4,6,4′-Trimethylangelicin Photoactivated by Blue Light Might Represent an Interesting Option for Photochemotherapy of Non-Invasive Bladder Carcinoma: An In Vitro Study on T24 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Cell Treatments
2.3. Cell Viability and Apoptosis
2.4. Immunofluorescence
2.5. Flow Cytometry
2.6. Western Blot
2.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.8. Statistical Analysis
3. Results
3.1. Antiproliferative Effects and Genotoxicity of TMA/BL
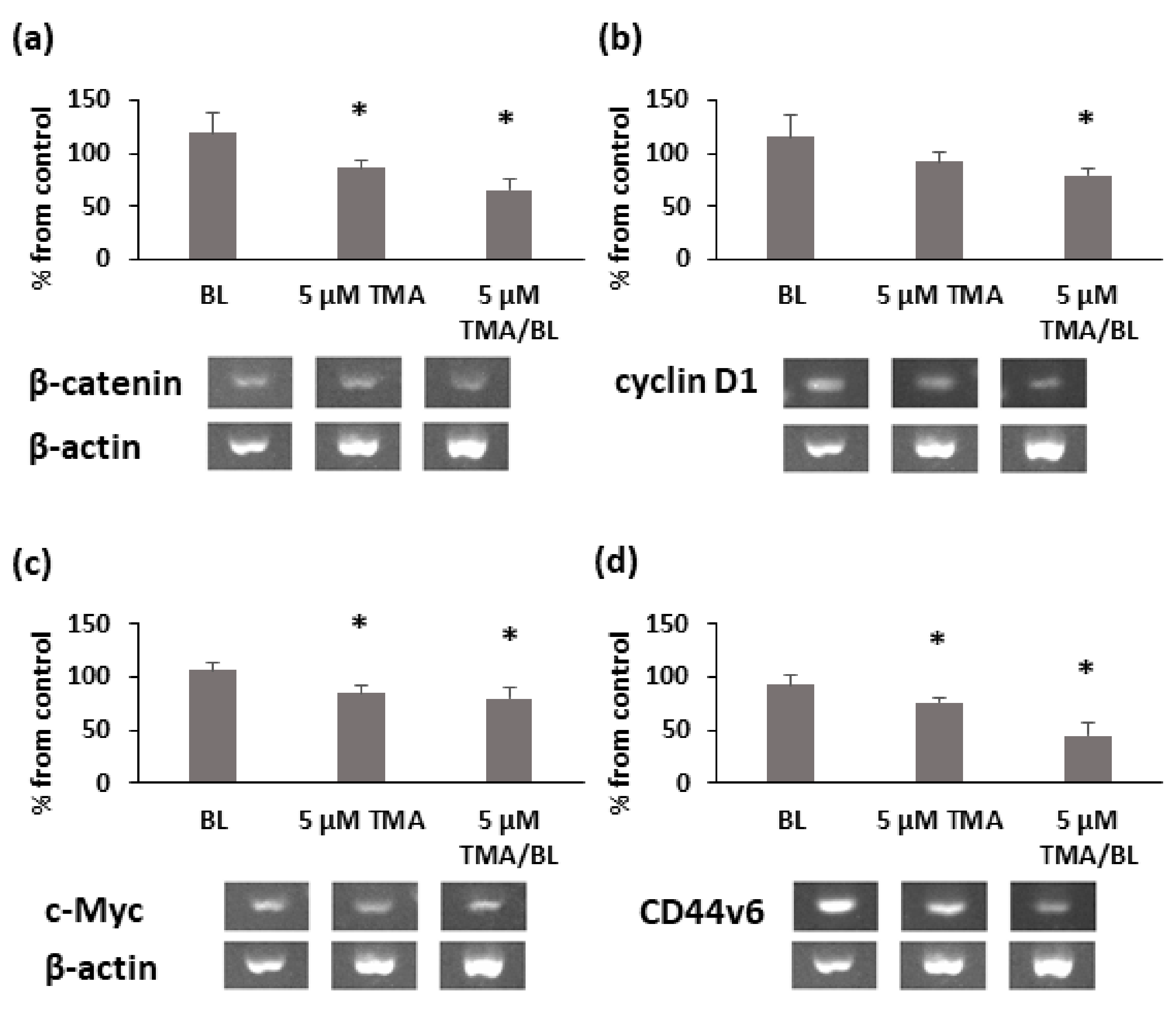
3.2. TMA/BL Affected the Canonical Wnt Pathway
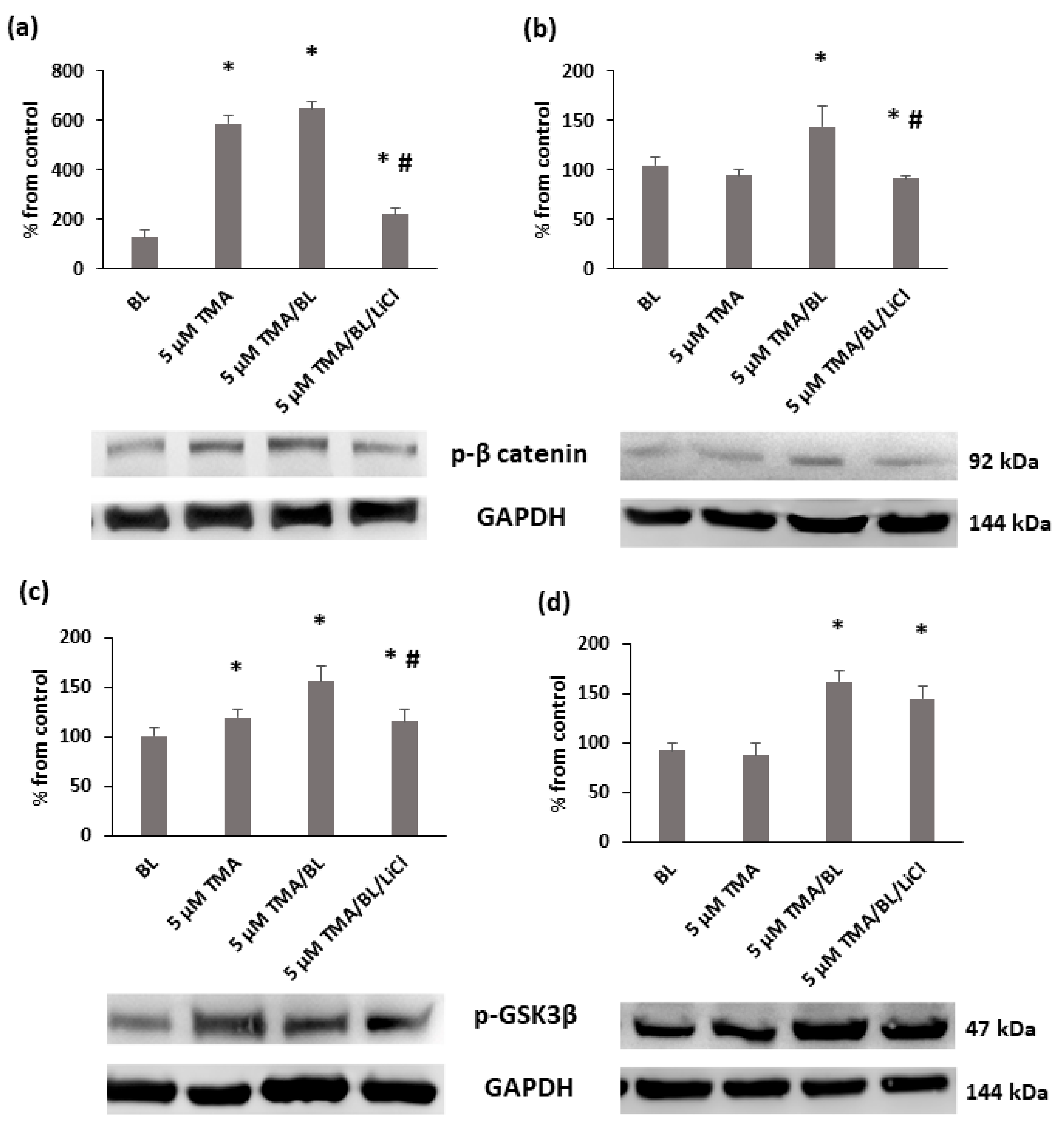
3.3. TMA/BL Affected the Canonical Wnt Pathway through the Modulation of GSK3β
3.4. TMA/BL Affected some Mitogen-Activated Protein Kinases (MAPKs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filonenko, E.V.; Kaprin, A.D.; Alekseev, B.Y.A.; Apolikhin, O.I.; Slovokhodov, E.K.; Ivanova-Radkevich, V.I.; Urlova, A.N. 5-Aminolevulinic acid in intraoperative photodynamic therapy of bladder cancer (results of multicenter trial). Photodiagn. Photodyn. Ther. 2016, 16, 106–109. [Google Scholar] [CrossRef]
- Yang, X.; Palasuberniam, P.; Kraus, D.; Chen, B. Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement. Int. J. Mol. Sci. 2015, 16, 25865–25880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, T.; Ogawa, T.; Yanagihara, C.; Tamai, I. Kinetic Evaluation of Determinant Factors for Cellular Accumulation of Protoporphyrin IX Induced by External 5-Aminolevulinic Acid for Photodynamic Cancer Therapy. J. Pharm. Sci. 2015, 104, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.J.; Pansa, M.F.; Vera, R.E.; Fernández-Zapico, M.E.; Vittar, N.B.R.; Rivarola, V.A. Transcriptional activation of HIF-1 by a ROS-ERK axis underlies the resistance to photodynamic therapy. PLoS ONE 2017, 12, e0177801. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Caffieri, S.; Dall’Acqua, F.; Di Lisa, F. PUVA-induced apoptosis involves mitochondrial dysfunction caused by the opening of the permeability transition pore. FEBS Lett. 2002, 522, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Caffieri, S.; Di Lisa, F.; Bolesani, F.; Facco, M.; Semenzato, G.; Dall’Acqua, F.; Canton, M. The mitochondrial effects of