Effects of Low-Dose Atorvastatin on the Peripheral Blood Mononuclear Cell Secretion of Angiogenic Factors in Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Protocol and Measurements
2.3. Statistical Analysis
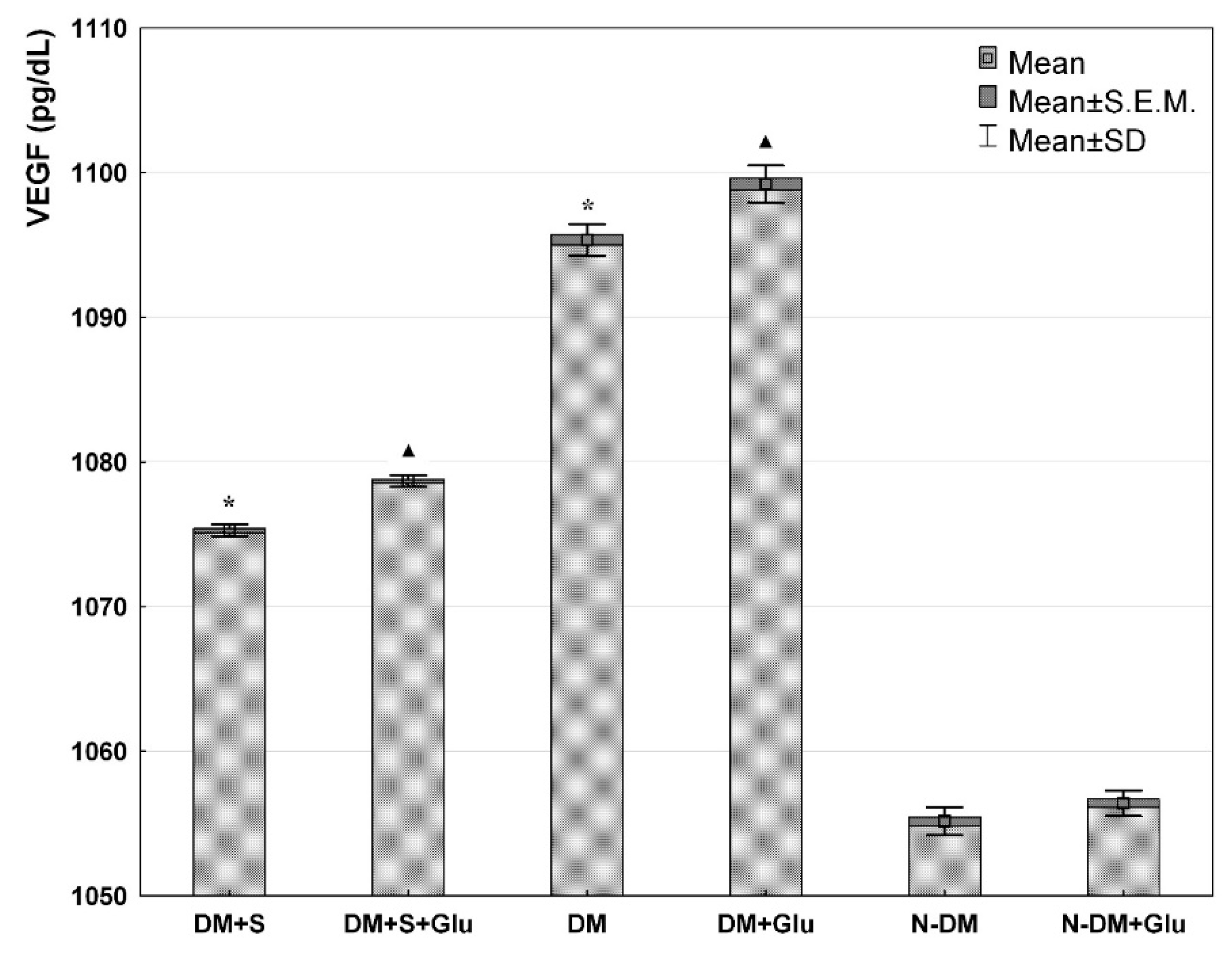
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno, P.R.; Purushothaman, M.; Purushothaman, K.-R. Plaque neovascularization: Defense mechanisms, betrayal, or a war in progress. Ann. N. Y. Acad. Sci. 2012, 1254, 7–17. [Google Scholar] [CrossRef]
- Michel, J.B.; Virmani, R.; Arbustini, E.; Pasterkamp, G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur. Heart J. 2011, 32, 1977–1985, 1985a, 1985b, 1985c. [Google Scholar] [CrossRef] [Green Version]
- Attanasio, S.; Schaer, G. Therapeutic angiogenesis for the management of refractory angina: Current concepts. Cardiovasc. Ther. 2011, 29, e1–e11. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, P.Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil, H.A.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; Fuller, J.H.; et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled tri-al. Lancet 2004, 364, 685–696. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, M.; Liao, Y.H.; Hu, Y.; Wu, M.; Wang, Q.; Qin, B.; Wang, H.; Zhu, Y.; Gao, X.M.; et al. Rosuvastatin enhances angiogenesis via eNOS-dependent mobilization of endothelial progenitor cells. PLoS ONE 2013, 8, e63126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garjani, A.; Rezazadeh, H.; Andalib, S.; Ziaee, M.; Doustar, Y.; Soraya, H.; Garjani, M.; Khorrami, A.; Asadpoor, M.; Maleki-Dizaji, N. Ambivalent effects of atorvastatin on angiogenesis, epidermal cell proliferation and tumorgenesis in animal models. Iran. Biomed. J. 2012, 16, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mück, A.O.; Seeger, H.; Wallwiener, D. Class-specific pro-apoptotic effect of statins on human vascular endothelial cells. Z. Kardiol. 2004, 93, 398–402. [Google Scholar] [CrossRef]
- Urbich, C.; Dernbach, E.; Zeiher, A.M.; Dimmeler, S. Double-edged role of statins in angiogenesis signaling. Circ. Res. 2002, 90, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, M.; Heeschen, C.; Glassford, A.J.; Cooke, J.P. Statins have biphasic effects on angiogenesis. Circulation 2002, 105, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 789–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Fujiyama, S.; Amano, K.; Uehira, K.; Yoshida, M.; Nishiwaki, Y.; Nozawa, Y.; Jin, D.; Takai, S.; Miyazaki, M.; Egashira, K.; et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ. Res. 2003, 93, 980–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Azfer, A.; Zhelyabovska, O.; Fatma, S.; Kolattukudy, P.E. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP). J. Biol. Chem. 2008, 283, 14542–14551. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, J.S.; Mallat, Z.; Duriez, M.; Tamarat, R.; Bureau, M.F.; Scherman, D.; Duverger, N.; Branellec, D.; Tedgui, A.; Levy, B.I. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 2000, 87, 448–552. [Google Scholar]
