl cyclase. Nat. Commun. 2018, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Scott, J.D. AKAP signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004, 5, 959–970. [Google Scholar] [CrossRef]
- Dessauer, C.W. Adenylyl cyclase–A-kinase anchoring protein complexes: The next dimension in cAMP signaling. Mol. Pharmacol. 2009, 76, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Bourque, K.; Jones-Tabah, J.; Devost, D.; Clarke, P.B.S.; Hébert, T.E. Exploring functional consequences of GPCR oligomerization requires a different lens. Prog. Mol. Biol. Transl. Sci. 2020, 169, 181–211. [Google Scholar]
- Limbird, L.E.; Meyts, P.D.; Lefkowitz, R.J. Beta-adrenergic receptors: Evidence for negative cooperativity. Biochem. Biophys. Res. Commun. 1975, 64, 1160–1168. [Google Scholar] [CrossRef]
- Maggio, R.; Vogel, Z.; Wess, J. Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular “cross-talk” between G-protein-linked receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 3103–3107. [Google Scholar] [CrossRef] [Green Version]
- Angers, S.; Salahpour, A.; Joly, E.; Hilairet, S.; Chelsky, D.; Dennis, M.; Bouvier, M. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc. Natl. Acad. Sci. USA 2000, 97, 3684–3689. [Google Scholar] [CrossRef]
- Albizu, L.; Cottet, M.; Kralikova, M.; Stoev, S.; Seyer, R.; Brabet, I.; Roux, T.; Bazin, H.; Bourrier, E.; Lamarque, L.; et al. Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 2010, 6, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, K.C.; Fanelli, F.; Huhtaniemi, I.T.; Hanyaloglu, A.C. Single molecule analysis of functionally asymmetric G protein-coupled receptor (GPCR) oligomers reveals diverse spatial and structural assemblies. J. Biol. Chem. 2015, 290, 3875–3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calebiro, D.; Rieken, F.; Wagner, J.; Sungkaworn, T.; Zabel, U.; Borzi, A.; Cocucci, E.; Zürn, A.; Lohse, M.J. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. USA 2013, 110, 743–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarselli, M.; Annibale, P.; McCormick, P.J.; Kolachalam, S.; Aringhieri, S.; Radenovic, A.; Corsini, G.U.; Maggio, R. Revealing G-protein-coupled receptor oligomerization at the single-molecule level through a nanoscopic lens: Methods, dynamics and biological function. FEBS J. 2016, 283, 1197–1217. [Google Scholar] [CrossRef] [PubMed]
- Kasai, R.S.; Suzuki, K.G.N.; Prossnitz, E.R.; Koyama-Honda, I.; Nakada, C.; Fujiwara, T.K.; Kusumi, A. Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J. Cell Biol. 2011, 192, 463–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, J.; Isbilir, A.; Sungkaworn, T.; Osberg, B.; Karathanasis, C.; Sunkara, V.; Grushevskyi, E.O.; Bock, A.; Annibale, P.; Heilemann, M.; et al. Single-molecule analysis reveals agonist-specific dimer formation of µ-opioid receptors. Nat. Chem. Biol. 2020, 16, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Bayburt, T.H.; Leitz, A.J.; Xie, G.; Oprian, D.D.; Sligar, S.G. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 2007, 282, 14875–14881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, O.P.; Gramse, V.; Kolbe, M.; Hofmann, K.P.; Heck, M. Monomeric G protein-coupled receptor rhodopsin in solution activates its G protein transducin at the diffusion limit. Proc. Natl. Acad. Sci. USA 2007, 104, 10859–10864. [Google Scholar] [CrossRef] [Green Version]
