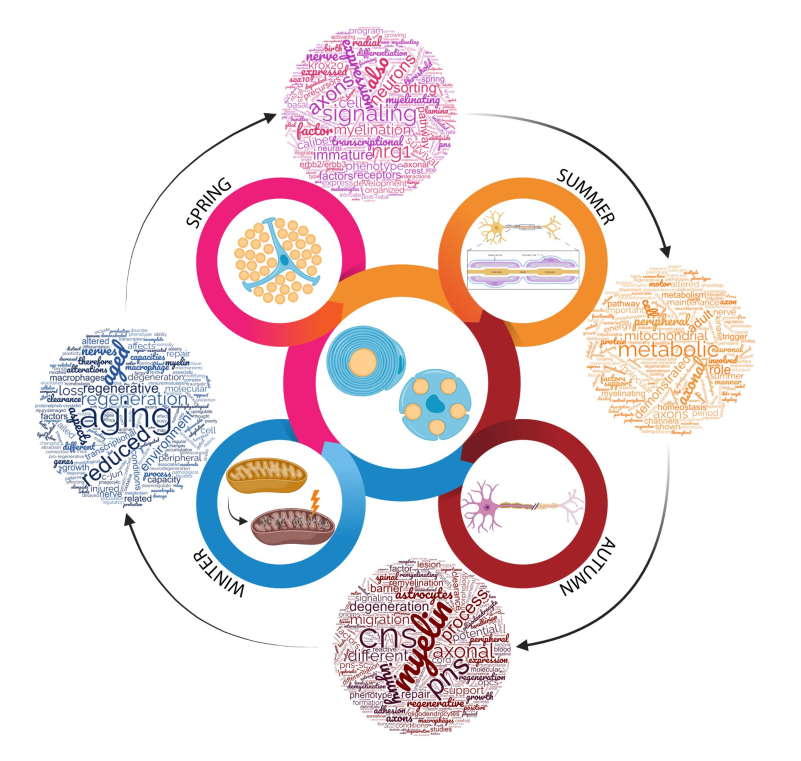
Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging
Abstract
:1. Introduction
2. Spring for Schwann Cells: The Development
3. Summer for Schwann Cells: Adult Homeostasis
4. Autumn for Schwann Cells: The Degeneration for a Potential Regeneration
5. Winter for Schwann Cells: Aging
6. Closing Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Scheib, J.; Höke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Mietto, B.S.; Kroner, X.A.; Girolami, E.I.; Santos-Nogueira, X.E.; Zhang, J.; David, S.; David, S. Role of IL-10 in Resolution of Inflammation and Functional Recovery after Peripheral Nerve Injury. J. Neurosci. 2015, 35, 16431–16442. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun Reprograms Schwann Cells of Injured Nerves to Generate a Repair Cell Essential for Regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; et al. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Viader, A.; Sasaki, Y.; Kim, S.; Strickland, A.; Workman, C.S.; Yang, K.; Gross, R.W.; Milbrandt, J. Aberrant Schwann Cell Lipid Metabolism Linked to Mitochondrial Deficits Leads to Axon Degeneration and Neuropathy. Neuron 2013, 77, 886–898. [Google Scholar] [CrossRef] [Green Version]
- Babetto, E.; Wong, K.M.; Beirowski, B. A glycolytic shift in Schwann cells supports injured axons. Nat. Neurosci. 2020, 23, 1215–1228. [Google Scholar] [CrossRef]
- Court, F.A.; Coleman, M.P. Mitochondria as a central sensor for axonal degenerative stimuli. Trends Neurosci. 2012, 35, 364–372. [Google Scholar] [CrossRef]
- Joseph, N.M.; Mukouyama, Y.-S.; Mosher, J.T.; Jaegle, M.; Crone, S.; Dormand, E.-L.; Lee, K.-F.; Meijer, D.; Anderson, D.J.; Morrison, S.J. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 2004, 131, 5599–5612. [Google Scholar] [CrossRef] [Green Version]
- Luo, R.; Gao, J.; Wehrle-Haller, B.; Henion, P.D. Molecular identification of distinct neurogenic and melanogenic neural crest sublineages. Development 2003, 130, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.; Brennan, A.; Morgan, L.; Mirsky, R.; Kent, A.; Hashimoto, Y.; Gavrilovic, J. The schwann cell precursor and its fate: A study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron 1994, 12, 509–527. [Google Scholar] [CrossRef]
- Weston, J.A. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev. Biol. 1963, 6, 279–310. [Google Scholar] [CrossRef]
- Le Douarin, N.M.; Teillet, M.-A.M. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 1974, 41, 162–184. [Google Scholar] [CrossRef]
- Piccolo, S.; Sasai, Y.; Lu, B.; De Robertis, E. Dorsoventral Patterning in Xenopus: Inhibition of Ventral Signals by Direct Binding of Chordin to BMP-4. Cell 1996, 86, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, L.B.; De Jesús-Escobar, J.M.; Harland, R.M. The Spemann Organizer Signal noggin Binds and Inactivates Bone Morphogenetic Protein 4. Cell 1996, 86, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Hemmati-Brivanlou, A. Neural Crest Induction by Xwnt7B in Xenopus. Dev. Biol. 1998, 194, 129–134. [Google Scholar] [CrossRef] [Green Version]
- García-Castro, M.I.; Marcelle, C.; Bronner-Fraser, M. Ectodermal Wnt Function as a Neural Crest Inducer. Science 2002, 297, 848–851. [Google Scholar] [CrossRef]
- LaBonne, C.; Bronner-Fraser, M. Neural crest induction in Xenopus: Evidence for a two-signal model. Development 1998, 125, 2403–2414. [Google Scholar] [CrossRef]
- Mayor, R.; Guerrero, N.; Martínez, C. Role of FGF and Noggin in Neural Crest Induction. Dev. Biol. 1997, 189, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Monsoro-Burq, A.-H.; Fletcher, R.B.; Harland, R.M. Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Dev. 2003, 130, 3111–3124. [Google Scholar] [CrossRef] [Green Version]
- Steventon, B.; Araya, C.; Linker, C.; Kuriyama, S.; Mayor, R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development 2009, 136, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, S.; Glavic, A.; Ruiz, P.; Mayor, R. Posteriorization by FGF, Wnt, and Retinoic Acid Is Required for Neural Crest Induction. Dev. Biol. 2002, 241, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Brennan, A.; Dean, C.; Zhang, A.; Cass, D.; Mirsky, R.; Jessen, K. Endothelins Control the Timing of Schwann Cell Generation in Vitro and in Vivo. Dev. Biol. 2000, 227, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.; Birchmeier, C. Erratum: Multiple essential functions of neuregulin in development. Nat. Cell Biol. 1995, 378, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.; Yamaai, T.; Garratt, A.; Riethmacher-Sonnenberg, E.; Kane, D.; Theill, L.E.; Birchmeier, C. Isoform-specific expression and function of neuregulin. Development 1997, 124, 3575–3586. [Google Scholar] [CrossRef]