novel apoptogenic molecules generated by psoralen photolysis as a crucial mechanism in PUVA therapy. Blood 2007, 109, 4988–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampronti, I.; Manzione, M.G.; Sacchetti, G.; Ferrari, D.; Spisani, S.; Bezzerri, V.; Finotti, A.; Borgatti, M.; Dechecchi, M.C.; Miolo, G.; et al. Differential Effects of Angelicin Analogues on NF- κ B Activity and IL-8 Gene Expression in Cystic Fibrosis IB3-1 Cells. Mediat. Inflamm. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laselva, O.; Molinski, S.; Casavola, V.; Bear, C.E. The investigational Cystic Fibrosis drug Trimethylangelicin directly modulates CFTR by stabilizing the first membrane-spanning domain. Biochem. Pharmacol. 2016, 119, 85–92. [Google Scholar] [CrossRef]
- Miolo, G.; Sturaro, G.; Cigolini, G.; Menilli, L.; Tasso, A.; Zago, I.; Conconi, M.T. 4,6,4′-trimethylangelicin shows high anti-proliferative activity on DU145 cells under both UVA and blue light. Cell Prolif. 2018, 51, e12430. [Google Scholar] [CrossRef] [Green Version]
- Toyooka, T.; Ishihama, M.; Ibuki, Y. Phosphorylation of Histone H2AX Is a Powerful Tool for Detecting Chemical Photogenotoxicity. J. Investig. Dermatol. 2011, 131, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Bjelland, S. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat. Res. Mol. Mech. Mutagen. 2003, 531, 37–80. [Google Scholar] [CrossRef] [PubMed]
- Gasparro, F.P.; Gattolin, P.; Olack, G.A.; Deckelbaum, L.I.; Sumpio, B.E. The excitation of 8-Methoxypsoralen with visible light: Reversed phase HPLC quantitation of monoadducts and cross-links. Photochem. Photobiol. 1993, 57, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-P.; Junttila, M.R.; Han, J.; Kähäri, V.-M.; Westermarck, J. p38 Mitogen-activated protein kinase pathway suppresses cell survival by inducing dephosphorylation of mitogen-activated protein/extracellular signal-regulated kinase kinase1,2. Cancer Res. 2003, 63, 3473–3477. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and Cancer Metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [Green Version]
- Wolfer, A.; Ramaswamy, S. MYC and Metastasis. Cancer Res. 2011, 71, 2034–2037. [Google Scholar] [CrossRef] [Green Version]
