Role of Small GTPase RhoA in DNA Damage Response
Abstract
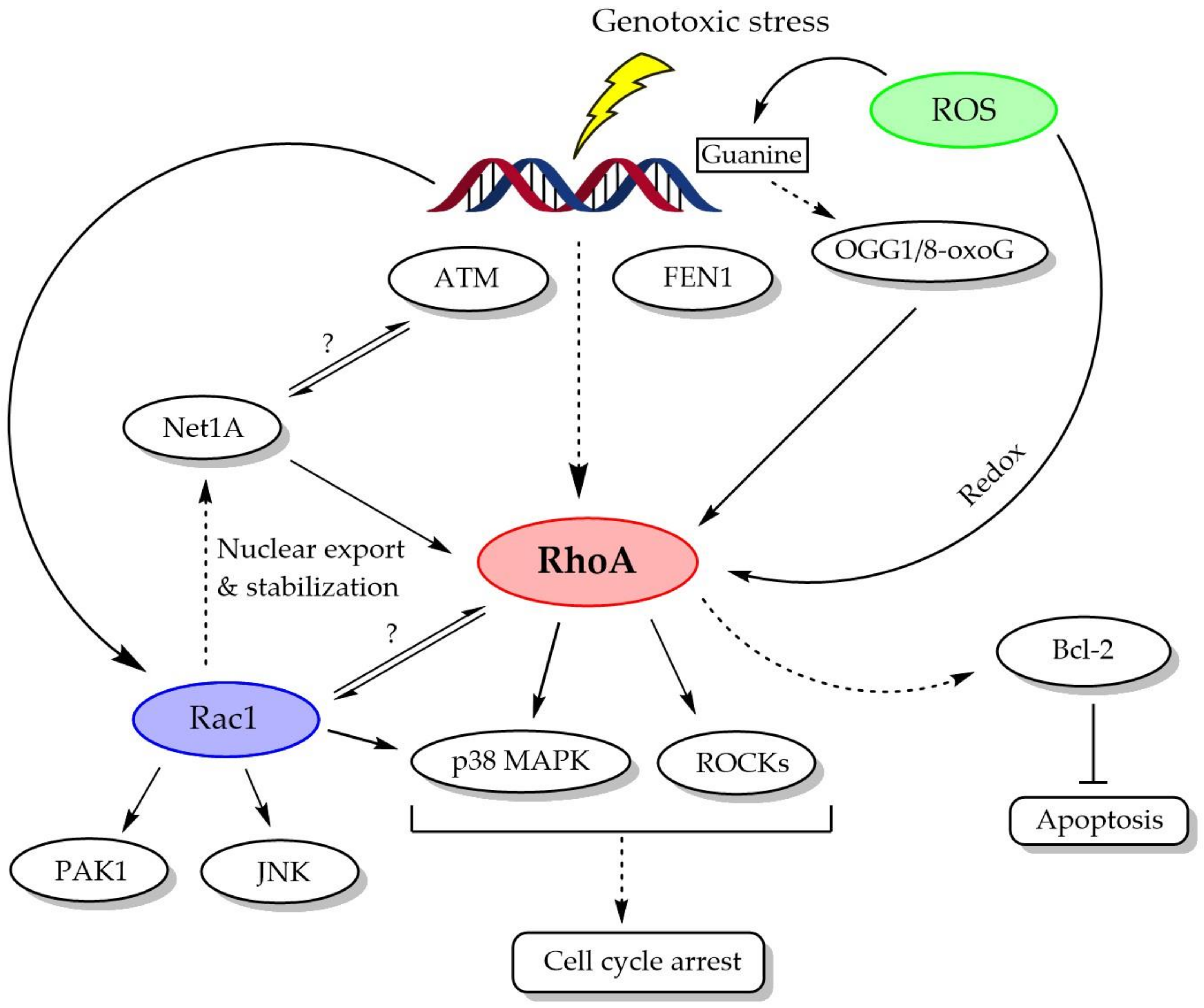
:1. Introduction
2. RhoA Activation in Response to DNA Damage
2.1. ATM and FEN1-Dependent RhoA Activation in DDR
2.2. Intracellular Reactive Oxygen Species (ROS)-Mediated RhoA Activation in DDR
| Mechanism | Cell Line | Genotoxic Agent | Reference |
|---|---|---|---|
| Ataxia telangiectasia-mutated (ATM)-dependent RhoA activation | HeLa, Human fibroblast | Haemophilus ducreyi cytolethal distending toxin (HdCDT), ionizing radiation (IR) | [24] |
| Flap structure-specific endonuclease 1 (FEN1)-dependent RhoA/p38 mitogen-activated protein kinase (MAPK) signaling | HeLa | Cytolethal distending toxin (CDT), IR | [32] |
| Reactive oxygen species (ROS)-mediated RhoA activation | REF-52 fibroblast, Rat2 fibroblast | Peroxide, Antimycin A 1 | [40] |
| 8-Oxoguanine DNA glycosylase-1/8-oxo-7,8-dihydroguanine (OGG1/8-oxoG) complex-mediated RhoA activation | MRC5 2, MEF 3, Mouse lung extract | GOx 4, 8-oxoG | [45] |
3. Rho-Specific GEFs: Net1 and Ect2 in DDR
3.1. Net1 in DDR
3.2. Ect2 in DDR
4. Possible Rac1/RhoA Interaction in DDR
4.1. Rac1 in DDR
4.2. Rac1/RhoA Interaction
4.3. Rac1/RhoA Interaction during DDR May Be Worthy of Special Attention
5. Downstream Pathways of RhoA Activation in DDR
5.1. RhoA and Cell Cycle Arrest in DDR
5.1.1. RhoA/p38 MAPK-Mediated Cell Cycle Arrest in DDR
5.1.2. Rho/ROCK-Mediated Cell Cycle Arrest in DDR
5.1.3. RhoA Interacts with p21 (Waf1/Cip1) and p27 (Kip1) CDK Inhibitors and May Contribute to Cell Cycle Arrest in DDR
5.1.4. RhoA Modulates Cyclin D1 and May Contribute to Cell Cycle Arrest in DDR
5.2. Rho Family Members and Cell Survival Signaling in DDR
5.2.1. RhoA
5.2.2. RhoB
5.2.3. RhoE
6. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATM | Ataxia telangiectasia mutated |
| ATR | Ataxia telangiectasia and rad3-related |
| AMN | Apoptotic microtubule network |
| AP-1 | Activator protein-1 |
| ATF2 | Activating Transcription factor 2 |
| AKT | Activation of PI3K/protein kinase B |
| BER | Base excision repair |
| BRCA | Breast cancer gene |
| BRIP1 | BRCA-1 interacting protein 1 |
| BRCT | BRCA-1 C-terminal |
| Bcl-2 | B-cell lymphoma 2 |
| Bim | Bcl-2 interacting mediator of cell death |
| Cdc42 | Cell division control protein 42 |
| CHK1/2 | Checkpoint kinase 1/2 |
| CDT | Cytolethal distending toxin |
| CRM1 | Chromosome region maintenance 1 |
| CDK | Cyclin-dependent kinase |
| Cox-2 | Cyclooxygenase 2 |
| DDR | DNA damage response |
| DSB | DNA double-strand break |
| Ect2 | Epithelial cell transforming sequence 2 |
| ERK | Extracellular signal-regulated kinase |
| EGF | Epidermal growth factor |
| EMT | Epithelial–mesenchymal transition |
| E2F | E2 promoter binding factor |
| FEN1 | Flap structure-specific endonuclease 1 |
| FA | Fanconi anemia |
| GEF | Guanine exchange factor |
| GAP | GTPase-activating factor |
| GDI | Guanine nucleotide dissociation inhibitor |
| GGPP | Geranylgeranyl pyrophosphate |
| H2AX | H2A histone family member X |
| HRR | Homologous recombination repair |
| HuR | Human antigen R |
| IR | Ionizing radiation |
| INK4 | Inhibitor of cyclin-dependent kinase 4 |
| JNK | c-Jun N-terminal kinase |
| LIMK | LIN-11, Isl1, and MEC-3 protein domain kinase |
| LMWPTP | Low-molecular-weight protein tyrosine phosphatase |
| mDia | Mammalian diaphanous-related formin |
| MLC | Myosin light chain |
| MRN | Mre-Rad50-Nbs1 |
| MAPK | Mitogen-activated protein kinase |
| MK2 | MAPK-activated protein kinase 2 |
| MORC2 | Microrchidia CW-type zinc finger 2 |
| Net1 | Neuroepithelial transforming gene 1 |
| NFκB | Nuclear factor kappa-B |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| NPM | Nucleophosmin |
| NHEJ | Non-homologous end joining |
| NOX | NADPH oxidase |
| OGG1 | 8-Oxoguanine DNA glycosylase-1 |
| 8-oxoG | 8-oxo-7,8-dihydroguanine |
| PAK1/2 | P21-activated kinase 1/2 |
| PIG3 | P53-inducible gene 3 |
| PCNA | Proliferating cell nuclear antigen |
| PI3K | Phosphatidylinositol 3-kinase |
| PIKK | Phosphatidylinositol 3-kinase-related kinase |
| RhoA | Ras homolog gene family member A |
| ROS/RNS | Reactive oxygen species/Reactive nitrogen species |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| ROCK | Rho-associated kinase |
| Rb | Retinoblastoma |
| SRF | Serum response factor |
| SSB | DNA single-strand break |
| SMA | α-smooth muscle actin |