- Riethmacher, D.; Sonnenberg-Riethmacher, E.; Brinkmann, V.; Yamaai, T.; Lewin, G.R.; Birchmeier, C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nat. Cell Biol. 1997, 389, 725–730. [Google Scholar] [CrossRef]
- Wolpowitz, D.; Mason, T.B.; Dietrich, P.; Mendelsohn, M.; A Talmage, D.; Role, L.W. Cysteine-Rich Domain Isoforms of the Neuregulin-1 Gene Are Required for Maintenance of Peripheral Synapses. Neuron 2000, 25, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Woodhoo, A.; Alonso, M.B.D.; Droggiti, A.; Turmaine, M.; D’Antonio, M.; Parkinson, D.B.; Wilton, D.K.; Al-Shawi, R.; Simons, P.; Shen, J.; et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci. 2009, 12, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Britsch, S.; Goerich, D.E.; Riethmacher, D.; Peirano, R.I.; Rossner, M.; Nave, K.-A.; Birchmeier, C.; Wegner, M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001, 15, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Sauka-Spengler, T.; Bronner-Fraser, M. A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 2008, 9, 557–568. [Google Scholar] [CrossRef]
- Dong, Z.; Brennan, A.; Liu, N.; Yarden, Y.; Lefkowitz, G.; Mirsky, R.; Jessen, K.R. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat schwann cell precursors. Neuron 1995, 15, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Winseck, A.K.; Caldero, J.; Ciutat, D.; Prevette, D.; Scott, S.A.; Wang, G.; Esquerda, J.E.; Oppenheim, R.W. In Vivo Analysis of Schwann Cell Programmed Cell Death in the Embryonic Chick: Regulation by Axons and Glial Growth Factor. J. Neurosci. 2002, 22, 4509–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, M.W.; Klein, R.D.; Fariñas, I.; Sauer, H.; Armanini, M.; Phillips, H.; Reichardt, L.F.; Ryan, A.M.; Carver-Moore, K.; Rosenthal, A. Renal and neuronal abnormalities in mice lacking GDNF. Nat. Cell Biol. 1996, 382, 76–79. [Google Scholar] [CrossRef]
- Morris, J.K.; Lin, W.; Hauser, C.; Marchuk, Y.; Getman, D.; Lee, K.-F. Rescue of the Cardiac Defect in ErbB2 Mutant Mice Reveals Essential Roles of ErbB2 in Peripheral Nervous System Development. Neuron 1999, 23, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Verdi, J.; Groves, A.; Fariñas, I.; Jones, K.; Marchionni, M.; Reichardt, L.; Anderson, D. A Reciprocal Cell–Cell Interaction Mediated by NT-3 and Neuregulins Controls the Early Survival and Development of Sympathetic Neuroblasts. Neuron 1996, 16, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Woldeyesus, M.T.; Britsch, S.; Riethmacher, D.; Xu, L.; Sonnenberg-Riethmacher, E.; Abou-Rebyeh, F.; Harvey, R.; Caroni, P.; Birchmeier, C. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999, 13, 2538–2548. [Google Scholar] [CrossRef] [Green Version]
- Dowsing, B.J.; Morrison, W.A.; Nicola, N.A.; Starkey, G.P.; Bucci, T.; Kilpatrick, T.J. Leukemia Inhibitory Factor Is an Autocrine Survival Factor for Schwann Cells. J. Neurochem. 1999, 73, 96–104. [Google Scholar] [CrossRef]
- Meier, C.; Parmantier, E.; Brennan, A.; Mirsky, R.; Jessen, K.R. Developing Schwann Cells Acquire the Ability to Survive without Axons by Establishing an Autocrine Circuit Involving Insulin-Like Growth Factor, Neurotrophin-3, and Platelet-Derived Growth Factor-BB. J. Neurosci. 1999, 19, 3847–3859. [Google Scholar] [CrossRef] [Green Version]
- Weiner, J.; Chun, J. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc. Natl. Acad. Sci. USA 1999, 96, 5233–5238. [Google Scholar] [CrossRef] [Green Version]
- Mukouyama, Y.-S.; Shin, D.; Britsch, S.; Taniguchi, M.; Anderson, D.J. Sensory Nerves Determine the Pattern of Arterial Differentiation and Blood Vessel Branching in the Skin. Cell 2002, 109, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Mukouyama, Y.-S.; Gerber, H.-P.; Ferrara, N.; Gu, C.; Anderson, D.J. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development 2005, 132, 941–952. [Google Scholar] [CrossRef] [Green Version]
- Parmantier, E.; Lynn, B.; Lawson, D.; Turmaine, M.; Namini, S.S.; Chakrabarti, L.; McMahon, A.P.; Jessen, K.R.; Mirsky, R. Schwann Cell–Derived Desert Hedgehog Controls the Development of Peripheral Nerve Sheaths. Neuron 1999, 23, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Webster, H.D.; Martin, J.R.; O’Connell, M.F. The relationships between interphase Schwann cells and axons before myelination: A quantitative electron microscopic study. Dev. Biol. 1973, 32, 401–416. [Google Scholar] [CrossRef]