- Morrish, F.; Neretti, N.; Sedivy, J.M.; Hockenbery, D.M. The oncogene c-Myc coordinates regulation of metabolic networks to enable rapid cell cycle entry. Cell Cycle 2008, 7, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Seiler, R.; Thalmann, G.N.; Rotzer, D.; Perren, A.; Fleischmann, A. CCND1/CyclinD1 status in metastasizing bladder cancer: A prognosticator and predictor of chemotherapeutic response. Mod. Pathol. 2014, 27, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Berx, G.; Van Roy, F. The E-cadherin/catenin complex: An important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 2001, 3, 289. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.; Dokken, B. Role of Glycogen Synthase Kinase-3 in Insulin Resistance and Type 2 Diabetes. Curr. Drug Targets 2006, 7, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, N.; Yan, F.; Jin, H.; Zhou, S.; Shi, J.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Duda, P.; Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Martelli, A.M.; Cocco, L.; Ratti, S.; Candido, S.; Libra, M.; Montalto, G.; et al. Targeting GSK3 and Associated Signaling Pathways Involved in Cancer. Cells 2020, 9, 1110. [Google Scholar] [CrossRef]
- Abdul, A.U.R.M.; De Silva, B.; Gary, R.K. The GSK3 kinase inhibitor lithium produces unexpected hyperphosphorylation of β-catenin, a GSK3 substrate, in human glioblastoma cells. Biol. Open 2018, 7, bio030874. [Google Scholar] [CrossRef] [Green Version]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.P.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Schmitt, M.; Metzger, M.; Gradl, D.; Davidson, G.; Orian-Rousseau, V. CD44 functions in Wnt signaling by regulating LRP6 localization and activation. Cell Death Differ. 2015, 22, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Foster, R.; Yang, X.; Feng, Y.; Shen, J.K.; Mankin, H.J.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget 2015, 6, 9313–9326. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc− and Thereby Promotes Tumor Growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Cristofolini, M.; Recchia, G.; Boi, S.; Piscioli, F.; Bordin, F.; Baccichetti, F.; Carlassare, F.; Tamaro, M.; Pani, B.; Babudri, N.; et al. 6-Methylangelicins: New monofunctional photochemotherapeutic agents for psoriasis. Br. J. Dermatol. 