- White, J.F.; Grodnitzky, J.; Louis, J.M.; Trinh, L.B.; Shiloach, J.; Gutierrez, J.; Northup, J.K.; Grisshammer, R. Dimerization of the class A G protein-couple d neurotensin receptor NTS1 alters G protein interaction. Proc. Natl. Acad. Sci. USA 2007, 104, 12199–12204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whorton, M.R.; Bokoch, M.P.; Rasmussen, S.G.F.; Huang, B.; Zare, R.N.; Kobilka, B.; Sunahara, R.K. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. USA 2007, 104, 7682–7687. [Google Scholar] [CrossRef] [Green Version]
- Milligan, G.; Ward, R.J.; Marsango, S. GPCR homo-oligomerization. Curr. Opin. Cell Biol. 2019, 57, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Maggio, R.; Novi, F.; Scarselli, M.; Corsini, G.U. The impact of G-protein-coupled receptor hetero-oligomerization on function and pharmacology. FEBS J. 2005, 272, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Gahbauer, S.; Böckmann, R.A. Membrane-Mediated Oligomerization of G Protein Coupled Receptors and Its Implications for GPCR Function. Front. Physiol. 2016, 7, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, R.; Innamorati, G.; Parenti, M. G protein-coupled receptor oligomerization provides the framework for signal discrimination. J. Neurochem. 2007, 103, 1741–1752. [Google Scholar] [CrossRef]
- Jordan, B.A.; Devi, L.A. G protein-coupled receptor heterodimerization modulates receptor function. Nature 1999, 399, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Israilova, M.; Tanaka, T.; Suzuki, F.; Morishima, S.; Muramatsu, I. Pharmacological characterization and cross talk of alpha1a- and alpha1b-adrenoceptors coexpressed in human embryonic kidney 293 cells. J. Pharmacol. Exp. Ther. 2004, 309, 259–266. [Google Scholar] [CrossRef]
- Hornigold, D.C.; Mistry, R.; Raymond, P.D.; Blank, J.L.; Challiss, J.R.A. Evidence for cross-talk between M2 and M3 muscarinic acetylcholine receptors in the regulation of second messenger and extracellular signal-regulated kinase signaling pathways in Chinese hamster ovary cells. Br. J. Pharmacol. 2003, 138, 1340–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novi, F.; Scarselli, M.; Corsini, G.U.; Maggio, R. The paired activation of the two components of the muscarinic M3 receptor dimer is required for induction of ERK1/2 phosphorylation. J. Biol. Chem. 2004, 297, 7476–7486. [Google Scholar] [CrossRef] [Green Version]
- Maggio, R.; Scarselli, M.; Novi, F.; Millan, M.J.; Corsini, G.U. Potent activation of dopamine D3/D2 heterodimers by the antiparkinsonian agents, S32504, pramipexole and ropinirole. J. Neurochem. 2003, 87, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Ferré, S.; Karcz-Kubicha, M.; Hope, B.T.; Popoli, P.; Burgueño, J.; Gutiérrez, M.A.; Casadó, V.; Fuxe, K.; Goldberg, S.R.; Lluis, C.; et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: Implications for striatal neuronal function. Proc. Natl. Acad. Sci. USA 2002, 99, 11940–11945. [Google Scholar] [CrossRef] [Green Version]
- Abadir, P.M.; Periasamy, A.; Carey, R.M.; Siragy, H.M. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension 2006, 48, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Yeo, A.; Samways, D.S.; Fowler, C.E.; Gunn-Moore, F.; Henderson, G. Coincident signalling between the Gi/Go-coupled delta-opioid receptor and the Gq-coupled m3 muscarinic receptor at the level of intracellular free calcium in SH-SY5Y cells. J. Neurochem. 2001, 76, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.; Gomes, I.; Devi, L.A. μ opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006, 148, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Vilardaga, J.P.; Nikolaev, V.O.; Lorenz, K.; Ferrandon, S.; Zhuang, Z.; Lohse, M.J. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat. Chem. Biol. 2008, 4, 126–131. [Google Scholar] [CrossRef] [PubMed]
