Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioinformatics Data and Tools
2.2. Cell Cultures
2.3. Gene Expression Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Molecular Characteristics of Galectin-16 Gene and Recombinant Protein
3.2. Expression Patterns and Functions of LGALS16 in Cells and Tissues
3.3. Transcriptional and Post-Transcriptional Regulation of LGALS16
3.3.1. Transcription Factors
3.3.2. miRNAs
3.4. LGALS16 and Human Diseases
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timoshenko, A.V. Towards molecular mechanisms regulating the expression of galectins in cancer cells. Cell. Mol. Life Sci. 2015, 72, 4327–4340. [Google Scholar] [CrossRef] [PubMed]
- Allo, V.C.M.; Toscano, M.A.; Pinto, N.; Rabinovich, G.A. Galectins: Key players at the frontiers of innate and adaptive immunity. Trends Glycosci. Glycotechnol. 2018, 30, SE97–SE107. [Google Scholar] [CrossRef] [Green Version]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tazhitdinova, R.; Timoshenko, A.V. The emerging role of galectins and O-GlcNAc homeostasis in processes of cellular differentiation. Cells 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Vladoiu, M.C.; Labrie, M.; St-Pierre, Y. Intracellular galectins in cancer cells: Potential new targets for therapy (Review). Int. J. Oncol. 2014, 44, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.J.; Haudek, K.C.; Voss, P.G.; Wang, J.L. Examination of the role of galectins in pre-mRNA splicing. Methods Mol. Biol. 2015, 1207, 431–449. [Google Scholar]
- Popa, S.J.; Stewart, S.E.; Moreau, K. Unconventional secretion of annexins and galectins. Semin. Cell Dev. Biol. 2018, 83, 42–50. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Baum, L.G. Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol. 2006, 417, 247–256. [Google Scholar]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef] [Green Version]
- Than, N.G.; Romero, R.; Kim, C.J.; McGowen, M.R.; Papp, Z.; Wildman, D.E. Galectins: Guardians of eutherian pregnancy at the maternal-fetal interface. Trends Endocrinol. Metab. 2012, 23, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736. [Google Scholar] [CrossRef] [Green Version]
- Than, N.G.; Romero, R.; Xu, Y.; Erez, O.; Xu, Z.; Bhatti, G.; Leavitt, R.; Chung, T.H.; El-Azzamy, H.; LaJeunesse, C.; et al. Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta 2014, 35, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef]