| Smurf1 | Smad ubiquitination regulatory factor 1 |
| ZEB1 | Zinc-finger-enhancer binding protein 1 |
References
- Narumiya, S.; Thumkeo, D. Rho Signaling Research: History, Current Status and Future Directions. FEBS Lett. 2018, 592, 1763–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haga, R.B.; Ridley, A.J. Rho GTPases: Regulation and Roles in Cancer Cell Biology. Small GTPases 2016, 7, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, M.; Ishizaki, T.; Boku, S.; Watanabe, N.; Fujita, A.; Iwamatsu, A.; Obinata, T.; Ohashi, K.; Mizuno, K.; Narumiya, S. Signaling from Rho to the Actin Cytoskeleton Through Protein Kinases ROCK and LIM-Kinase. Science 1999, 285, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Marshall, C.J.; Olson, M.F. RAS and RHO GTPases in G1-Phase Cell-Cycle Regulation. Nat. Rev. Mol. Cell Biol. 2004, 5, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Clayton, N.S.; Ridley, A.J. Targeting Rho GTPase Signaling Networks in Cancer. Front. Cell Dev. Biol. 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Kamai, T.; Yamanishi, T.; Shirataki, H.; Takagi, K.; Asami, H.; Ito, Y.; Yoshida, K.-I. Overexpression of RhoA, Rac1, and Cdc42 GTPases Is Associated with Progression in Testicular Cancer. Clin. Cancer Res. 2004, 10, 4799–4805. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Dou, K.; Xiang, H.; Song, Z.; Zhao, Q.; Chen, Y.; Li, Y. Involvement of RhoA in Progression of Human Hepatocellular Carcinoma. J. Gastroenterol. Hepatol. 2007, 22, 1916–1920. [Google Scholar] [CrossRef]
- Campbell, H.; Fleming, N.; Roth, I.; Mehta, S.; Wiles, A.; Williams, G.; Vennin, C.; Arsic, N.; Parkin, A.; Pajic, M.; et al. Δ133p53 Isoform Promotes Tumour Invasion and Metastasis via Interleukin-6 Activation of JAK-STAT and RhoA-ROCK Signalling. Nat. Commun. 2018, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, S.; Wang, Z.; Wang, F.; Cao, X.; Wu, Q.; Zhao, C.; Ma, H.; Ye, F.; Wang, H.; et al. Supervillin Promotes Epithelial-Mesenchymal Transition and Metastasis of Hepatocellular Carcinoma in Hypoxia via Activation of the RhoA/ROCK-ERK/P38 Pathway. J. Exp. Clin. Cancer Res. 2018, 37, 128. [Google Scholar] [CrossRef]
- Ren, T.; Zheng, B.; Huang, Y.; Wang, S.; Bao, X.; Liu, K.; Guo, W. Osteosarcoma Cell Intrinsic PD-L2 Signals Promote Invasion and Metastasis via the RhoA-ROCK-LIMK2 and Autophagy Pathways. Cell Death Dis. 2019, 10, 261. [Google Scholar] [CrossRef]
- Zuo, Y.; Ulu, A.; Chang, J.T.; Frost, J.A. Contributions of the RhoA Guanine Nucleotide Exchange Factor Net1 to Polyoma Middle T Antigen-Mediated Mammary Gland Tumorigenesis and Metastasis. Breast Cancer Res. 2018, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, G.; Figy, C.; Yeung, M.; Yeung, K.C. Reduced RhoA Expression Enhances Breast Cancer Metastasis with a Concomitant Increase in CCR5 and CXCR4 Chemokines Signaling. Sci. Rep. 2019, 9, 16351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Wu, Y.; Yang, M.; Wang, Z.; Zhang, H.; Jiang, X.; Chen, M.; Jin, T.; Wang, T. IRX5 Promotes Colorectal Cancer Metastasis by Negatively Regulating the Core Components of the RHOA Pathway. Mol. Carcinog. 2019, 58, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Povea-Cabello, S.; Oropesa-Ávila, M.; De la Cruz-Ojeda, P.; Villanueva-Paz, M.; De la Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somlyo, A.P.; Somlyo, A.V. Signal Transduction by G-Proteins, Rho-Kinase and Protein Phosphatase to Smooth Muscle and Non-Muscle Myosin II. J. Physiol. 2000, 522, 177–185. [Google Scholar] [CrossRef]
- Kamijo, K.; Ohara, N.; Abe, M.; Uchimura, T.; Hosoya, H.; Lee, J.-S.; Miki, T. Dissecting the Role of Rho-Mediated Signaling in Contractile Ring Formation. Mol. Biol. Cell 2006, 17, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, C.B.; Wheatley, S.P.; Ahmed, S.; Wang, Y. The Small GTP-Binding Protein Rho Regulates Cortical Activities in Cultured Cells during Division. J. Cell Biol. 1999, 144, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, S.; Oceguera-Yanez, F.; Kato, T.; Okamoto, M.; Yonemura, S.; Terada, Y.; Ishizaki, T.; Narumiya, S. Cdc42 and MDia3 Regulate Microtubule Attachment to Kinetochores. Nature 2004, 428, 767–771. [Google Scholar] [CrossRef]
- Yoshizaki, H.; Ohba, Y.; Parrini, M.-C.; Dulyaninova, N.G.; Bresnick, A.R.; Mochizuki, N.; Matsuda, M. Cell Type-Specific Regulation of RhoA Activity during Cytokinesis. J. Biol. Chem. 2004, 279, 44756–44762. [Google Scholar] [CrossRef] [Green Version]