- George, S.R.; Fan, T.; Xie, Z.; Tse, R.; Tam, V.; Varghese, G.; O’Dowd, B.F. Oligomerization of μ- and δ-opioid receptors. Generation of novel functional properties. J. Biol. Chem. 2000, 275, 26128–26135. [Google Scholar] [CrossRef] [Green Version]
- Mellado, M.; Rodriguez-Frade, J.M.; Vila-Coro, A.J.; Fernandez, S.; Martin de Ana, A.; Jones, D.R.; Toran, J.L.; Martinez, A.C. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001, 20, 2497–2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.P.; So, C.H.; Rashid, A.J.; Varghese, G.; Cheng, R.; Lanca, A.J.; O’Dowd, B.F.; George, S.R. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 2004, 279, 35671–35678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearn, C.S.; Blake-Palmer, K.; Daniel, E.; Mackie, K.; Glass, M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 2005, 67, 1697–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breit, A.; Gagnidze, K.; Devi, L.A.; Lagacé, M.; Bouvier, M. Simultaneous activation of the delta opioid receptor (deltaOR)/sensory neuron-specific receptor-4 (SNSR-4) hetero-oligomer by the mixed bivalent agonist bovine adrenal medulla peptide 22 activates SNSR-4 but inhibits deltaOR signaling. Mol. Pharmacol. 2006, 70, 686–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Le Trong, I.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baneres, J.L.; Parello, J. Structure-based analysis of GPCR function: Evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J. Mol. Biol. 2003, 329, 815–829. [Google Scholar] [CrossRef]
- Chinault, S.L.; Overton, M.C.; Blumer, K.J. Subunits of a yeast oligomeric G protein-coupled receptor are activated independently by agonist but function in concert to activate G protein heterotrimers. J. Biol. Chem. 2004, 279, 16091–16100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrick-Davis, K.; Grinde, E.; Harrigan, T.J.; Mazurkiewicz, J.E. Inhibition of serotonin 5-HT2C receptor function through heterodimerization: Receptor dimers bind two molecules of ligand and one G-protein. J. Biol. Chem. 2005, 280, 40144–40151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhang, Z.; Moreno-Delgado, D.; Dalton, J.A.; Rovira, X.; Trapero, A.; Goudet, C.; Llebaria, A.; Giraldo, J.; Yuan, Q.; et al. Allosteric control of an asymmetric transduction in a G protein-coupled receptor heterodimer. Elife 2017, 6, e26985. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Levitz, J. Glutametergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Liberto, V.; Mudò, G.; Belluardo, N. Crosstalk between receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCR) in the brain: Focus on heteroreceptor complexes and related functional neurotrophic effects. Neuropharmacology 2019, 152, 67–77. [Google Scholar] [CrossRef]
- Perroy, J.; Raynaud, F.; Homburger, V.; Rousset, M.C.; Telley, L.; Bockaert, J.; Fagni, L. Direct interaction enables cross-talk between ionotropic and group I metabotropic glutamate receptors. J. Biol. Chem. 2008, 283, 6799–6805. [Google Scholar] [CrossRef] [Green Version]
- Moutin, E.; Raynaud, F.; Roger, J.; Pellegrino, E.; Homburger, V.; Bertaso, F.; Ollendorff, V.; Bockaert, J.; Fagni, L.; Perroy, J. Dynamic remodeling of scaffold interactions in dendritic spines controls synaptic excitability. J. Cell Biol. 2012, 198, 251–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bontempi, L.; Savoia, P.; Bono, F.; Fiorentini, C.; Missale, C. Dopamine D3 and acetylcholine nicotinic receptor heteromerization in midbrain dopamine neurons: Relevance for neuroplasticity. Eur. Neuropsychopharmacol. 2017, 27, 313–324. [Google Scholar] [CrossRef]