- Blois, S.M.; Dveksler, G.; Vasta, G.R.; Freitag, N.; Blanchard, V.; Barrientos, G. Pregnancy galectinology: Insights into a complex network of glycan binding proteins. Front. Immunol. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Messeguer, X.; Escudero, R.; Farré, D.; Núñez, O.; Martínez, J.; Albà, M.M. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 2002, 18, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Farré, D.; Roset, R.; Huerta, M.; Adsuara, J.E.; Roselló, L.; Albà, M.M.; Messeguer, X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003, 31, 3651–3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.S.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013, 41, W169–W173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quillet, A.; Saad, C.; Ferry, G.; Anouar, Y.; Vergne, N.; Lecroq, T.; Dubessy, C. Improving bioinformatics prediction of microRNA targets by ranks aggregation. Front. Genet. 2020, 10, 1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjöstedt, E.; Asplund, A. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timoshenko, A.V. Chitin hydrolysate stimulates VEGF-C synthesis by MDA-MB-231 breast cancer cells. Cell Biol. Int. 2011, 35, 281–286. [Google Scholar] [CrossRef]
- Renaud, S.J.; Chakraborty, D.; Mason, C.W.; Rumi, M.A.; Vivian, J.L.; Soares, M.J. OVO-like 1 regulates progenitor cell fate in human trophoblast development. Proc. Natl. Acad. Sci. USA 2015, 112, E6175–E6184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timoshenko, A.V.; Lanteigne, J.; Kozak, K. Extracellular stress stimuli alter galectin expression profiles and adhesion characteristics of HL-60 cells. Mol. Cell Biochem. 2016, 413, 137–143. [Google Scholar] [CrossRef]
- Sherazi, A.A.; Jariwala, K.A.; Cybulski, A.N.; Lewis, J.W.; Karagiannis, J.; Cumming, R.C.; Timoshenko, A.V. Effects of global O-GlcNAcylation on galectin gene-expression profiles in human cancer cell lines. Anticancer Res. 2018, 38, 6691–6697. [Google Scholar] [CrossRef] [PubMed]
- Ely, A.Z.; Moon, J.M.; Sliwoski, G.R.; Sangha, A.K.; Shen, X.-X.; Labella, A.L.; Meiler, J.; Capra, J.A.; Rokas, A. The impact of natural selection on the evolution and function of placentally expressed galectins. Genome Biol. Evol. 2019, 11, 2574–2592. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.F. SINEs and LINEs: Highly repeated short and long interspersed sequences in mammalian genomes. Cell 1982, 28, 433–434. [Google Scholar] [CrossRef]