- Feltri, M.L.; Poitelon, Y.; Previtali, S.C. How Schwann Cells Sort Axons. Neuroscience 2016, 22, 252–265. [Google Scholar] [CrossRef]
- Poitelon, Y.; Lopez-Anido, C.; Catignas, K.; Berti, C.; Palmisano, M.; Williamson, C.; Ameroso, D.; Abiko, K.; Hwang, Y.; Gregorieff, A.; et al. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat. Neurosci. 2016, 19, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Berti, C.; Bartesaghi, L.; Ghidinelli, M.; Zambroni, D.; Figlia, G.; Chen, Z.-L.; Quattrini, A.; Wrabetz, L.; Feltri, M.L. Non-redundant function of dystroglycan and β1 integrins in radial sorting of axons. Development 2011, 138, 4025–4037. [Google Scholar] [CrossRef] [Green Version]
- Grigoryan, T.; Stein, S.; Qi, J.; Wende, H.; Garratt, A.N.; Nave, K.-A.; Birchmeier, C.; Birchmeier, W. Wnt/Rspondin/β-catenin signals control axonal sorting and lineage progression in Schwann cell development. Proc. Natl. Acad. Sci. USA 2013, 110, 18174–18179. [Google Scholar] [CrossRef] [Green Version]
- Pellegatta, M.; De Arcangelis, A.; D’Urso, A.; Nodari, A.; Zambroni, D.; Ghidinelli, M.; Matafora, V.; Williamson, C.; Georges-Labouesse, E.; Kreidberg, J.; et al. α6β1 and α7β1 integrins are required in Schwann cells to sort axons. J. Neurosci. 2013, 33, 17995–18007. [Google Scholar] [CrossRef] [Green Version]
- Rasi, K.; Hurskainen, M.; Kallio, M.; Stavén, S.; Sormunen, R.; Heape, A.M.; Avila, R.L.; Kirschner, D.; Muona, A.; Tolonen, U.; et al. Lack of Collagen XV Impairs Peripheral Nerve Maturation and, When Combined with Laminin-411 Deficiency, Leads to Basement Membrane Abnormalities and Sensorimotor Dysfunction. J. Neurosci. 2010, 30, 14490–14501. [Google Scholar] [CrossRef]
- Wallquist, W.; Plantman, S.; Thams, S.; Thyboll, J.; Kortesmaa, J.; Lännergren, J.; Domogatskaya, A.; Ögren, S.O.; Risling, M.; Hammarberg, H.; et al. Impeded Interaction between Schwann Cells and Axons in the Absence of Laminin 4. J. Neurosci. 2005, 25, 3692–3700. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Bierman, J.; Tarumi, Y.S.; Zhong, Y.-P.; Rangwala, R.; Proctor, T.M.; Miyagoe-Suzuki, Y.; Takeda, S.; Miner, J.H.; Sherman, L.S.; et al. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J. Cell Biol. 2005, 168, 655–666. [Google Scholar] [CrossRef] [Green Version]
- Taveggia, C.; Zanazzi, G.; Petrylak, A.; Yano, H.; Rosenbluth, J.; Einheber, S.; Xu, X.; Esper, R.M.; Loeb, J.A.; Shrager, P.; et al. Neuregulin-1 Type III Determines the Ensheathment Fate of Axons. Neuron 2005, 47, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Michailov, G.V.; Sereda, M.W.; Brinkmann, B.G.; Fischer, T.M.; Haug, B.; Birchmeier, C.; Role, L.; Lai, C.; Schwab, M.H.; Nave, K.-A. Axonal Neuregulin-1 Regulates Myelin Sheath Thickness. Science 2004, 304, 700–703. [Google Scholar] [CrossRef] [Green Version]
- Ghislain, J.; Desmarquet-Trin-Dinh, C.; Jaegle, M.; Meijer, D.; Charnay, P.; Frain, M. Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development 2002, 129, 155–166. [Google Scholar] [CrossRef]
- Ghislain, J.; Charnay, P. Control of myelination in Schwann cells: aKrox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep. 2006, 7, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Kuhlbrodt, K.; Herbarth, B.; Sock, E.; Enderich, J.; Hermans-Borgmeyer, I.; Wegner, M. Cooperative Function of POU Proteins and SOX Proteins in Glial Cells. J. Biol. Chem. 1998, 273, 16050–16057. [Google Scholar] [CrossRef] [Green Version]
- Kao, S.-C.; Wu, H.; Xie, J.; Chang, C.-P.; Ranish, J.A.; Graef, I.A.; Crabtree, G.R. Calcineurin/NFAT Signaling Is Required for Neuregulin-Regulated Schwann Cell Differentiation. Science 2009, 323, 651–654. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Kim, J.Y.; Dupree, J.; Tewari, A.; Melendez-Vasquez, C.; Svaren, J.; Casaccia, P. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat. Neurosci. 2010, 13, 1472–1480. [Google Scholar] [CrossRef] [Green Version]
- Decker, L.; Desmarquet-Trin-Dinh, C.; Taillebourg, E.; Ghislain, J.; Vallat, J.-M.; Charnay, P. Peripheral Myelin Maintenance Is a Dynamic Process Requiring Constant Krox20 Expression. J. Neurosci. 2006, 26, 9771–9779. [Google Scholar] [CrossRef] [Green Version]
- Murphy, P.; Topilko, P.; Schneider-Maunoury, S.; Seitanidou, T.; Evercooren, A.B.-V.; Charnay, P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development 1996, 122, 2847–2857. [Google Scholar] [CrossRef]