1990, 122, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Clingen, P.H.; Wu, J.Y.-H.; Miller, J.; Mistry, N.; Chin, F.; Wynne, P.; Prise, K.M.; Hartley, J.A. Histone H2AX phosphorylation as a molecular pharmacological marker for DNA interstrand crosslink cancer chemotherapy. Biochem. Pharmacol. 2008, 76, 19–27. [Google Scholar] [CrossRef] [PubMed]






| Gene | Primer Sequence | Accession |
|---|---|---|
| β-Actin | F-TGACGTGGACATCCGCAAAG R-TGGAAGGTGGACAGCGAGG | NM_001101 |
| β-Catenin | F-CTTCACCTGACAGATCCAAGTC R-CCTTCCATCCCTTCCTGTTTAG | NM_001904 |
| cyclin D1 | F-GCGGAGGAGAACAAACAGAT R-GAGGGCGGATTGGAAATGA | NM_053056.2 |
| c-myc | F-CTCCACACATCAGCACAACTA R-TGTCCAACTTGACCCTCTTG | NM_002467 |
| CD44v6 | F-TCCAGGCAACTCCTA R-CAGCTGTCCCTGTTG | NM_001202555.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturaro, G.; Tasso, A.; Menilli, L.; Di Liddo, R.; Miolo, G.; Conconi, M.T. 4,6,4′-Trimethylangelicin Photoactivated by Blue Light Might Represent an Interesting Option for Photochemotherapy of Non-Invasive Bladder Carcinoma: An In Vitro Study on T24 Cells. Biomolecules 2021, 11, 158. https://doi.org/10.3390/biom11020158
Sturaro G, Tasso A, Menilli L, Di Liddo R, Miolo G, Conconi MT. 4,6,4′-Trimethylangelicin Photoactivated by Blue Light Might Represent an Interesting Option for Photochemotherapy of Non-Invasive Bladder Carcinoma: An In Vitro Study on T24 Cells. Biomolecules. 2021; 11(2):158. https://doi.org/10.3390/biom11020158
Chicago/Turabian StyleSturaro, Giulio, Alessia Tasso, Luca Menilli, Rosa Di Liddo, Giorgia Miolo, and Maria Teresa Conconi. 2021. "4,6,4′-Trimethylangelicin Photoactivated by Blue Light Might Represent an Interesting Option for Photochemotherapy of Non-Invasive Bladder Carcinoma: An In Vitro Study on T24 Cells" Biomolecules 11, no. 2: 158. https://doi.org/10.3390/biom11020158
APA StyleSturaro, G., Tasso, A., Menilli, L., Di Liddo, R., Miolo, G., & Conconi, M. T. (2021). 4,6,4′-Trimethylangelicin Photoactivated by Blue Light Might Represent an Interesting Option for Photochemotherapy of Non-Invasive Bladder Carcinoma: An In Vitro Study on T24 Cells. Biomolecules, 11(2), 158. https://doi.org/10.3390/biom11020158