- Weckselblatt, B.; Rudd, M.K. Human structural variation: Mechanisms of chromosome rearrangements. Trends Genet. 2015, 31, 587–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Y.; Yao, Y.; Ayala, G.J.; Li, X.; Han, Q.; Zhang, W.; Xu, X.; Tai, G.; Mayo, K.H.; Zhou, Y.; et al. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel mediated lymphocyte activity. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129755. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, C.S. The placenta-brain-axis. J. Neurosci. Res. 2021, 99, 271–283. [Google Scholar] [CrossRef]
- Inamochi, Y.; Mochizuki, K.; Goda, T. Histone code of genes induced by co-treatment with a glucocorticoid hormone agonist and a p44/42 MAPK inhibitor in human small intestinal Caco-2 cells. Biochim. Biophys. Acta 2014, 1840, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [Green Version]
- Balsa, E.; Perry, E.A.; Bennett, C.F.; Jedrychowski, M.; Gygi, S.P.; Doench, J.G.; Puigserver, P. Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat. Commun. 2020, 11, 2714. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Giuffrida, D.; Zenerino, C.; Piazzese, A.; Olearo, E.; Todros, T.; Rolfo, A. JunB/Cyclin-D1 imbalance in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise. Placenta 2014, 35, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.M.; Romero, R.; Kim, J.S.; Tarca, A.L.; Kim, S.K.; Draghici, S.; Kusanovic, J.P.; Gotsch, F.; Mittal, P.; Hassan, S.S.; et al. Region-specific gene expression profiling: Novel evidence for biological heterogeneity of the human amnion. Biol. Reprod. 2008, 79, 954–961. [Google Scholar] [CrossRef] [Green Version]
- Knyazev, E.N.; Zakharova, G.S.; Astakhova, L.A.; Tsypina, I.M.; Tonevitsky, A.G.; Sukhikh, G.T. Metabolic reprogramming of trophoblast cells in response to hypoxia. Bull. Exp. Biol. Med. 2019, 166, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Winkler, E.A.; Zhou, S.; Birk, H.; Guo, D.; Koch, M.J.; Stapleton, C.J.; Spiegelman, D.; Dionne-Laporte, A.; Dion, P.A.; et al. Identification of a rare BMP pathway mutation in a non-syndromic human brain arteriovenous malformation via exome sequencing. Hum. Genome Var. 2018, 5, 18001. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Pang, C.; Song, L.; Guo, F.; Sun, H. Activating transcription factor 3 is overexpressed in human glioma and its knockdown in glioblastoma cells causes growth inhibition both in vitro and in vivo. Int. J. Mol. Med. 2015, 35, 1561–1573. [Google Scholar] [CrossRef] [Green Version]