- Topilko, P.; Schneider-Maunoury, S.; Levi, G.; Evercooren, A.B.-V.; Chennoufi, A.B.Y.; Seitanidou, T.; Babinet, C.; Charnay, P. Krox-20 controls myelination in the peripheral nervous system. Nat. Cell Biol. 1994, 371, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Beirowski, B. The LKB1-AMPK and mTORC1 Metabolic Signaling Networks in Schwann Cells Control Axon Integrity and Myelination: Assembling and upholding nerves by metabolic signaling in Schwann cells. BioEssays 2018, 41, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mietto, B.S.; Mostacada, K.; Maria, A.; Martinez, B. Neurotrauma and Inflammation: CNS and PNS Responses. Mediat. Inflamm. 2015, 2015, 251204. [Google Scholar] [CrossRef] [PubMed]
- de Waegh, S.M.; Lee, V.M.-Y.; Brady, S.T. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 1992, 68, 451–463. [Google Scholar] [CrossRef]
- Yin, X.; Crawford, T.O.; Griffin, J.W.; Tu, P.-H.; Lee, V.M.-Y.; Li, C.; Roder, J.; Trapp, B.D. Myelin-Associated Glycoprotein Is a Myelin Signal that Modulates the Caliber of Myelinated Axons. J. Neurosci. 1998, 18, 1953–1962. [Google Scholar] [CrossRef] [Green Version]
- Deerinck, T.J.; Levinson, S.R.; Bennett, G.V.; Ellisman, M.H. Clustering of Voltage-Sensitive Sodium Channels on Axons Is Independent of Direct Schwann Cell Contact in the Dystrophic Mouse. J. Neurosci. 1997, 17, 5080–5088. [Google Scholar] [CrossRef] [Green Version]
- Hinson, A.; Gu, X.; Dib-Hajj, S.; Black, J.; Waxman, S. Schwann cells modulate sodium channel expression in spinal sensory neurons in vitro. Glia 1997, 21, 339–349. [Google Scholar] [CrossRef]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Höke, A. Schwann cell phenotype is regulated by axon modality and central—Location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Hoke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann Cells Express Motor and Sensory Phenotypes That Regulate Axon Regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef] [Green Version]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Viader, A.; Golden, J.; Baloh, R.H.; Schmidt, R.E.; Hunter, D.A.; Milbrandt, J. Schwann Cell Mitochondrial Metabolism Supports Long-Term Axonal Survival and Peripheral Nerve Function. J. Neurosci. 2011, 31, 10128–10140. [Google Scholar] [CrossRef]
- Kline, R.A.; Dissanayake, K.N.; Hurtado, M.L.; Martínez, N.; Ahl, A.; Mole, A.J.; Lamont, D.J.; Court, F.A.; Ribchester, R.R.; Wishart, T.; et al. Altered mitochondrial bioenergetics are responsible for the delay in Wallerian degeneration observed in neonatal mice. Neurobiol. Dis. 2019, 130, 104496. [Google Scholar] [CrossRef]
- Kalichman, M.W.; Powell, H.C.; Mizisin, A.P. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol. 1998, 95, 47–56. [Google Scholar] [CrossRef]
- Mietto, B.S.; Jhelum, P.; Schulz, K.; David, S. Schwann cells provide iron to axonal mitochondria and its role in nerve regeneration. J. Neurosci. 2021, 41, 7300–7313. [Google Scholar] [CrossRef]
- Beirowski, B.; Babetto, E.; Golden, J.; Chen, Y.-J.; Yang, K.; Gross, R.W.; Patti, G.J.; Milbrandt, J. Metabolic regulator LKB1 is crucial for Schwann cell–mediated axon maintenance. Nat. Neurosci. 2014, 17, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Lutz, A.B.; Barres, B.A. Contrasting the Glial Response to Axon Injury in the Central and Peripheral Nervous Systems. Dev. Cell 2014, 28, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Stassart, R.M.; Woodhoo, A. Axo-glial interaction in the injured PNS. Dev. Neurobiol. 2021, 81, 490–506. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; Van Der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Griffin, J.W.; Thompson, W.J. Biology and pathology of nonmyelinating Schwann cells. Glia 2008, 56, 1518–1531. [Google Scholar] [CrossRef]
- Waller, A. XX. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. 1850, 140, 423–429. [Google Scholar] [CrossRef]
- Bignami, A.; Ralston, H.J. The cellular reaction to wallerian degeneration in the central nervous system of the cat. Brain Res. 1969, 13, 444–461. [Google Scholar] [CrossRef]
- George, R.; Griffin, J.W. Delayed Macrophage Responses and Myelin Clearance during Wallerian Degeneration in the Central Nervous System: The Dorsal Radiculotomy Model. Exp. Neurol. 1994, 129, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lampert, P.W.; Cressman, M.R. Fine-structural changes of myelin sheaths after axonal degeneration in the spinal cord of rats. Am. J. Pathol. 1966, 49, 1139–1155. [Google Scholar]