- Matera, C.; Bono, F.; Pelucchi, S.; Collo, G.; Bontempi, L.; Gotti, C.; Zoli, M.; De Amici, M.; Missale, C.; Fiorentini, C.; et al. The novel hybrid agonist HyNDA-1 targets the D3R-nAChR heteromeric complex in dopaminergic neurons. Biochem. Pharmacol. 2019, 163, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Daub, H.; Weiss, F.U.; Wallasch, C.; Ullrich, A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996, 379, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, L.E.; Hill, S.J. Transactivation of G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs): Recent insights using luminescence and fluorescence technologies. Curr. Opin. Endocr. Metab. Res. 2021, 16, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.M.; Liu, Z.; Meier, K.E. Positive and negative cross-talk between lysophosphatidic acid receptor 1, free fatty acid receptor 4, and epidermal growth factor receptor in human prostate cancer cells. J. Pharmacol. Exp. Therapeut. 2016, 359, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Benito, S.; Moreno, E.; Seijo-Vila, M.; Tundidor, I.; Andradas, C.; Caffarel, M.M.; Caro-Villalobos, M.; Urigüen, L.; Diez-Alarcia, R.; Moreno-Bueno, G.; et al. Therapeutic targeting of HER2-CB2R heteromers in HER2-positive breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3863–3872. [Google Scholar] [CrossRef] [Green Version]
- Flajolet, M.; Wang, Z.; Futter, M.; Shen, W.; Nuangchamnong, N.; Bendor, J.; Wallach, I.; Nairn, A.C.; Surmeier, D.J.; Greengard, P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat. Neurosci. 2008, 11, 1402–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarselli, M.; Annibale, P.; Radenovic, A. Cell type-specific β2-adrenergic receptor clusters identified using photoactivated localization microscopy are not lipid raft related, but depend on actin cytoskeleton integrity. J. Biol. Chem. 2012, 287, 16768–16780. [Google Scholar] [CrossRef] [Green Version]
- Marsango, S.; Caltabiano, G.; Jiménez-Rosés, M.; Millan, M.J.; Pediani, J.D.; Ward, R.J.; Milligan, G. A molecular basis for selective antagonist destabilization of dopamine D3 receptor quaternary organization. Sci. Rep. 2017, 7, 1–17. [Google Scholar]
- Ward, R.J.; Pediani, J.D.; Marsango, S.; Jolly, R.; Stoneman, M.R.; Biener, G.; Handel, T.M.; Raicu, V.; Milligan, G. Chemokine receptor CXCR4 oligomerization is disrupted selectively by the antagonist ligand IT1t. J. Biol. Chem. 2021, 296, 100139. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.D.; Bort, E.T.; Grose, R.P.; McCormick, P.J.; Simoncelli, S. Quantitative super-resolution imaging for the analysis of GPCR oligomerization. Biomolecules 2021, 11, 1503. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Lisanti, M.P. The caveolin proteins. Genome Biol. 2004, 5, 214. [Google Scholar] [CrossRef] [Green Version]
- Stern, C.M.; Mermelstein, P.G. Caveolin regulation of neuronal intracellular signaling. Cell Mol. Life Sci. 2010, 67, 3785–3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Root, K.T.; Julien, J.A.; Glover, K.J. Secondary structure of caveolins: A mini review. Biochem. Soc. Trans. 2019, 47, 1489–1498. [Google Scholar] [CrossRef]
- Liu, P.; Rudick, M.; Anderson, R.G.W. Multiple functions of caveolin-1. J. Biol. Chem. 2002, 277, 41295–41298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirama, T.; Das, R. Quantitative Image Analysis of the Spatial Organization and Mobility of Caveolin Aggregates at the Plasma Membrane. Methods Mol. Biol. 2020, 2169, 53–62. [Google Scholar] [PubMed]