- Garces de Los Favos Alonso, I.; Liang, H.C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The role of activator protein-1 (AP-1) family members in CD30-positive lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Shankar, K.; Kang, P.; Zhong, Y.; Borengasser, S.J.; Wingfield, C.; Saben, J.; Gomez-Acevedo, H.; Thakali, K.M. Transcriptomic and epigenomic landscapes during cell fusion in BeWo trophoblast cells. Placenta 2015, 36, 1342–1351. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Richardson, B.D.; Hubert, M.A.; Handwerger, S. Isolation and characterization of the human syncytin gene promoter. Biol. Reprod. 2004, 70, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Liu, J.; Kong, N.; Jiang, Y.; Jiang, R.; Zhen, X.; Zhou, J.; Li, C.; Sun, H.; et al. ATF3 deficiency impairs the proliferative-secretory phase transition and decidualization in RIF patients. Cell Death Dis. 2021, 12, 387. [Google Scholar] [CrossRef]
- Jadhav, K.; Zhang, Y. Activating transcription factor 3 in immune response and metabolic regulation. Liver Res. 2017, 1, 96–102. [Google Scholar] [CrossRef]
- Moslehi, R.; Mills, J.L.; Signore, C.; Kumar, A.; Ambroggio, X.; Dzutsev, A. Integrative transcriptome analysis reveals dysregulation of canonical cancer molecular pathways in placenta leading to preeclampsia. Sci. Rep. 2013, 3, 2407. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, S.; Mizuta, T.; Fujimoto, M.; Ohte, S.; Osawa, K.; Miyamoto, A.; Yoneyama, K.; Murata, E.; Machiya, A.; Jimi, E.; et al. Smad9 is a new type of transcriptional regulator in bone morphogenetic protein signaling. Sci. Rep. 2014, 4, 7596. [Google Scholar] [CrossRef]
- Heo, K.S.; Fujiwara, K.; Abe, J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ. J. 2011, 75, 2722–2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.K.; Sheller-Miller, S.; Salomon, C.; Garbhini Study Team. Circulating exosomal miRNA profile during term and preterm birth pregnancies: A longitudinal study. Endocrinology 2019, 160, 249–275. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, Y.; Roy, B.; Dwivedi, Y. Altered miRNA landscape of the anterior cingulate cortex is associated with potential loss of key neuronal functions in depressed brain. Eur. Neuropsychopharmacol. 2020, 40, 70–84. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, H.; Xie, W.; Meng, F.; Zhang, K.; Jiang, Y.; Zhang, X.; Zhang, J. Altered microRNA profiles in plasma exosomes from mesial temporal lobe epilepsy with hippocampal sclerosis. Oncotarget 2017, 8, 4136–4146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, B.; Zhou, M.X.; Zhou, F.K.; Luo, X.M.; Zhong, S.X.; Zhou, Y.F.; Qin, Y.S.; Li, P.P.; Qin, C. Exosome-derived miRNAs as biomarkers of the development and progression of intracranial aneurysms. J. Atheroscler. Thromb. 2020, 27, 545–610. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Gong, S.; Gaccioli, F.; Dopierala, J.; Sovio, U.; Cook, E.; Volders, P.J.; Martens, L.; Kirk, P.D.W.; Richardson, S.; Smith, G.C.S.; et al. The RNA landscape of the human placenta in health and disease. Nat. Commun. 2021, 12, 2639. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.E.; Muench, K.L.; Robinson, B.G.; Wang, A.; Palmer, T.D.; Winn, V.D. Examining Sex Differences in the Human Placental Transcriptome During the First Fetal Androgen Peak. Reprod Sci. 2021, 28, 801–818. [Google Scholar] [CrossRef]
- Vastrad, B.; Vastrad, C. Bioinformatics analyses of significant genes, related pathways and candidate prognostic biomarkers in Alzheimer’s disease. BioRxiv 2021. [Google Scholar] [CrossRef]
- Zhao, B.; Shan, Y.; Yang, Y.; Zhaolong, Y.; Li, T.; Wang, X.; Luo, T.; Zhu, Z.; Sullivan, P.; Zhao, H.; et al. Transcriptome-wide association analysis of brain structures yields insights into pleiotropy with complex neuropsychiatric traits. Nat. Commun. 2021, 12, 2878. [Google Scholar] [CrossRef] [PubMed]
- Čokić, V.P.; Mojsilović, S.; Jauković, A.; Kraguljac-Kurtović, N.; Mojsilović, S.; Šefer, D.; Mitrović Ajtić, O.; Milošević, V.; Bogdanović, A.; Đikić, D.; et al. Gene expression profile of circulating CD34(+) cells and granulocytes in chronic myeloid leukemia. Blood Cells Mol. Dis. 2015, 55, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues-Peres, R.M.; de Carvalho, B.S.; Anurag, M.; Lei, J.T.; Conz, L.; Gonçalves, R.; Cardoso Filho, C.; Ramalho, S.; de Paiva, G.R.; Derchain, S.; et al. Copy number alterations associated with clinical features in an underrepresented population with breast cancer. Mol. Genet. Genomic. Med. 2019, 7, e00750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.X.; Rasga, C.; Marques, A.R.; Martiniano, H.F.M.C.; Asif, M.; Vilela, J.; Oliveira, G.; Vicente, A.M. A role for gene-environment interactions in Autism Spectrum Disorder is suggested by variants in genes regulating exposure to environmental factors. BioRxiv 2019. [Google Scholar] [CrossRef]