- Perry, V.H.; Brown, M.C.; Gordon, S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J. Exp. Med. 1987, 165, 1218–1223. [Google Scholar] [CrossRef]
- Beirowski, B.; Adalbert, R.; Wagner, D.; Grumme, D.S.; Addicks, K.; Ribchester, R.R.; Coleman, M.P. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Griffin, J.W.; George, R.; Lobato, C.; Tyor, W.R.; Li, C.Y.; Glass, J.D. Macrophage responses and myelin clearance during Wallerian degeneration: Relevance to immune-mediated demyelination. J. Neuroimmunol. 1992, 40, 153–165. [Google Scholar] [CrossRef]
- Becerra, J.L.; Puckett, W.R.; Hiester, E.D.; Quencer, R.M.; E Marcillo, A.; Post, M.J.; Bunge, R.P. MR-pathologic comparisons of wallerian degeneration in spinal cord injury. Am. J. Neuroradiol. 1995, 16, 125–133. [Google Scholar]
- Buss, A.; Brook, G.A.; Kakulas, B.; Martin, D.; Franzen, R.; Schoenen, J.; Noth, J.; Schmitt, A.B. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 2004, 127, 34–44. [Google Scholar] [CrossRef]
- Mietto, B.S.; Jürgensen, S.; Alves, L.; Pecli, C.; Narciso, M.S.; Assunção-Miranda, I.; Villa-Verde, D.M.S.; Lima, F.R.D.S.; De Menezes, J.R.L.; Benjamim, C.F.; et al. Lack of galectin-3 speeds Wallerian degeneration by altering TLR and pro-inflammatory cytokine expressions in injured sciatic nerve. Eur. J. Neurosci. 2013, 37, 1682–1690. [Google Scholar] [CrossRef]
- Lutz, A.B.; Chung, W.-S.; Sloan, S.; Carson, G.A.; Zhou, L.; Lovelett, E.; Posada, S.; Zuchero, J.B.; Barres, B.A. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc. Natl. Acad. Sci. USA 2017, 114, E8072–E8080. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.Y.; Shin, Y.K.; Park, S.Y.; Park, J.Y.; Lee, H.J.; Yoo, Y.H.; Kim, J.K.; Park, H.T. Autophagic myelin destruction by Schwann cells during wallerian degeneration and segmental demyelination. Glia 2016, 64, 730–742. [Google Scholar] [CrossRef]
- Napoli, I.; Noon, L.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; et al. A Central Role for the ERK-Signaling Pathway in Controlling Schwann Cell Plasticity and Peripheral Nerve Regeneration In Vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.P.; Zhang, D.P.; Mak, K.S.; Bonder, D.E.; Pomeroy, S.L.; Kim, H.A. Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: Axon-dependent removal of newly generated Schwann cells by apoptosis. Mol. Cell. Neurosci. 2008, 38, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Parkinson, D.B.; Bhaskaran, A.; Arthur-Farraj, P.; Noon, L.A.; Woodhoo, A.; Lloyd, A.C.; Feltri, M.L.; Wrabetz, L.; Behrens, A.; Mirsky, R.; et al. c-Jun is a negative regulator of myelination. J. Cell Biol. 2008, 181, 625–637. [Google Scholar] [CrossRef] [Green Version]
- del Río-Hortega, P. La glía de escasa radiaciones (oligodendroglia) [Glia with many processes (oligoden-droglia)]. Trab. Lab. Histol. Patol. 1921, 1, 1–43. [Google Scholar]
- Penfield, M.D.W.; Oxon, B.S.C. Oligodendroglia and its relation to classical neuroglia. Brain 1924, 47, 430. [Google Scholar] [CrossRef]
- Roots, B.I. The Evolution of Myelinating Cells. In Neuron-Glia Interrelations During Phylogeny: I. Phylogeny and Ontogeny of Glial Cells; Vernadakis, A., Roots, B.I., Eds.; Humana Press: Totowa, NJ, USA, 1995; pp. 223–248. [Google Scholar] [CrossRef]
- Chen, M.S.; Huber, A.B.; Van Der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nat. Cell Biol. 2000, 403, 434–439. [Google Scholar] [CrossRef]
- Bandtlow, C.; Zachleder, T.; Schwab, M. Oligodendrocytes arrest neurite growth by contact inhibition. J. Neurosci. 1990, 10, 3837–3848. [Google Scholar] [CrossRef] [Green Version]
- McKerracher, L.; David, S.; Jackson, D.; Kottis, V.; Dunn, R.; Braun, P. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 1994, 13, 805–811. [Google Scholar] [CrossRef]
- Barres, B.A.; Jacobson, M.D.; Schmidt, I.R.; Sendtnerf, M.; Raff, M.C. Does oligodendrocyte survival depend on axons? Curr. Biol. 1993, 3, 489–497. [Google Scholar] [CrossRef]
- Raine, C.S. On the occurrence of Schwann cells within the normal central nervous system. J. Neurocytol. 1976, 5, 371–380. [Google Scholar] [CrossRef]
- Koeppen, A.H.; Ordinario, A.T.; Barron, K.D. Aberrant Intramedullary Peripheral Nerve Fibers. Arch. Neurol. 1968, 18, 567–573. [Google Scholar] [CrossRef]
- Blakemore, W.F. Ethidium Bromide Induced Demyelination in the Spinal Cord of the Cat. Neuropathol. Appl. Neurobiol. 1982, 8, 365–375. [Google Scholar] [CrossRef]
- Canto, M.C.D.; Lipton, H.L. Schwann cell remyelination and recurrent demyelination in the central nervous system of mice infected with attenuated Theiler’s virus. Am. J. Pathol. 1980, 98, 101–122. [Google Scholar]