- Li, S.; Couet, J.; Lisanti, M.P. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. J. Biol. Chem. 1996, 271, 29182–29190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razani, B.; Rubin, C.S.; Michael, P.; Lisanti, M.P. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J. Biol. Chem. 1999, 274, 26353–26360. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.G. Caveolae: Where incoming and outgoing messengers meet. Proc. Natl. Acad. Sci. USA 1993, 90, 10909–10913. [Google Scholar] [CrossRef] [Green Version]
- Smart, E.J.; Graf, G.A.; McNiven, M.A.; Sessa, W.C.; Engelman, J.A.; Scherer, P.E.; Okamoto, T.; Lisanti, M.P. Caveolins liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 1999, 19, 7289–7304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insel, P.A.; Head, B.P.; Ostrom, R.S.; Patel, H.H.; Swaney, J.S.; Tang, C.M.; Roth, D.M. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann. N. Y. Acad. Sci. 2005, 1047, 166–172. [Google Scholar] [CrossRef]
- Schwencke, C.; Okumura, S.; Yamamoto, M.; Geng, Y.J.; Ishikawa, Y. Colocalization of beta-adrenergic receptors and caveolin within the plasma membrane. J. Cell. Biochem. 1999, 75, 64–72. [Google Scholar] [CrossRef]
- Schwencke, C.; Yamamoto, M.; Okumura, S.; Toya, Y.; Kim, S.J.; Ishikawa, Y. Compartmentation of cyclic adenosine 3′,5′-monophosphate signaling in caveolae. Mol. Endocrinol. 1999, 13, 1061–1070. [Google Scholar] [PubMed] [Green Version]
- Ostrom, R.S.; Violin, J.D.; Coleman, S.; Insel, P.A. Selective enhancement of beta-adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: Colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol. Pharmacol. 2000, 57, 1075–1079. [Google Scholar] [PubMed]
- Chakrabarti, S.; Chang, A.; Liu, N.J.; Gintzler, A.R. Chronic opioid treatment augments caveolin-1 scaffolding: Relevance to stimulatory mu-opioid receptor adenylyl cyclase signaling. J. Neurochem. 2016, 139, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemal, H.; Patel, F.; Murray, P.A. Insel Caveolae as Organizers of Pharmacologically Relevant Signal Transduction Molecules. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 359–391. [Google Scholar]
- Kauk, M.; Hoffmann, C. Intramolecular and Intermolecular FRET Sensors for GPCRs—Monitoring Conformational Changes and Beyond. Trends Pharmacol. Sci. 2018, 39, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hlavackova, V.; Zabel, U.; Frankova, D.; Bätz, J.; Hoffmann, C.; Prezeau, L.; Pin, J.P.; Blahos, J.; Lohse, M.J. Sequential inter- and intrasubunit rearrangements during activation of dimeric metabotropic glutamate receptor 1. Sci. Signal. 2012, 5, ra59. [Google Scholar] [CrossRef] [PubMed]
- Sleno, R.; Devost, D.; Pétrin, D.; Zhang, A.; Bourque, K.; Shinjo, Y.; Aoki, J.; Inoue, A.; Hébert, T.E. Conformational biosensors reveal allosteric interactions between heterodimeric AT1 angiotensin and prostaglandin F2α receptors. J. Biol. Chem. 2017, 292, 12139–12152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Meng, J.; Xu, C.; Liu, J. Multiple GPCR functional assays based on resonance energy transfer sensors. Front. Cell Dev. Biol. 2021, 9, 611443. [Google Scholar] [CrossRef]
- Erdmann, R.S.; Baguley, S.W.; Richens, J.H.; Wissner, R.F.; Xi, Z.; Allgeyer, E.S.; Zhong, S.; Thompson, A.D.; Lowe, N.; Butler, R.; et al. Labeling Strategies Matter for Super-Resolution Microscopy: A Comparison between HaloTags and SNAP-tags. Cell Chem. Biol. 2019, 26, 584–592.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, W.B.; Geggier, P.; Holsey, M.D.; Gilmore, G.T.; Pati, A.K.; Meszaros, J.; Terry, D.S.; Mathiasen, S.; Kaliszewski, M.J.; McCauley, M.D.; et al. Single-molecule FRET imaging of GPCR dimers in living cells. Nat. Methods 2021, 18, 397–405. [Google Scholar] [CrossRef]