- Arthur, S.E.; Jiang, A.; Grande, B.M.; Alcaide, M.; Cojocaru, R.; Rushton, C.K.; Mottok, A.; Hilton, L.K.; Kumar Lat, P.; Zhao, E.Y. Genome-wide discovery of somatic regulatory variants in diffuse large B-cell lymphoma. Nat. Commun. 2018, 9, 4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keaton, J.M.; Hellwege, J.N.; Ng, M.C.; Palmer, N.D.; Pankow, J.S.; Fornage, M.; Wilson, J.G.; Correa, A.; Rasmussen-Torvik, L.J.; Rotter, J.I.; et al. Genome-wide interaction with insulin secretion loci reveals novel loci for type 2 diabetes in African Americans. PLoS ONE 2016, 11, e0159977. [Google Scholar] [CrossRef]
- Cheng, P.; Feng, F.; Yang, H.; Jin, S.; Lai, C.; Wang, Y.; Bi, J. Detection and significance of exosomal mRNA expression profiles in the cerebrospinal fluid of patients with meningeal carcinomatosis. J. Mol. Neurosci. 2021, 71, 790–803. [Google Scholar] [CrossRef]


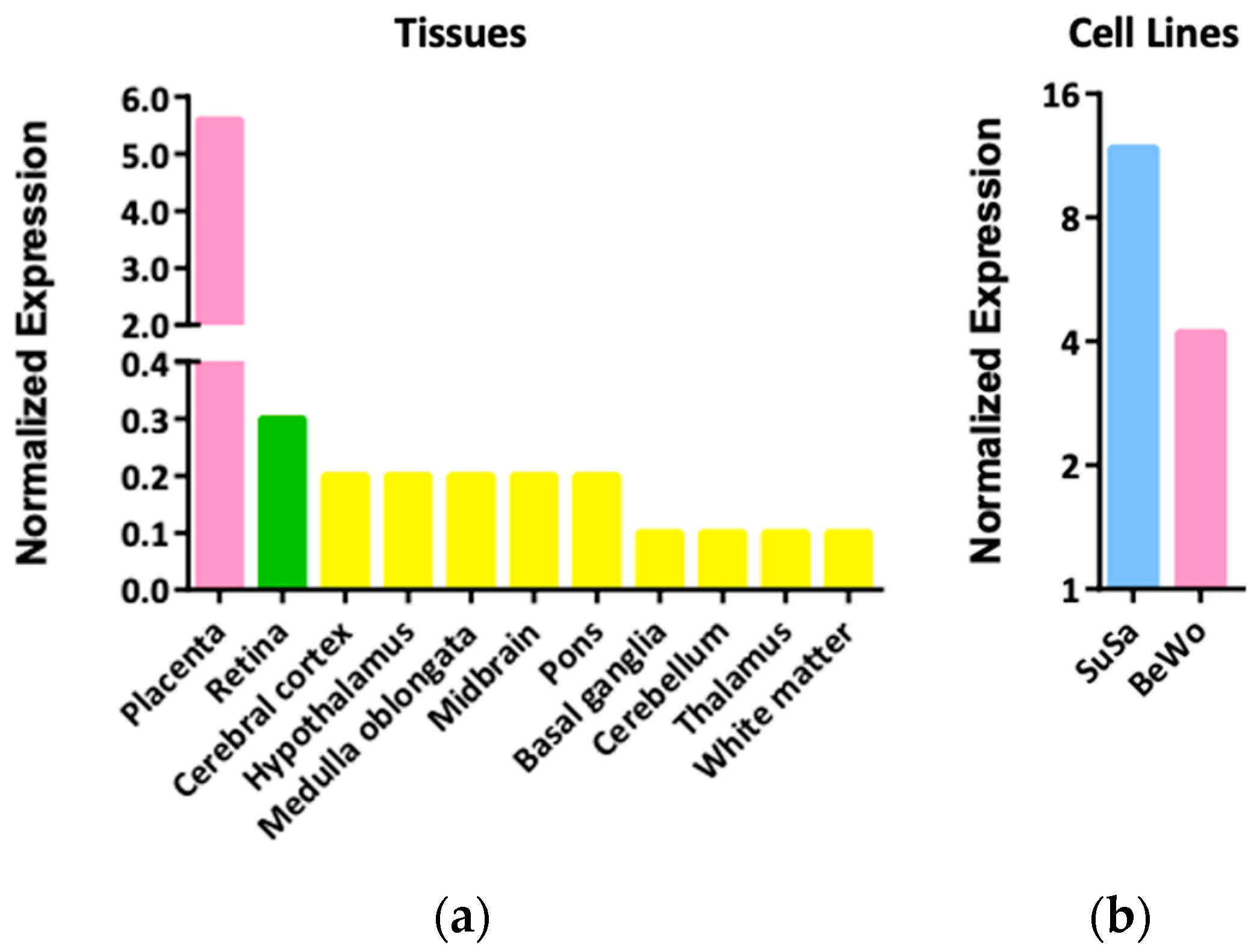



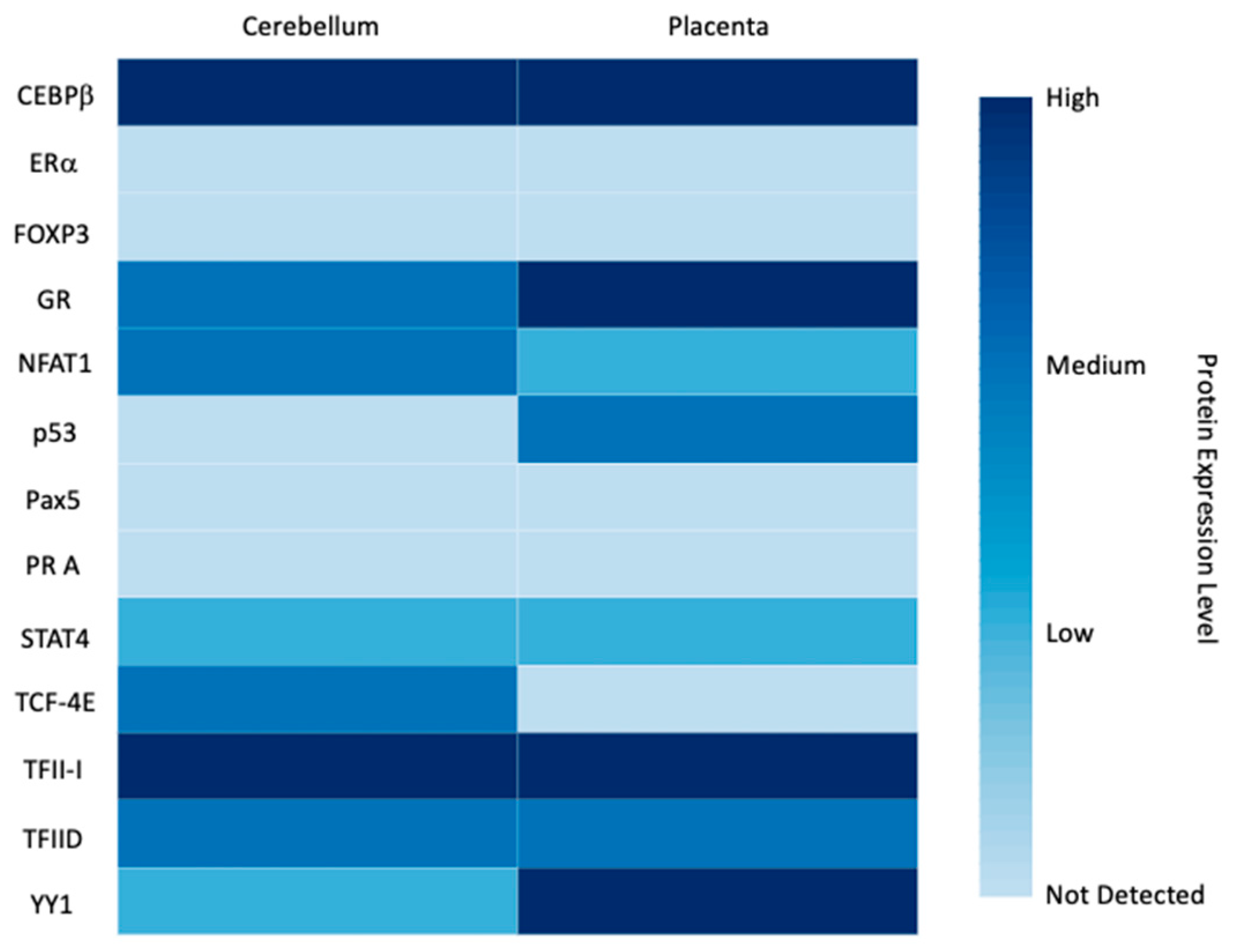

| Names of Cells or Tissues | GEO Accession Number | ACTB | LGALS1 | LGALS16 | Sample Size |
|---|---|---|---|---|---|
| Acetabular labrum cells | GDS5427 a | 12.682 ± 0.150 | 12.057 ± 0.107 | 2.949 ± 0.0093 | 3 |
| Acute lymphoblastic leukemia cell line RS4;11 | GDS4043 b | 13.861 ± 0.017 | 11.487 ± 0.033 | 0.4023 ± 0.607 | 2 |
| Acute myeloblastic leukemia cell line Kasumi-1 | GDS5600 a | 11.965 ± 0.025 | 6.455 ± 0.299 | 2.918 ± 0.036 | 3 |
| Acute promyelocytic leukemia cell line NB4 | GDS4180 a | 13.130 ± 0.035 | 10.823 ± 0.031 | 3.650 ± 0.108 | 3 |
| Adipocyte progenitor cells (subcutaneous) | GDS5171 a | 13.523 ± 0.038 | 13.397 ± 0.112 | 4.597 ± 0.251 | 6 |
| Adipocyte progenitors from deep neck | GDS5171 a | 13.469 ± 0.057 | 13.208 ± 0.177 | 4.505 ± 0.094 | 6 |
| Bone marrow CD34+ cells (chronic myeloid leukemia) | GDS4756 a | 13.524 | 11.137 | 3.050 | 1 |
| Bone marrow plasma cells | GDS4968 a | 11.990 ± 0.226 | 8.714 ± 0.515 | 3.052 ± 0.257 | 5 |
| Brain frontal cortex | GDS4758 a | 13.402 ± 0.125 | 96.333 ± 0.840 | 4.632 ± 0.249 | 18 |
| Brain hippocampus | GDS4758 a | 13.477 ± 0.130 | 11.133 ± 0.375 | 4.659 ± 0.300 | 10 |
| Brain hippocampus | GDS4879 a | 12.113 ± 0.409 | 9.076 ± 0.232 | 3.177 ± 0.177 | 19 |
| Brain temporal cortex | GDS4758 a | 13.560 ± 0.131 | 11.189 ± 0.280 | 4.749 ± 0.193 | 19 |
| Breast cancer cell line MCF-7 | GDS2759 b | 15.884 ± 0.030 | 13.752 ± 0.153 | 6.053 ± 0.237 | 2 |
| Breast cancer cell line MCF-7 | GDS4972 a | 13.029 ± 0.038 | 12.439 ± 0.083 | 3.892 ± 0.066 | 3 |