- Trapp, B.D.; Itoyama, Y.; MacIntosh, T.D.; Quarles, R.H. P2Protein in Oligodendrocytes and Myelin of the Rabbit Central Nervous System. J. Neurochem. 1983, 40, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Itoyama, Y.; Ohnishi, A.; Tateishi, J.; Kuroiwa, Y.; De Webster, H.F. Spinal cord multiple sclerosis lesions in Japanese patients: Schwann cell remyelination occurs in areas that lack glial fibrillary acidic protein (GFAP). Acta Neuropathol. 1985, 65, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Walter, G.F.; Gerhard, L. The expression of nerve growth factor receptor on Schwann cells and the effect of these cells on the regeneration of axons in traumatically injured human spinal cord. Acta Neuropathol. 1996, 91, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Waxman, S.G.; Smith, K. Remyelination of dorsal column axons by endogenous Schwann cells restores the normal pattern of Nav1.6 and Kv1.2 at nodes of Ranvier. Brain 2006, 129, 1319–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blight, A.R.; Young, W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J. Neurol. Sci. 1989, 91, 15–34. [Google Scholar] [CrossRef]
- Felts, P.; Smith, K. Conduction properties of central nerve fibers remyelinated by Schwann cells. Brain Res. 1992, 574, 178–192. [Google Scholar] [CrossRef]
- Gilmore, S.A. Autoradiographic studies of intramedullary schwann cells in irradiated spinal cords of immature rats. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1971, 171, 517–527. [Google Scholar] [CrossRef]
- Gilmore, S.A.; Sims, T.J.; Heard, J.K. Autoradiographic and ultrastructural studies of areas of spinal cord occupied by Schwann cells and Schwann cell myelin. Brain Res. 1982, 239, 365–375. [Google Scholar] [CrossRef]
- Griffiths, I.R.; McCulloch, M.C. Nerve fibres in spinal cord impact injuries: Part 1. Changes in the myelin sheath during the initial 5 weeks. J. Neurol. Sci. 1983, 58, 335–349. [Google Scholar] [CrossRef]
- Guest, J.D.; Hiester, E.D.; Bunge, P.R. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 2005, 192, 384–393. [Google Scholar] [CrossRef]
- Jasmin, L.; Janni, G.; Moallem, T.M.; Lappi, D.A.; Ohara, P.T. Schwann Cells Are Removed from the Spinal Cord after Effecting Recovery from Paraplegia. J. Neurosci. 2000, 20, 9215–9223. [Google Scholar] [CrossRef] [Green Version]
- Raine, C.S.; Traugott, U.; Stone, S.H. Glial bridges and Schwann cell migration during chronic demyelination in the C.N.S. J. Neurocytol. 1978, 7, 541–553. [Google Scholar] [CrossRef]
- Sims, T.J.; Durgun, M.B.; Gilmore, S.A. Schwann cell invasion of ventral spinal cord: The effect of irradiation on astrocyte barriers. J. Neuropathol. Exp. Neurol. 1998, 57, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Bachelin, C.; Zujovic, V.; Buchet, D.; Mallet, J.; Evercooren, A.B.-V. Ectopic expression of polysialylated neural cell adhesion molecule in adult macaque Schwann cells promotes their migration and remyelination potential in the central nervous system. Brain 2010, 133, 406–420. [Google Scholar] [CrossRef]
- Evercooren, A.B.; Avellana-Adalid, V.; Younes-Chennoufi, A.B.; Gansmüller, A.; Nait-Oumesmar, B.; Vignais, L. Cell-cell interactions during the migration of myelin-forming cells transplanted in the demye-linated spinal cord. Glia 1996, 16, 147–164. [Google Scholar] [CrossRef]
- Blakemore, W.F. The effect of sub-dural nerve tissue transplatation on the spinal cord of the rat. Neuropathol. Appl. Neurobiol. 1980, 6, 433–447. [Google Scholar] [CrossRef]
- Blakemore, W.F. Limited remyelination of CNS axons by Schwann cells transplanted into the sub-arachnoid space. J. Neurol. Sci. 1984, 64, 265–276. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Crang, A.J. The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. J. Neurol. Sci. 1985, 70, 207–223. [Google Scholar] [CrossRef]
- Girard, C.; Bemelmans, A.; Dufour, N.; Mallet, J.; Bachelin, C.; Nait-Oumesmar, B.; Evercooren, A.B.-V.; Lachapelle, F. Grafts of Brain-Derived Neurotrophic Factor and Neurotrophin 3-Transduced Primate Schwann Cells Lead to Functional Recovery of the Demyelinated Mouse Spinal Cord. J. Neurosci. 2005, 25, 7924–7933. [Google Scholar] [CrossRef] [Green Version]
- Pearse, D.D.; Sanchez, A.R.; Pereira, F.C.; Andrade, C.M.; Puzis, R.; Pressman, Y.; Golden, K.; Kitay, B.M.; Blits, B.; Wood, P.M.; et al. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia 2007, 55, 976–1000. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Plemel, J.R.; Lee, M.; Stratton, J.A.; Manesh, S.B.; Liu, J.; Ramer, L.M.; Kang, S.H.; Bergles, D.E.; et al. Myelinogenic Plasticity of Oligodendrocyte Precursor Cells following Spinal Cord Contusion Injury. J. Neurosci. 2017, 37, 8635–8654. [Google Scholar] [CrossRef]