- Ronco, C.; Martin, A.R.; Demange, L.; Benhida, R. ATM, ATR, CHK1, CHK2 and WEE1 Inhibitors in Cancer and Cancer Stem Cells. Med. Chem. Commun. 2017, 8, 295–319. [Google Scholar] [CrossRef]
- Cw, Z.; Rj, Z.; Ly, X.; Em, L. Rho GTPases: Promising Candidates for Overcoming Chemotherapeutic Resistance. Available online: https://pubmed.ncbi.nlm.nih.gov/31981606/ (accessed on 7 October 2020).
- Guidi, R.; Guerra, L.; Levi, L.; Stenerlöw, B.; Fox, J.G.; Josenhans, C.; Masucci, M.G.; Frisan, T. Chronic Exposure to the Cytolethal Distending Toxins of Gram-Negative Bacteria Promotes Genomic Instability and Altered DNA Damage Response: Bacterial Toxin and Genomic Instability. Cell Microbiol. 2013, 15, 98–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The Multiple Mechanisms That Regulate P53 Activity and Cell Fate. Nat. Rev. Mol. Cell. Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Frisan, T.; Cortes-Bratti, X.; Chaves-Olarte, E.; Stenerlow, B.; Thelestam, M. The Haemophilus Ducreyi Cytolethal Distending Toxin Induces DNA Double-Strand Breaks and Promotes ATM-Dependent Activation of RhoA. Cell Microbiol. 2003, 5, 695–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Jia, J.; Finger, L.D.; Guo, Z.; Zer, C.; Shen, B. Functional Regulation of FEN1 Nuclease and Its Link to Cancer. Nucleic Acids Res. 2011, 39, 781–794. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, L.; Bambara, R.A. Flap Endonuclease 1. Annu. Rev. Biochem. 2013, 82, 119–138. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Zhu, L.; Jiang, X.; Wang, Y.; Wang, Y.; Wang, X.; Chen, B. Curcumin Increases Breast Cancer Cell Sensitivity to Cisplatin by Decreasing FEN1 Expression. Oncotarget 2018, 9, 11268–11278. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Che, X.; Liu, Y.-P.; Qu, X.-J.; Xu, L.; Zhao, C.-Y.; Zheng, C.-L.; Hou, K.-Z.; Teng, Y. FEN1 Knockdown Improves Trastuzumab Sensitivity in Human Epidermal Growth Factor 2-Positive Breast Cancer Cells. Exp. Ther. Med. 2017, 14, 3265–3272. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Liu, R.; Wang, M.; Kumar, A.K.; Pan, F.; He, L.; Hu, Z.; Guo, Z. MicroRNA-140 Impedes DNA Repair by Targeting FEN1 and Enhances Chemotherapeutic Response in Breast Cancer. Oncogene 2020, 39, 234–247. [Google Scholar] [CrossRef]
- He, L.; Luo, L.; Zhu, H.; Yang, H.; Zhang, Y.; Wu, H.; Sun, H.; Jiang, F.; Kathera, C.S.; Liu, L.; et al. FEN1 Promotes Tumor Progression and Confers Cisplatin Resistance in Non-Small-Cell Lung Cancer. Mol. Oncol. 2017, 11, 640–654. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Xiao, Y.; Ma, X.; He, W.; Kang, J.; Peng, Z.; Wang, L.; Li, Z. MiR-193b Increases the Chemosensitivity of Osteosarcoma Cells by Promoting FEN1-Mediated Autophagy. Onco Targets Ther. 2019, 12, 10089–10098. [Google Scholar] [CrossRef] [Green Version]
- Guerra, L.; Guidi, R.; Slot, I.; Callegari, S.; Sompallae, R.; Pickett, C.L.; Astrom, S.; Eisele, F.; Wolf, D.; Sjogren, C.; et al. Bacterial Genotoxin Triggers FEN1-Dependent RhoA Activation, Cytoskeleton Remodeling and Cell Survival. J. Cell Sci. 2011, 124, 2735–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Cui, Y.; Niedernhofer, L.J.; Wang, Y. Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage. Chem. Res. Toxicol. 2016, 29, 2008–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabello-Verrugio, C.; Ruiz-Ortega, M.; Mosqueira, M.; Simon, F. Oxidative Stress in Disease and Aging: Mechanisms and Therapies. Oxid. Med. Cell Longev. 2016, 2016, 8786564. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative Stress and Male Infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Dada, R. Oxidative Stress: Major Executioner in Disease Pathology, Role in Sperm DNA Damage and Preventive Strategies. Front. BioSci. Sch. Ed. 2017, 9, 420–447. [Google Scholar] [CrossRef] [Green Version]
- Houston, B.J.; Nixon, B.; Martin, J.H.; De Iuliis, G.N.; Trigg, N.A.; Bromfield, E.G.; McEwan, K.E.; Aitken, R.J. Heat Exposure Induces Oxidative Stress and DNA Damage in the Male Germ Line. Biol. Reprod. 2018, 98, 593–606. [Google Scholar] [CrossRef]
- Sertan Copoglu, U.; Virit, O.; Hanifi Kokacya, M.; Orkmez, M.; Bulbul, F.; Binnur Erbagci, A.; Semiz, M.; Alpak, G.; Unal, A.; Ari, M.; et al. Increased Oxidative Stress and Oxidative DNA Damage in Non-Remission Schizophrenia Patients. Psychiatry Res. 2015, 229, 200–205. [Google Scholar] [CrossRef]