| Breast cancer cell line MCF-7 | GDS4090 a | 13.087 ± 0.019 | 9.566 ± 0.100 | 2.827 ± 0.405 | 3 |
| Breast cancer cell line MDA-MB-231 | GDS4800 a | 13.875 ± 0.007 | 13.565 ± 0.042 | 5.189 ± 0.085 | 3 |
| Bronchial smooth muscle primary cells | GDS4803 a | 11.629 ± 0.175 | 11.533 ± 0.041 | 3.181 ± 0.095 | 3 |
| Bronchopulmonary neuroendocrine cell line NCI-H727 | GDS4330 a | 11.978 | 5.715 | 3.808 | 1 |
| Burkitt lymphoma cell line Namalwa | GDS4978 a | 13.468 ± 0.187 | 8.005 ± 0.073 | 3.916 ± 0.297 | 3 |
| Burkitt lymphoma cell line Raji | GDS4978 a | 13.367 ± 0.093 | 8.052 ± 0.141 | 3.962 ± 0.019 | 3 |
| Colorectal adenocarcinoma cell line SW620 | GDS5416 e | 16.400 ± 0.362 | 17.280 ± 0.043 | 2.766 ± 0.554 | 2 |
| Embryonic kidney cell line HEK-293 | GDS4233 a | 10.330 ± 0.050 | 7.109 ± 0.098 | 3.757 ± 0.328 | 4 |
| Endothelial progenitor cells | GDS3656 c | 15.397 ± 0.174 | 13.845 ± 0.457 | 8.018 ± 0.103 | 11 |
| Esophagus biopsies | GDS4350 a | 12.617 ± 0.230 | 8.062 ± 0.507 | 3.255 ± 0.208 | 8 |
| Gastrointestinal neuroendocrine cell line KRJ-1 | GDS4330 a | 12.135 | 9.592 | 2.859 | 1 |
| Germinal center B cells | GDS4977 a | 9.793 ± 0.373 | 8.438 ± 0.225 | 6.723 ± 0.538 | 5 |
| Gingival fibroblasts | GDS5811 a | 13.628 ± 0.101 | 13.770 ± 0.174 | 3.674 ± 0.140 | 2 |
| Heart (left ventricle) | GDS4772 a | 11.293 ± 0.361 | 10.672 ± 0.377 | 2.941 ± 0.030 | 5 |
| Heart (left ventricle) | GDS4314 a | 12.142 ± 0.365 | 11.052 ± 0.223 | 3.344 ± 0.154 | 5 |
| Heart (right ventricular) | GDS5610 a | 11.930 ± 0.255 | 10.934 ± 0.044 | 3.637 ± 0.181 | 2 |
| Hepatocellular carcinoma cell line HepG2 | GDS5340 a | 13.259 ± 0.039 | 11.256 ± 0.054 | 4.281 ± 0.327 | 3 |
| Microglia cell line HMO6 | GDS4151 a | 13.545 | 12.231 | 2.979 | 1 |
| Keratinocytes | GDS4426 a | 12.679 ± 0.056 | 11.147 ± 0.236 | 3.804 ± 0.138 | 6 |
| Lung carcinoma cell line A549 | GDS4997 a | 10.970 ± 0.044 | 12.187 ± 0.049 | 2.418 ± 0.072 | 3 |
| Lung carcinoma cell line H460 | GDS5247 a | 12.504 ± 0.043 | 11.111 ± 0.063 | 3.439 ± 0.117 | 3 |
| Lung microvascular endothelial cell line CC-2527 | GDS2987 b | 32,061 ± 7366 | 15,158 ± 2227 | 8.100 ± 9.051 | 2 |
| Lymphoblastoid cell line TK6 | GDS4915 a | 13.365 ± 0.061 | 11.161 ± 0.323 | 4.005 ± 0.327 | 2 |
| Lymphoblastoid cell line TK6 | GDS4916 a | 13.940 ± 0.058 | 12.023 ± 0.130 | 4.061 ± 0.357 | 2 |
| Medulloblastoma tumor tissue | GDS4469 a | 13.099 ± 0.302 | 9.490 ± 0.801 | 4.005 ± 0.839 | 15 |