- Garcia-Diaz, B.; Bachelin, C.; Coulpier, F.; Gerschenfeld, G.; Deboux, C.; Zujovic, V.; Charnay, P.; Topilko, P.; Evercooren, A.B.-V. Blood vessels guide Schwann cell migration in the adult demyelinated CNS through Eph/ephrin signaling. Acta Neuropathol. 2019, 138, 457–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nievergall, E.; Lackmann, M.; Janes, P.W. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell. Mol. Life Sci. 2012, 69, 1813–1842. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, N.; Bachelin, C.; Zujovic, V.; Hilaire, M.; Baldwin, K.; Follis, R.M.; Giger, R.; Carter, B.D.; Evercooren, A.B.-V.; Filbin, M.T. Myelin-Associated Glycoprotein Inhibits Schwann Cell Migration and Induces Their Death. J. Neurosci. 2017, 37, 5885–5899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakemore, W.F.; Crang, A.J.; Patterson, R.C. Schwann cell remyelination of CNS axons following injection of cultures of CNS cells into areas of persistent demyelination. Neurosci. Lett. 1987, 77, 20–24. [Google Scholar] [CrossRef]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-Resident Glial Progenitor/Stem Cells Produce Schwann Cells as well as Oligodendrocytes during Repair of CNS Demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef] [Green Version]
- Kegler, K.; Imbschweiler, I.; Ulrich, R.; Kovermann, P.; Fahlke, C.; Deschl, U.; Kalkuhl, A.; Baumgärnter, W.; Wewetzer, K. CNS Schwann cells display oligodendrocyte precursor-like potassium channel activation and antigenic expression in vitro. J. Neural Transm. 2014, 121, 569–581. [Google Scholar] [CrossRef]
- Ulanska-Poutanen, J.; Mieczkowski, J.; Zhao, C.; Konarzewska, K.; Kaza, B.; Pohl, H.B.; Bugajski, Ł.; Kaminska, B.; Franklin, R.J.; Zawadzka, M. Injury-induced perivascular niche supports alternative differentiation of adult rodent CNS progenitor cells. eLife 2018, 7, 1–25. [Google Scholar] [CrossRef]
- Talbott, J.F.; Loy, D.N.; Liu, Y.; Qiu, M.S.; Bunge, M.B.; Rao, M.S.; Whittemore, S.R. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp. Neurol. 2005, 192, 11–24. [Google Scholar] [CrossRef] [Green Version]
- de Castro, G.M.; Deja, N.A.; Ma, D.; Zhao, C.; Franklin, R.J.M. Astrocyte Activation via Stat3 Signaling Determines the Balance of Oligodendrocyte versus Schwann Cell Remyelination. Am. J. Pathol. 2015, 185, 2431–2440. [Google Scholar] [CrossRef] [Green Version]
- Fraher, J.P. The CNS—PNS transitional zone of the rat. Morphometric studies at cranial and spinal levels. Prog. Neurobiol. 1992, 38, 261–316. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Wanner, I.B.; Anderson, M.A.; Song, B.; Levine, J.; Fernandez, A.; Gray-Thompson, Z.; Ao, Y.; Sofroniew, M.V. Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. J. Neurosci. 2013, 33, 12870–12886. [Google Scholar] [CrossRef] [Green Version]
- Liuzzi, F.J.; Lasek, R.J. Astrocytes Block Axonal Regeneration in Mammals by Activating the Physiological Stop Pathway. Science 1987, 237, 642–645. [Google Scholar] [CrossRef]
- Rudge, J.S.; Silver, J. Inhibition of neurite outgrowth on astroglial scars in vitro. J. Neurosci. 1990, 10, 3594–3603. [Google Scholar] [CrossRef]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [Green Version]
- Clemente, C.D.; Windle, W.F. Regeneration of severed nerve fibers in the spinal cord of the adult cat. J. Comp. Neurol. 1954, 101, 691–731. [Google Scholar] [CrossRef]
- Brown, J.O.; McCouch, G.P. Abortive regeneration of the transected spinal cord. J. Comp. Neurol. 1947, 87, 131–137. [Google Scholar] [CrossRef]
- Windle, W.F.; Clemente, C.D.; Chambers, W.W. Inhibition of formation of a glial barrier as a means of permitting a peripheral nerve to grow into the brain. J. Comp. Neurol. 1952, 96, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Frame, M.C.; Barnett, S.C. N-cadherin differentially determines Schwann cell and olfactory ensheathing cell adhesion and migration responses upon contact with astrocytes. Mol. Cell. Neurosci. 2005, 28, 253–263. [Google Scholar] [CrossRef]