- Wu, W.K.; Llewellyn, O.P.; Bates, D.O.; Nicholson, L.B.; Dick, A.D. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology 2010, 215, 796–803. [Google Scholar] [CrossRef]
- Voest, E.E.; Kenyon, B.M.; O’Reilly, M.S.; Truitt, G.; D’Amato, R.J.; Folkman, J. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 1995, 87, 581–586. [Google Scholar] [CrossRef]
- Albini, A.; Brigati, C.; Ventura, A.; Lorusso, G.; Pinter, M.; Morini, M.; Mancino, A.; Sica, A.; Noonan, D.M. Angiostatin anti-angiogenesis requires IL-12: The innate immune system as a key target. J. Transl. Med. 2009, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Mazighi, M.; Pellé, A.; Gonzalez, W.; Mtairag, E.M.; Philippe, M.; Hénin, D.; Michel, J.B.; Feldman, L.J. IL-10 inhibits vascular smooth muscle cell activation in vitro and in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H866–H871. [Google Scholar] [CrossRef] [PubMed]
- Holven, K.B.; Halvorsen, B.; Bjerkeli, V.; Damås, J.K.; Retterstøl, K.; Mørkrid, L.; Ose, L.; Aukrust, P.; Nenseter, M.S. Impaired inhibitory effect of interleukin-10 on the balance between matrix metalloproteinase-9 and its inhibitor in mononuclear cells from hyperhomocysteinemic subjects. Stroke 2006, 37, 1731–1736. [Google Scholar] [CrossRef] [Green Version]
- Jaumdally, R.J.; Goon, P.K.; Varma, C.; Blann, A.D.; Lip, G.Y. Effects of atorvastatin on circulating CD34+/CD133+/CD45- progenitor cells and indices of angiogenesis (vascular endothelial growth factor and the angiopoietins 1 and 2) in atherosclerotic vascular disease and diabetes mellitus. J. Intern. Med. 2010, 267, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.P.; Huikuri, H.; et al. Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); Document Reviewers. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovas-cular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2013, 34, 3035–3087. [Google Scholar]
- Dworacka, M.; Winiarska, H.; Szymanska, M.; Kuczynski, S.; Szczawinska, K.; Wierusz-Wysocka, B. 1,5-anhydro-D-glucitol: A novel marker of glucose excursions. Int. J. Clin. Pract. Suppl. 2002, 129, 40–44. [Google Scholar]
- Weykamp, C.; John, W.G.; Mosca, A.; Hoshino, T.; Little, R.; Jeppsson, J.-O.; Goodall, I.; Miedema, K.; Myers, G.; Reinauer, H.; et al. The IFCC Reference Measurement System for HbA1c: A 6-year progress report. Clin. Chem. 2008, 54, 240–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haasdijk, R.A.; Den Dekker, W.K.; Cheng, C.; Tempel, D.; Szulcek, R.; Bos, F.L.; Hermkens, D.M.; Chrifi, I.; Brandt, M.M.; Van Dijk, C.; et al. THSD1 preserves vascular integrity and protects against intraplaque haemorrhaging in ApoE-/- mice. Cardiovasc. Res. 2016, 110, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Nishimoto-Hazuku, A.; Hirase, T.; Ide, N.; Ikeda, Y.; Node, K. Simvastatin stimulates vascular endothelial growth factor production by hypoxia-inducible factor-1alpha upregulation in endothelial cells. J. Cardiovasc. Pharmacol. 2008, 51, 267–273. [Google Scholar] [CrossRef]