- Stumpf, A.D.; Hoffmann, C. Optical probes based on G protein-coupled receptors—Added work or added value? Br. J. Pharmacol. 2016, 173, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zürn, A.; Klenk, C.; Zabel, U.; Reiner, S.; Lohse, M.J.; Hoffmann, C. Site-specific, orthogonal labeling of proteins in intact cells with two small biarsenical fluorophores. Bioconjug. Chem. 2010, 21, 853–859. [Google Scholar] [CrossRef]
- Rasmussen, S.G.; DeVree, B.T.; Zou, Y.; Kruse, A.C.; Chung, K.Y.; Kobilka, T.S.; Thian, F.S.; Chae, P.S.; Pardon, E.; Calinski, D.; et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 2011, 477, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haga, K.; Kruse, A.C.; Asada, H.; Yurugi-Kobayashi, T.; Shiroishi, M.; Zhang, C.; Weis, W.I.; Okada, T.; Kobilka, B.K.; Haga, T.; et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 2012, 482, 547–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, A.C.; Weiss, D.R.; Rossi, M.; Hu, J.; Hu, K.; Eitel, K.; Gmeiner, P.; Wess, J.; Kobilka, B.K.; Shoichet, B.K. Muscarinic receptors as model targets and antitargets for structure-based ligand discovery. Mol. Pharmacol. 2013, 84, 528–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Scheerer, P.; Hofmann, K.P.; Choe, H.W.; Ernst, O.P. Crystal structure of the ligand-free Gprotein-coupled receptor opsin. Nature 2008, 454, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Chien, E.Y.; Mol, C.D.; Fenalti, G.; Liu, W.; Katritch, V.; Abagyan, R.; Brooun, A.; Wells, P.; Bi, F.C.; et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 2010, 330, 1066–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, H.W.; Kim, Y.J.; Park, J.H.; Morizumi, T.; Pai, E.F.; Krauss, N.; Hofmann, K.P.; Scheerer, P.; Ernst, O.P. Crystal structure of metarhodopsin II. Nature 2011, 471, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.I.; Kobilka, B.K.; Granier, S. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 2012, 485, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.Y.; Mezei, M.; Cui, M. Computational approaches for modeling GPCR dimerization. Curr. Pharm. Biotechnol. 2014, 15, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carli, M.; Kolachalam, S.; Aringhieri, S.; Rossi, M.; Giovannini, L.; Maggio, R.; Scarselli, M. Dopamine D2 Receptors Dimers: How can we Pharmacologically Target Them? Curr. Neuropharmacol. 2018, 16, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Fasciani, I.; Marampon, F.; Maggio, R.; Scarselli, M. The First Negative Allosteric Modulator for Dopamine D(2) and D(3) Receptors, SB269652 May Lead to a New Generation of Antipsychotic Drugs. Mol. Pharmacol. 2017, 91, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, R.; Fasciani, I.; Carli, M.; Petragnano, F.; Marampon, F.; Rossi, M.; Scarselli, M. Integration and Spatial Organization of Signaling by G Protein-Coupled Receptor Homo- and Heterodimers. Biomolecules 2021, 11, 1828. https://doi.org/10.3390/biom11121828
Maggio R, Fasciani I, Carli M, Petragnano F, Marampon F, Rossi M, Scarselli M. Integration and Spatial Organization of Signaling by G Protein-Coupled Receptor Homo- and Heterodimers. Biomolecules. 2021; 11(12):1828. https://doi.org/10.3390/biom11121828
Chicago/Turabian StyleMaggio, Roberto, Irene Fasciani, Marco Carli, Francesco Petragnano, Francesco Marampon, Mario Rossi, and Marco Scarselli. 2021. "Integration and Spatial Organization of Signaling by G Protein-Coupled Receptor Homo- and Heterodimers" Biomolecules 11, no. 12: 1828. https://doi.org/10.3390/biom11121828