- Raza, M.U.; Tufan, T.; Wang, Y.; Hill, C.; Zhu, M.-Y. DNA Damage in Major Psychiatric Diseases. Neurotox. Res. 2016, 30, 251–267. [Google Scholar] [CrossRef]
- Aghajanian, A.; Wittchen, E.S.; Campbell, S.L.; Burridge, K. Direct Activation of RhoA by Reactive Oxygen Species Requires a Redox-Sensitive Motif. PLoS ONE 2009, 4, e8045. [Google Scholar] [CrossRef] [Green Version]
- Margolin, Y.; Cloutier, J.-F.; Shafirovich, V.; Geacintov, N.E.; Dedon, P.C. Paradoxical Hotspots for Guanine Oxidation by a Chemical Mediator of Inflammation. Nat. Chem. Biol. 2006, 2, 365–366. [Google Scholar] [CrossRef]
- Mitra, S.; Izumi, T.; Boldogh, I.; Bhakat, K.K.; Hill, J.W.; Hazra, T.K. Choreography of Oxidative Damage Repair in Mammalian Genomes. Free Radic. Biol. Med. 2002, 33, 15–28. [Google Scholar] [CrossRef]
- Nishimura, S. Involvement of Mammalian OGG1(MMH) in Excision of the 8-Hydroxyguanine Residue in DNA. Free Radic. Biol. Med. 2002, 32, 813–821. [Google Scholar] [CrossRef]
- Sassa, A.; Beard, W.A.; Prasad, R.; Wilson, S.H. DNA Sequence Context Effects on the Glycosylase Activity of Human 8-Oxoguanine DNA Glycosylase. J. Biol. Chem. 2012, 287, 36702–36710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Hosoki, K.; Bacsi, A.; Radak, Z.; Hegde, M.L.; Sur, S.; Hazra, T.K.; Brasier, A.R.; Ba, X.; Boldogh, I. 8-Oxoguanine DNA Glycosylase-1-Mediated DNA Repair Is Associated with Rho GTPase Activation and α-Smooth Muscle Actin Polymerization. Free Radic. Biol. Med. 2014, 73, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Boldogh, I.; Hajas, G.; Aguilera-Aguirre, L.; Hegde, M.L.; Radak, Z.; Bacsi, A.; Sur, S.; Hazra, T.K.; Mitra, S. Activation of Ras Signaling Pathway by 8-Oxoguanine DNA Glycosylase Bound to Its Excision Product, 8-Oxoguanine. J. Biol. Chem. 2012, 287, 20769–20773. [Google Scholar] [CrossRef] [Green Version]
- German, P.; Szaniszlo, P.; Hajas, G.; Radak, Z.; Bacsi, A.; Hazra, T.K.; Hegde, M.L.; Ba, X.; Boldogh, I. Activation of Cellular Signaling by 8-Oxoguanine DNA Glycosylase-1-Initiated DNA Base Excision Repair. DNA Repair 2013, 12, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Hajas, G.; Bacsi, A.; Aguilera-Aguirre, L.; Hegde, M.L.; Tapas, K.H.; Sur, S.; Radak, Z.; Ba, X.; Boldogh, I. 8-Oxoguanine DNA Glycosylase-1 Links DNA Repair to Cellular Signaling via the Activation of the Small GTPase Rac1. Free Radic. Biol. Med. 2013, 61, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, A.J.; Golding, S.E.; Khalil, A.; Valerie, K. DNA Double-Strand Break—Induced pro-Survival Signaling. Radiother. Oncol. 2011, 101, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Casey, P.J.; Kumar, A.P.; Pervaiz, S. Deciphering the Signaling Networks Underlying Simvastatin-Induced Apoptosis in Human Cancer Cells: Evidence for Non-Canonical Activation of RhoA and Rac1 GTPases. Cell Death Dis. 2013, 4, e568. [Google Scholar] [CrossRef] [Green Version]
- Chalamalasetty, R.B.; Hümmer, S.; Nigg, E.A.; Silljé, H.H.W. Influence of Human Ect2 Depletion and Overexpression on Cleavage Furrow Formation and Abscission. J. Cell. Sci. 2006, 119, 3008–3019. [Google Scholar] [CrossRef] [Green Version]
- Guerra, L.; Carr, H.S.; Richter-Dahlfors, A.; Masucci, M.G.; Thelestam, M.; Frost, J.A.; Frisan, T. A Bacterial Cytotoxin Identifies the RhoA Exchange Factor Net1 as a Key Effector in the Response to DNA Damage. PLoS ONE 2008, 3, e2254. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Hall, A. The Rho Exchange Factor Net1 Is Regulated by Nuclear Sequestration. J. Biol. Chem. 2002, 277, 14581–14588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bork, P.; Hofmann, K.; Bucher, P.; Neuwald, A.F.; Altschul, S.F.; Koonin, E.V. A Superfamily of Conserved Domains in DNA Damage-Responsive Cell Cycle Checkpoint Proteins. FASEB J. 1997, 11, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srougi, M.C.; Burridge, K. The Nuclear Guanine Nucleotide Exchange Factors Ect2 and Net1 Regulate RhoB-Mediated Cell Death after DNA Damage. PLoS ONE 2011, 6, e17108. [Google Scholar] [CrossRef] [PubMed]
- Dubash, A.D.; Guilluy, C.; Srougi, M.C.; Boulter, E.; Burridge, K.; García-Mata, R. The Small GTPase RhoA Localizes to the Nucleus and Is Activated by Net1 and DNA Damage Signals. PLoS ONE 2011, 6, e17380. [Google Scholar] [CrossRef] [Green Version]