- Koyuturk, M.; Ersoz, M.; Altiok, N. Simvastatin induces proliferation inhibition and apoptosis in C6 glioma cells via c-jun N-terminal kinase. Neurosci. Lett. 2004, 370, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Nandy, D.; Mukhopadhyay, D.; Basu, A. Both vascular endothelial growth factor and soluble Flt-1 are increased in type 2 diabetes but not in impaired fasting glucose. J. Investig. Med. 2010, 58, 804–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimering, M.B.; Anderson, R.J.; Ge, L.; Moritz, T.E.; Investigators for the VADT. Increased plasma basic fibroblast growth factor is associated with coronary heart disease in adult type 2 diabetes mellitus. Metabolism 2011, 60, 284–291. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: A malignant transformation. Cardiovasc. Diabetol. 2004, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Awad, O.; Dedkov, E.I.; Jiao, C.; Bloomer, S.; Tomanek, R.J.; Schatteman, G.C. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Praidou, A.; Androudi, S.; Brazitikos, P.; Karakiulakis, G.; Papakonstantinou, E.; Dimitrakos, S. Angiogenic growth factors and their inhibitors in diabetic retinopathy. Curr. Diabetes Rev. 2010, 6, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Khazaei, M. Imbalance of angiogenesis in diabetic complications: The mechanisms. Int. J. Prev. Med. 2012, 3, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Rizk, S.M.; Ibrahim, I.A.; El-Emady, Y.F. Alterations in circulating angiogenic and anti-angiogenic factors in type 2 diabetic patients with neuropathy. Cell Biochem. Funct. 2014, 32, 155–163. [Google Scholar] [CrossRef]
- Dworacka, M.; Krzyżagórska, E.; Wesołowska, A.; Borowska, M.; Iskakova, S.; Dworacki, G. Statins in low doses reduce VEGF and bFGF serum levels in patients with type 2 diabetes mellitus. Pharmacology 2014, 93, 32–38. [Google Scholar] [CrossRef]
- Dworacka, M.; Krzyżagórska, E.; Wesołowska, A.; Zharmakhanova, G.; Iskakova, S.; Dworacki, G. Circulating monocyte chemotactic protein 1 (MCP-1), vascular cell adhesion molecule 1 (VCAM-1) and angiogenin in type 2 diabetic patients treated with statins in low doses. Eur. J. Pharmacol. 2014, 740, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Bláha, V.; Andrýs, C.; Smahelová, A.; Knízek, J.; Hyspler, R.; Solichová, D.; Bláha, M.; Zadák, Z. Effect of atorvastatin on soluble CD14, CD40 Ligand, sE- and sP-selectins and MCP-1 in patients with type 2 diabetes mellitus: Relationship to cholesterol turnover. Pharmacol. Res. 2006, 54, 421–428. [Google Scholar] [CrossRef]