- Lavdas, A.A.; Franceschini, I.; Dubois-Dalcq, M.; Matsas, R. Schwann cells genetically engineered to express PSA show enhanced migratory potential without impairment of their myelinating ability in vitro. Glia 2006, 53, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Chen, J.; Lavdas, A.A.; Thomaidou, D.; Schachner, M.; Matsas, R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 2007, 130, 2159–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilby, M.J.; Muir, E.M.; Fok-Seang, J.; Gour, B.J.; Blaschuk, O.W.; Fawcett, J.W. N-Cadherin Inhibits Schwann Cell Migration on Astrocytes. Mol. Cell. Neurosci. 1999, 14, 66–84. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.; Lindsay, S.L.; Pantiru, A.; Guimond, S.E.; Fagoe, N.; Verhaagen, J.; Turnbull, J.E.; Riddell, J.S.; Barnett, S.C. Sulfatase-mediated manipulation of the astrocyte-Schwann cell interface. Glia 2017, 65, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Afshari, F.T.; Kwok, J.C.; Fawcett, J.W. Astrocyte-Produced Ephrins Inhibit Schwann Cell Migration via VAV2 Signaling. J. Neurosci. 2010, 30, 4246–4255. [Google Scholar] [CrossRef] [Green Version]
- Grimpe, B.; Pressman, Y.; Bunge, M.B.; Silver, J. The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol. Cell. Neurosci. 2005, 28, 18–29. [Google Scholar] [CrossRef]
- Lakatos, A.; Barnett, S.C.; Franklin, R.J.M. Olfactory ensheathing cells induce less host astrocyte response and chondroitin sulphate proteoglycan expression than schwann cells following transplantation into adult cns white matter. Exp. Neurol. 2003, 184, 237–246. [Google Scholar] [CrossRef]
- Plant, G.W.; Bates, M.L.; Bunge, M.B. Inhibitory Proteoglycan Immunoreactivity Is Higher at the Caudal Than the Rostral Schwann Cell Graft-Transected Spinal Cord Interface. Mol. Cell. Neurosci. 2001, 17, 471–487. [Google Scholar] [CrossRef]
- Ghirnikar, R.S.; Eng, L.F. Chondroitin sulfate proteoglycan staining in astrocyte-schwann cell co-cultures. Glia 1995, 14, 145–152. [Google Scholar] [CrossRef]
- Verdier, V.; Csardi, G.; Smit, A.B.; Verheijen, M.H.G.; Bergmann, S.; Chrast, R.; De Preux-Charles, A.-S.; Médard, J.-J. Aging of myelinating glial cells predominantly affects lipid metabolism and immune response pathways. Glia 2012, 60, 751–760. [Google Scholar] [CrossRef]
- Hamilton, R.; Walsh, M.; Singh, R.; Rodriguez, K.; Gao, X.; Rahman, M.; Chaudhuri, A.; Bhattacharya, A. Oxidative damage to myelin proteins accompanies peripheral nerve motor dysfunction in aging C57BL/6 male mice. J. Neurol. Sci. 2016, 370, 47–52. [Google Scholar] [CrossRef]
- Lim, E.-M.F.; Musa, A.; Frederick, A.; Ousman, S.S. AlphaB-crystallin expression correlates with aging deficits in the peripheral nervous system. Neurobiol. Aging 2017, 53, 138–149. [Google Scholar] [CrossRef]
- Painter, M.W.; Lutz, A.B.; Cheng, Y.-C.; Latremoliere, A.; Duong, K.; Miller, C.M.; Posada, S.; Cobos, E.J.; Zhang, A.X.; Wagers, A.J.; et al. Diminished Schwann Cell Repair Responses Underlie Age-Associated Impaired Axonal Regeneration. Neuron 2014, 83, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Lichtman, J.W. Motor Axon Regeneration and Muscle Reinnervation in Young Adult and Aged Animals. J. Neurosci. 2013, 33, 19480–19491. [Google Scholar] [CrossRef]
- Wagstaff, L.J.; Gomez-Sanchez, J.A.; Fazal, S.V.; Otto, G.W.; Kilpatrick, A.M.; Michael, K.; Wong, L.Y.; Ma, K.H.; Turmaine, M.; Svaren, J.; et al. Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun. eLife 2021, 10, 1–32. [Google Scholar] [CrossRef]
- Stratton, J.; Eaton, S.; Rosin, N.L.; Jawad, S.; Holmes, A.; Yoon, G.; Midha, R.; Biernaskie, J. Macrophages and Associated Ligands in the Aged Injured Nerve: A Defective Dynamic That Contributes to Reduced Axonal Regrowth. Front. Aging Neurosci. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Scheib, J.L.; Höke, A. An attenuated immune response by Schwann cells and macrophages inhibits nerve regeneration in aged rats. Neurobiol. Aging 2016, 45, 1–9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardella-Silva, G.; Mietto, B.S.; Ribeiro-Resende, V.T. Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging. Biomolecules 2021, 11, 1887. https://doi.org/10.3390/biom11121887
Sardella-Silva G, Mietto BS, Ribeiro-Resende VT. Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging. Biomolecules. 2021; 11(12):1887. https://doi.org/10.3390/biom11121887
Chicago/Turabian StyleSardella-Silva, Gabriela, Bruno Siqueira Mietto, and Victor Túlio Ribeiro-Resende. 2021. "Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging" Biomolecules 11, no. 12: 1887. https://doi.org/10.3390/biom11121887
APA StyleSardella-Silva, G., Mietto, B. S., & Ribeiro-Resende, V. T. (2021). Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging. Biomolecules, 11(12), 1887. https://doi.org/10.3390/biom11121887