- Dutertre, M.; Gratadou, L.; Dardenne, E.; Germann, S.; Samaan, S.; Lidereau, R.; Driouch, K.; de la Grange, P.; Auboeuf, D. Estrogen Regulation and Physiopathologic Significance of Alternative Promoters in Breast Cancer. Cancer Res. 2010, 70, 3760–3770. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, E.; Vasilaki, E.; Vorvis, C.; Iliopoulos, D.; Moustakas, A.; Kardassis, D.; Stournaras, C. Differential Regulation of the Two RhoA-Specific GEF Isoforms Net1/Net1A by TGF-β and MiR-24: Role in Epithelial-to-Mesenchymal Transition. Oncogene 2012, 31, 2862–2875. [Google Scholar] [CrossRef]
- Menon, S.; Oh, W.; Carr, H.S.; Frost, J.A. Rho GTPase–Independent Regulation of Mitotic Progression by the RhoGEF Net1. MBoC 2013, 24, 2655–2667. [Google Scholar] [CrossRef]
- Carr, H.S.; Morris, C.A.; Menon, S.; Song, E.H.; Frost, J.A. Rac1 Controls the Subcellular Localization of the Rho Guanine Nucleotide Exchange Factor Net1A To Regulate Focal Adhesion Formation and Cell Spreading. Mol. Cell. Biol. 2013, 33, 622–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, W.; Frost, J.A. Rho GTPase Independent Regulation of ATM Activation and Cell Survival by the RhoGEF Net1A. Cell Cycle 2014, 13, 2765–2772. [Google Scholar] [CrossRef] [Green Version]
- Mizuarai, S.; Yamanaka, K.; Kotani, H. Mutant P53 Induces the GEF-H1 Oncogene, a Guanine Nucleotide Exchange Factor-H1 for RhoA, Resulting in Accelerated Cell Proliferation in Tumor Cells. Cancer Res. 2006, 66, 6319–6326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberts, A.S.; Qin, H.; Carr, H.S.; Frost, J.A. PAK1 Negatively Regulates the Activity of the Rho Exchange Factor NET1. J. Biol. Chem. 2005, 280, 12152–12161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, T.; Takaki, T.; Itadani, H.; Kotani, H. RB Silencing Compromises the DNA Damage-Induced G2/M Checkpoint and Causes Deregulated Expression of the ECT2 Oncogene. Oncogene 2007, 26, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, D.R.; Rossman, K.L.; Der, C.J. Rho Guanine Nucleotide Exchange Factors: Regulators of Rho GTPase Activity in Development and Disease. Oncogene 2014, 33, 4021–4035. [Google Scholar] [CrossRef] [Green Version]
- Fields, A.P.; Justilien, V.; Murray, N.R. The Chromosome 3q26 OncCassette: A Multigenic Driver of Human Cancer. Adv. Biol. Regul. Regul. 2016, 60, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Justilien, V.; Fields, A.P. Ect2 Links the PKCι-Par6α Complex to Rac1 Activation and Cellular Transformation. Oncogene 2009, 28, 3597–3607. [Google Scholar] [CrossRef] [Green Version]
- Huff, L.P.; DeCristo, M.J.; Trembath, D.; Kuan, P.F.; Yim, M.; Liu, J.; Cook, D.R.; Miller, C.R.; Der, C.J.; Cox, A.D. The Role of Ect2 Nuclear RhoGEF Activity in Ovarian Cancer Cell Transformation. Genes Cancer 2013, 4, 460–475. [Google Scholar] [CrossRef]
- Saito, S.; Liu, X.-F.; Kamijo, K.; Raziuddin, R.; Tatsumoto, T.; Okamoto, I.; Chen, X.; Lee, C.-C.; Lorenzi, M.V.; Ohara, N.; et al. Deregulation and Mislocalization of the Cytokinesis Regulator ECT2 Activate the Rho Signaling Pathways Leading to Malignant Transformation. J. Biol. Chem. 2004, 279, 7169–7179. [Google Scholar] [CrossRef] [Green Version]
- Scoumanne, A.; Chen, X. The Epithelial Cell Transforming Sequence 2, a Guanine Nucleotide Exchange Factor for Rho GTPases, Is Repressed by P53 via Protein Methyltransferases and Is Required for G1-S Transition. Cancer Res. 2006, 66, 6271–6279. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Tian, P.; Liu, Y. P53 and Protein Phosphorylation Regulate the Oncogenic Role of Epithelial Cell Transforming 2 (ECT2). Med. Sci. Monit. 2017, 23, 3154–3160. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Xiang, J.; Li, B.; Liu, H. The Dynamic Behavior of Ect2 in Response to DNA Damage. Sci. Rep. 2016, 6, 24504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, G.; Kaina, B. Rac1 GTPase, a Multifunctional Player in the Regulation of Genotoxic Stress Response. Cell Cycle 2013, 12, 2521–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, G.; Henninger, C. Rho GTPases: Novel Players in the Regulation of the DNA Damage Response? Biomolecules 2015, 5, 2417–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Yépez, E.A.; Saldívar-Cerón, H.I.; Villamar-Cruz, O.; Pérez-Plasencia, C.; Arias-Romero, L.E. P21 Activated Kinase 1: Nuclear Activity and Its Role during DNA Damage Repair. DNA Repair 2018, 65, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.; Henninger, C.; Schad, A.; Lackner, K.J.; Kaina, B.; Fritz, G. Inhibition of Rac1 Signaling by Lovastatin Protects against Anthracycline-Induced Cardiac Toxicity. Cell Death Dis. 2011, 2, e190. [Google Scholar] [CrossRef]