- Wong, B.W.; Wong, D.; Luo, H.; McManus, B.M. Vascular endothelial growth factor-D is overexpressed in human cardiac allograft vasculopathy and diabetic atherosclerosis and induces endothelial permeability to low-density lipoproteins in vitro. J. Heart Lung Transplant. 2011, 30, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Panutsopulos, D.; Zafiropoulos, A.; Krambovitis, E.; Kochiadakis, G.E.; Igoumenidis, N.E.; Spandidos, D.A. Peripheral monocytes from diabetic patients with coronary artery disease display increased bFGF and VEGF mRNA expression. J. Transl. Med. 2003, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.; Hsu, Y.C.; Tseng, C.C.; Wang, F.S.; Lin, C.L.; Wang, J.Y. Simvastatin alleviates diabetes-induced VEGF-mediated nephropathy via the modulation of Ras signaling pathway. Ren. Fail. 2008, 30, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Khatri, J.J.; Johnson, C.; Magid, R.; Lessner, S.M.; Laude, K.M.; Dikalov, S.I.; Harrison, D.G.; Sung, H.J.; Rong, Y.; Galis, Z.S. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 2004, 109, 520–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Ran, Y.; Chen, X.; Li, C.; Cheng, S.; Liu, J. Pleiotropic Effects of Simvastatin on the Regulation of Potassium Channels in Monocytes. Front. Pharmacol. 2020, 11, 101. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Langer, A.; Ur, E.; Calciu, C.D.; Leiter, L.A. Inflammatory biomarkers CRP, MCP-1, serum amyloid alpha and interleukin-18 in patients with HTN and dyslipidemia: Impact of diabetes mellitus on metabolic syndrome and the effect of statin therapy. Hypertens. Res. 2013, 36, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, M.; Diomede, L.; Sironi, M.; Massimiliano, L.; Sottocorno, M.; Polentarutti, N.; Guglielmotti, A.; Albani, D.; Bruno, A.; Fruscella, P.; et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab. Investig. 2000, 80, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Hattori, Y.; Hattori, S.; Sato, N.; Kasai, K. High-glucose-induced nuclear factor kappaB activation in vascular smooth muscle cells. Cardiovasc. Res. 2000, 46, 188–197. [Google Scholar] [CrossRef]
- Graier, W.F.; Posch, K.; Fleischhacker, E.; Wascher, T.C.; Kostner, G.M. Increased superoxide anion formation in endothelial cells during hyperglycemia: An adaptive response or initial step of vascular dysfunction? Diabetes Res. Clin. Pract. 1999, 45, 153–160. [Google Scholar] [CrossRef]
- Ortego, M.; Bustos, C.; Hernández-Presa, M.A.; Tuñón, J.; Díaz, C.; Hernández, G.; Egido, J. Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis 1999, 147, 253–261. [Google Scholar] [CrossRef]
- Diomede, L.; Albani, D.; Sottocorno, M.; Donati, M.B.; Bianchi, M.; Fruscella, P.; Salmona, M. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 39–50. [Google Scholar] [CrossRef]