| Melanoma cell line A-375 | GDS5085 a | 13.888 ± 0.011 | 13.474 ± 0.101 | 4.618 ± 0.045 | 3 |
| Melanoma cell line FEMX-I | GDS3489 d | 16.04 ± 0.354 | 16.04 ± 0.354 | 0.550 ± 1.061 | 2 |
| Melanoma cell line Hs294T | GDS5670 a | 11.353 ± 0.245 | 10.349 ± 0.097 | 2.149 ± 0.585 | 2 |
| Microglia cell line HMO6 | GDS4151 a | 13.545 | 12.231 | 2.979 | 1 |
| Myotubes from musculus obliquus internus | GDS5378 a | 13.224 ± 0.099 | 12.925 ± 0.114 | 2.840 ± 0.057 | 4 |
| Pancreatic neuroendocrine cell line QGP-1 | GDS4330 a | 12.057 | 5.749 | 3.031 | 1 |
| Peripheral blood CD34+ cells (chronic myeloid leukemia) | GDS4756 a | 13.414 ± 0.049 | 11.144 ± 0.578 | 2.974 ± 0.140 | 2 |
| Peripheral blood CD4+ T cells | GDS5544 a | 13.598 ± 0.053 | 9.707 ± 0.247 | 4.584 ± 0.126 | 4 |
| Peripheral blood cells | GDS4240 a | 11.825 ± 0.084 | 7.307 ± 0.154 | 1.506 ± 0.112 | 7 |
| Renal adenocarcinoma cell line 786-O | GDS5810 a | 12.902 ± 0.030 | 12.809 ± 0.015 | 5.753 ± 0.031 | 2 |
| Retinal pigment epithelia primary cells | GDS4224 a | 13.407 ± 0.110 | 11.842 ± 0.449 | 3.468 ± 0.367 | 4 |
| Retinal pigmented epithelium cell line ARPE-19 | GDS4224 a | 13.288 | 11.946 | 3.646 | 1 |
| Skeletal muscle (vastus lateralis) primary cells | GDS4920 a | 13.649 ± 0.084 | 13.385 ± 0.114 | 4.609 ± 0.136 | 12 |
| Skeletal muscle tissue | GDS4841 a | 9.400 ± 0.190 | 11.486 ± 0.247 | 2.786 ± 0.355 | 5 |
| Skin cancer cell line RT3Sb | GDS5381 a | 13.409 ± 0.062 | 8.775 ± 0.114 | 3.539 ± 0.252 | 4 |
| Skin epidermis | GDS3806 c | 15.139 ± 0.141 | 9.534 ± 0.370 | 7.909 ± 0.469 | 7 |
| Visceral adipose tissue (omentum) | GDS4857 a | 11.875 ± 0.352 | 11.488 ± 0.416 | 4.666 ± 0.754 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminker, J.D.; Timoshenko, A.V. Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues. Biomolecules 2021, 11, 1909. https://doi.org/10.3390/biom11121909
Kaminker JD, Timoshenko AV. Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues. Biomolecules. 2021; 11(12):1909. https://doi.org/10.3390/biom11121909
Chicago/Turabian StyleKaminker, Jennifer D., and Alexander V. Timoshenko. 2021. "Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues" Biomolecules 11, no. 12: 1909. https://doi.org/10.3390/biom11121909
APA StyleKaminker, J. D., & Timoshenko, A. V. (2021). Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues. Biomolecules, 11(12), 1909. https://doi.org/10.3390/biom11121909