- Bopp, A.; Wartlick, F.; Henninger, C.; Kaina, B.; Fritz, G. Rac1 Modulates Acute and Subacute Genotoxin-Induced Hepatic Stress Responses, Fibrosis and Liver Aging. Cell Death Dis. 2013, 4, e558. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Moreno, V.; Gaggioli, C.; Yeo, M.; Albrengues, J.; Wallberg, F.; Viros, A.; Hooper, S.; Mitter, R.; Féral, C.C.; Cook, M.; et al. ROCK and JAK1 Signaling Cooperate to Control Actomyosin Contractility in Tumor Cells and Stroma. Cancer Cell 2011, 20, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Tozluoğlu, M.; Tournier, A.L.; Jenkins, R.P.; Hooper, S.; Bates, P.A.; Sahai, E. Matrix Geometry Determines Optimal Cancer Cell Migration Strategy and Modulates Response to Interventions. Nat. Cell Biol. 2013, 15, 751–762. [Google Scholar] [CrossRef]
- Nguyen, L.K.; Kholodenko, B.N.; von Kriegsheim, A. Rac1 and RhoA: Networks, Loops and Bistability. Small Gtpases 2018, 9, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, G.A.; Zhou, B.; Cox, A.D.; Campbell, S.L. Rho GTPases, Oxidation, and Cell Redox Control. Small Gtpases 2014, 5, e28579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taddei, M.L.; Parri, M.; Mello, T.; Catalano, A.; Levine, A.D.; Raugei, G.; Ramponi, G.; Chiarugi, P. Integrin-Mediated Cell Adhesion and Spreading Engage Different Sources of Reactive Oxygen Species. Antioxid. Redox Signal. 2007, 9, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Bokoch, G.M.; Knaus, U.G. NADPH Oxidases: Not Just for Leukocytes Anymore! Trends Biochem. Sci. 2003, 28, 502–508. [Google Scholar] [CrossRef]
- Nimnual, A.S.; Taylor, L.J.; Bar-Sagi, D. Redox-Dependent Downregulation of Rho by Rac. Nat. Cell Biol. 2003, 5, 236–241. [Google Scholar] [CrossRef]
- Jansen, S.; Gosens, R.; Wieland, T.; Schmidt, M. Paving the Rho in Cancer Metastasis: Rho GTPases and Beyond. Pharm. Ther. 2018, 183, 1–21. [Google Scholar] [CrossRef]
- Seo, M.; Cho, C.-H.; Lee, Y.-I.; Shin, E.-Y.; Park, D.; Bae, C.-D.; Lee, J.W.; Lee, E.-S.; Juhnn, Y.-S. Cdc42-Dependent Mediation of UV-Induced P38 Activation by G Protein Βγ Subunits. J. Biol. Chem. 2004, 279, 17366–17375. [Google Scholar] [CrossRef] [Green Version]
- Bagrodia, S.; Dérijard, B.; Davis, R.J.; Cerione, R.A. Cdc42 and PAK-Mediated Signaling Leads to Jun Kinase and P38 Mitogen-Activated Protein Kinase Activation. J. Biol. Chem. 1995, 270, 27995–27998. [Google Scholar] [CrossRef] [Green Version]
- Coso, O.A.; Chiariello, M.; Yu, J.C.; Teramoto, H.; Crespo, P.; Xu, N.; Miki, T.; Gutkind, J.S. The Small GTP-Binding Proteins Rac1 and Cdc42 Regulate the Activity of the JNK/SAPK Signaling Pathway. Cell 1995, 81, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Ulu, A.; Oh, W.; Zuo, Y.; Frost, J.A. Stress-Activated MAPKs and CRM1 Regulate the Subcellular Localization of Net1A to Control Cell Motility and Invasion. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Song, E.H.; Oh, W.; Ulu, A.; Carr, H.S.; Zuo, Y.; Frost, J.A. Acetylation of the RhoA GEF Net1A Controls Its Subcellular Localization and Activity. J. Cell Sci. 2015, 128, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Carr, H.S.; Frost, J.A. Timing Is Everything. Cell Adh. Migr. 2013, 7, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Kang, Y.; Khare, V.; Jin, Z.-Y.; Kang, M.-Y.; Yoon, Y.; Hyun, J.-W.; Chung, M.-H.; Cho, S.-I.; Jun, J.Y.; et al. The P53-Inducible Gene 3 (PIG3) Contributes to Early Cellular Response to DNA Damage. Oncogene 2010, 29, 1431–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herraiz, C.; Calvo, F.; Pandya, P.; Cantelli, G.; Rodriguez-Hernandez, I.; Orgaz, J.L.; Kang, N.; Chu, T.; Sahai, E.; Sanz-Moreno, V. Reactivation of P53 by a Cytoskeletal Sensor to Control the Balance Between DNA Damage and Tumor Dissemination. J. Natl. Cancer Inst. 2015, 108, djv289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The P38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinissen, M.J.; Chiariello, M.; Gutkind, J.S. Regulation of Gene Expression by the Small GTPase Rho through the ERK6 (P38γ) MAP Kinase Pathway. Genes Dev. 2001, 15, 535–553. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, H.C.; Aslanian, A.S.; Lees, J.A.; Yaffe, M.B. P53 Deficient Cells Rely on ATM and ATR-Mediated Checkpoint Signaling through the P38 MAPK/MK2pathway for Survival after DNA Damage. Cancer Cell 2007, 11, 175–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kümper, S.; Mardakheh, F.K.; McCarthy, A.; Yeo, M.; Stamp, G.W.; Paul, A.; Worboys, J.; Sadok, A.; Jørgensen, C.; Guichard, S.; et al. Rho-Associated Kinase (ROCK) Function Is Essential for Cell Cycle Progression, Senescence and Tumorigenesis. eLife 2016, 5, e12203. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple Functions of P21 in Cell Cycle, Apoptosis and Transcriptional Regulation after DNA Damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Croft, D.R.; Crighton, D.; Samuel, M.S.; Lourenco, F.C.; Munro, J.; Wood, J.; Bensaad, K.; Vousden, K.H.; Sansom, O.J.; Ryan, K.M.; et al. P53-Mediated Transcriptional Regulation and Activation of the Actin Cytoskeleton Regulatory RhoC to LIMK2 Signaling Pathway Promotes Cell Survival. Cell Res. 2011, 21, 666–682. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-F.; Takenaka, K.; Nakanishi, A.; Miki, Y. BRCA2 and Nucleophosmin Coregulate Centrosome Amplification and Form a Complex with the Rho Effector Kinase ROCK2. Cancer Res. 2011, 71, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yu, X.; Li, G.; Yuan, R.; Wang, Q.; Tang, P.; Wu, L.; Liu, X.; Peng, X.; Shao, J. Rock2 Regulates Cdc25A through Ubiquitin Proteasome System in Hepatocellular Carcinoma Cells. Exp. Cell Res. 2012, 318, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.-P.; Regner, J.; Theil, A.; Erlmann, P.; Holeiter, G.; Jähne, R.; Schmid, S.; Hausser, A.; Olayioye, M.A. DLC1 Interacts with 14-3-3 Proteins to Inhibit RhoGAP Activity and Block Nucleocytoplasmic Shuttling. J. Cell. Sci. 2009, 122, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Nishimura, D.; Wu, R.-C.; Amano, M.; Iso, T.; Kedes, L.; Nishida, H.; Kaibuchi, K.; Hamamori, Y. Nuclear Rho Kinase, ROCK2, Targets P300 Acetyltransferase. J. Biol. Chem. 2006, 281, 15320–15329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhou, Y.; Wang, K.; Li, T.; Zhang, M.; Yang, Y.; Wang, R.; Hu, R. ROCK2 Confers Acquired Gemcitabine Resistance in Pancreatic Cancer Cells by Upregulating Transcription Factor ZEB1. Cancers 2019, 11, 1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaki, J.H.; Espinha, G.; Magalhaes, Y.T.; Forti, F.L. Modulation of RhoA GTPase Activity Sensitizes Human Cervix Carcinoma Cells to γ -Radiation by Attenuating DNA Repair Pathways. Oxidative Med. Cell. Longev. 2016, 2016, 6012642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Zhou, B.; Liu, X.; Heung, Y.; Chau, J.; Chu, E.; Li, S.; Jiang, C.; Un, F.; Yen, Y. Ribonucleotide Reductase Small Subunit P53R2 Facilitates P21 Induction of G1 Arrest under UV Irradiation. Cancer Res. 2007, 67, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Soria, G.; Speroni, J.; Podhajcer, O.L.; Prives, C.; Gottifredi, V. P21 Differentially Regulates DNA Replication and DNA-Repair-Associated Processes after UV Irradiation. J. Cell. Sci. 2008, 121, 3271–3282. [Google Scholar] [CrossRef] [Green Version]
- Assoian, R.K. Stopping and Going with P27kip1. Dev. Cell 2004, 6, 458–459. [Google Scholar] [CrossRef] [Green Version]
- Larrea, M.D.; Liang, J.; Da Silva, T.; Hong, F.; Shao, S.H.; Han, K.; Dumont, D.; Slingerland, J.M. Phosphorylation of P27Kip1 Regulates Assembly and Activation of Cyclin D1-Cdk4. Mol. Cell. Biol. 2008, 28, 6462–6472. [Google Scholar] [CrossRef] [Green Version]
- Phillips, A.H.; Li, O.; Gay, A.; Besson, A.; Kriwacki, R.W. Mapping Interactions between P27 and RhoA That Stimulate Cell Migration. J. Mol. Biol. 2018, 430, 751–758. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Q.; Xu, F.; Xue, Y.; Zhen, Z.; Deng, Y.; Liu, M.; Chen, J.; Liu, S.; Qiu, M.; et al. RhoA Regulates G1-S Progression of Gastric Cancer Cells by Modulation of Multiple INK4 Family Tumor Suppressors. Mol. Cancer Res. 2009, 7, 570–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liontos, M.; Velimezi, G.; Pateras, I.S.; Angelopoulou, R.; Papavassiliou, A.G.; Bartek, J.; Gorgoulis, V.G. The Roles of P27Kip1 and DNA Damage Signalling in the Chemotherapy-Induced Delayed Cell Cycle Checkpoint. J. Cell Mol. Med. 2010, 14, 2264–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris-Hanon, O.; Furmento, V.A.; Rodríguez-Varela, M.S.; Mucci, S.; Fernandez-Espinosa, D.D.; Romorini, L.; Sevlever, G.E.; Scassa, M.E.; Videla-Richardson, G.A. The Cell Cycle Inhibitors P21Cip1 and P27Kip1 Control Proliferation but Enhance DNA Damage Resistance of Glioma Stem Cells. Neoplasia 2017, 19, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Jirawatnotai, S.; Sittithumcharee, G. Paradoxical Roles of Cyclin D1 in DNA Stability. DNA Repair 2016, 42, 56–62. [Google Scholar] [CrossRef]