- Hernández, C.; Francisco, G.; Ciudin, A.; Chacón, P.; Montoro, B.; Llaverias, G.; Blanco-Vaca, F.; Simó, R. Effect of atorvastatin on lipoprotein (a) and interleukin-10: A randomized placebo-controlled trial. Diabetes Metab. 2011, 37, 124–130. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Li, J.; Dong, M.; Yang, J.; An, G.; Qin, W.; Gao, F.; Zhang, C.; Zhang, Y. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol. Med. 2012, 18, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.L.; Quinto, B.M.; Queiroz, K.C.; Iizuka, I.J.; Monte, J.C.; Dalboni, M.A.; Durão, M.S.; Cendoroglo Neto, M.; dos Santos, O.F.; Batista, M.C. Effects of simvastatin on cytokines secretion from mononuclear cells from critically ill patients with acute kidney injury. Cytokine 2011, 54, 144–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naoumova, R.P.; Patel, D.D.; O’Neill, F.H.; Thompson, G.R.; Knight, B.L. Treatment with atorvastatin alters the ratio of interleukin-12/interleukin-10 gene expression. Eur. J. Clin. Investig. 2003, 33, 88–91. [Google Scholar] [CrossRef]
- Kriegel, M.A.; Tretter, T.; Blank, N.; Schiller, M.; Gabler, C.; Winkler, S.; Kalden, J.R.; Lorenz, H.M. Interleukin-4 supports interleukin-12-induced proliferation and interferon-gamma secretion in human activated lymphoblasts and T helper type 1 cells. Immunology 2006, 119, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Gu, J.; Li, S.L.; Reddy, M.A.; Natarajan, R.; Nadler, J.L. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 2006, 147, 2518–2525. [Google Scholar] [CrossRef]



| Parameters | DM+S n = 10 (5M/5F) | DM n = 10 (5M/5F) | N-DM n = 10 (5M/5F) |
|---|---|---|---|
| 1,5-AG (mg/L) | 13.5 ± 2.0 | 14.2 ± 2.7 | 23.3 ± 2.5 |
| HbA1c (%) | 7.1 ± 0.4 | 7.1 ± 0.5 | 5.3 ± 0.4 |
| Total cholesterol (mg/dL) | 179.7 ± 15.1 | 240.3 ± 24.2 | 176.3 ± 19.8 |
| LDL (mg/dL) | 91.0 ± 16.5 | 132.1 ± 16.3 | 101.2 ± 11.5 |
| HDL (mg/dL) | 54.7 ± 18.6 | 56.7 ± 16.2 | 54.9 ± 14.6 |
| TG (mg/dL) | 144.7 ± 19.1 | 158.1 ± 16.0 | 123.4 ± 19.6 |
| Values expressed as Mean ± SD; DM+S—statin-treated patients with type 2 diabetes; DM—statin-free patients with type 2 diabetes; N-DM—non-diabetic control | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesołowska, A.; Winiarska, H.; Owoc, J.; Borowska, M.; Domagała, J.; Mikołajczak, P.Ł.; Iskakova, S.; Dworacki, G.; Dworacka, M. Effects of Low-Dose Atorvastatin on the Peripheral Blood Mononuclear Cell Secretion of Angiogenic Factors in Type 2 Diabetes. Biomolecules 2021, 11, 1885. https://doi.org/10.3390/biom11121885
Wesołowska A, Winiarska H, Owoc J, Borowska M, Domagała J, Mikołajczak PŁ, Iskakova S, Dworacki G, Dworacka M. Effects of Low-Dose Atorvastatin on the Peripheral Blood Mononuclear Cell Secretion of Angiogenic Factors in Type 2 Diabetes. Biomolecules. 2021; 11(12):1885. https://doi.org/10.3390/biom11121885
Chicago/Turabian StyleWesołowska, Anna, Hanna Winiarska, Jakub Owoc, Magdalena Borowska, Joanna Domagała, Przemysław Łukasz Mikołajczak, Saule Iskakova, Grzegorz Dworacki, and Marzena Dworacka. 2021. "Effects of Low-Dose Atorvastatin on the Peripheral Blood Mononuclear Cell Secretion of Angiogenic Factors in Type 2 Diabetes" Biomolecules 11, no. 12: 1885. https://doi.org/10.3390/biom11121885