- Di Sante, G.; Di Rocco, A.; Pupo, C.; Casimiro, M.C.; Pestell, R.G. Hormone-Induced DNA Damage Response and Repair Mediated by Cyclin D1 in Breast and Prostate Cancer. Oncotarget 2017, 8, 81803–81812. [Google Scholar] [CrossRef]
- Welsh, C.F. Rho GTPases as Key Transducers of Proliferative Signals in G1 Cell Cycle Regulation. Breast Cancer Res. Treat. 2004, 84, 33–42. [Google Scholar] [CrossRef]
- Iordanov, M.S.; Choi, R.J.; Ryabinina, O.P.; Dinh, T.-H.; Bright, R.K.; Magun, B.E. The UV (Ribotoxic) Stress Response of Human Keratinocytes Involves the Unexpected Uncoupling of the Ras-Extracellular Signal-Regulated Kinase Signaling Cascade from the Activated Epidermal Growth Factor Receptor. Mol. Cell. Biol. 2002, 22, 5380–5394. [Google Scholar] [CrossRef] [Green Version]
- Boswell, S.A.; Ongusaha, P.P.; Nghiem, P.; Lee, S.W. The Protective Role of a Small GTPase RhoE against UVB-Induced DNA Damage in Keratinocytes. J. Biol. Chem. 2007, 282, 4850–4858. [Google Scholar] [CrossRef] [Green Version]
- Villalonga, P.; Guasch, R.M.; Riento, K.; Ridley, A.J. RhoE Inhibits Cell Cycle Progression and Ras-Induced Transformation. Mol. Cell. Biol. 2004, 24, 7829–7840. [Google Scholar] [CrossRef] [Green Version]
- Fiorentini, C.; Matarrese, P.; Straface, E.; Falzano, L.; Fabbri, A.; Donelli, G.; Cossarizza, A.; Boquet, P.; Malorni, W. Toxin-Induced Activation of Rho GTP-Binding Protein Increases Bcl-2 Expression and Influences Mitochondrial Homeostasis. Exp. Cell Res. 1998, 242, 341–350. [Google Scholar] [CrossRef]
- Fromigué, O.; Haÿ, E.; Modrowski, D.; Bouvet, S.; Jacquel, A.; Auberger, P.; Marie, P.J. RhoA GTPase Inactivation by Statins Induces Osteosarcoma Cell Apoptosis by Inhibiting P42/P44-MAPKs-Bcl-2 Signaling Independently of BMP-2 and Cell Differentiation. Cell Death Differ. 2006, 13, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- BRIP1 Inhibits the Tumorigenic Properties of Cervical Cancer by Regulating RhoA GTPase Activity—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26870246/ (accessed on 30 December 2020).
- Espinha, G.; Osaki, J.H.; Costa, E.T.; Forti, F.L. Inhibition of the RhoA GTPase Activity Increases Sensitivity of Melanoma Cells to UV Radiation Effects. Oxidative Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.; Choi, J.-H.; Won, M.; Kang, C.-M.; Gyun, M.-R.; Park, H.-M.; Kim, C.-H.; Chung, K.-S. The Activation of P38 MAPK Primarily Contributes to UV-Induced RhoB Expression by Recruiting the c-Jun and P300 to the Distal CCAAT Box of the RhoB Promoter. Biochem. Biophys. Res. Commun. 2011, 409, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.M.; Ridley, A.J. The RhoB Small GTPase in Physiology and Disease. Small Gtpases 2018, 9, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, K.; Wu, Y.; Pervaiz, S. LY303511 Enhances TRAIL Sensitivity of SHEP-1 Neuroblastoma Cells via Hydrogen Peroxide-Mediated Mitogen-Activated Protein Kinase Activation and up-Regulation of Death Receptors. Cancer Res. 2009, 69, 1941–1950. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.H.; Iskandar, K.B.; Yadav, S.K.; Hirpara, J.L.; Loh, T.; Pervaiz, S. Simultaneous Induction of Non-Canonical Autophagy and Apoptosis in Cancer Cells by ROS-Dependent ERK and JNK Activation. PLoS ONE 2010, 5, e9996. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Seen, D.; Zheng, C.; Zeng, R.; Li, E. Role of Small GTPase RhoA in DNA Damage Response. Biomolecules 2021, 11, 212. https://doi.org/10.3390/biom11020212
Cheng C, Seen D, Zheng C, Zeng R, Li E. Role of Small GTPase RhoA in DNA Damage Response. Biomolecules. 2021; 11(2):212. https://doi.org/10.3390/biom11020212
Chicago/Turabian StyleCheng, Chibin, Daniel Seen, Chunwen Zheng, Ruijie Zeng, and Enmin Li. 2021. "Role of Small GTPase RhoA in DNA Damage Response" Biomolecules 11, no. 2: 212. https://doi.org/10.3390/biom11020212
APA StyleCheng, C., Seen, D., Zheng, C., Zeng, R., & Li, E. (2021). Role of Small GTPase RhoA in DNA Damage Response. Biomolecules, 11(2), 212. https://doi.org/10.3390/biom11020212







