TRPM4 in Cancer—A New Potential Drug Target
Abstract
:1. Introduction
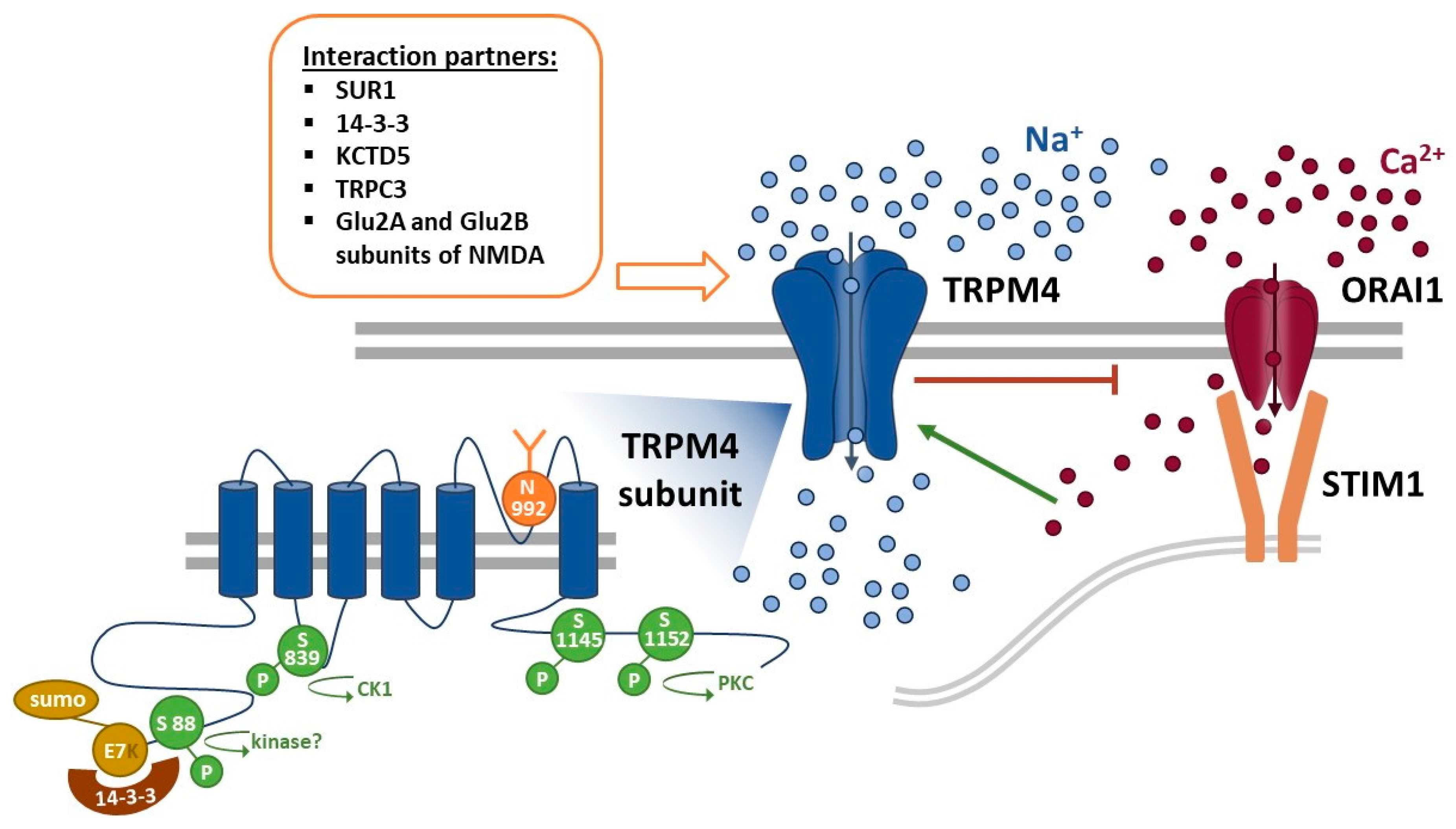
2. General Mechanisms of TRPM4
3. TRPM4 Expression in Cancer
4. Functional Role of TRPM4 in Different Types of Cancer
4.1. Prostate Cancer
4.2. Colorectal Cancer
4.3. Cervical Cancer
4.4. Endometrial Cancer
4.5. Breast Cancer
4.6. Other Cancers
5. Potential as a Drug Target
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Launay, P.; Fleig, A.; Perraud, A.-L.; Scharenberg, A.M.; Penner, R.; Kinet, J.-P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002, 109, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Guinamard, R.; Sallé, L.; Simard, C. The Non-selective Monovalent Cationic Channels TRPM4 and TRPM5. Adv. Exp. Med. Biol. 2011, 704, 147–171. [Google Scholar] [CrossRef]
- Vennekens, R.; Nilius, B. Insights into TRPM4 Function, Regulation and Physiological Role. In Botulinum Toxin Therapy; Springer Nature: Berlin, Germany, 2007; pp. 269–285. [Google Scholar]
- Autzen, H.E.; Myasnikov, A.; Campbell, M.G.; Asarnow, D.; Julius, D.; Cheng, Y. Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 2018, 359, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Li, Z.; Li, J.; Santa-Cruz, A.; Sanchez-Martinez, S.; Zhang, J.; Clapham, D.E. Structure of full-length human TRPM4. Proc. Natl. Acad. Sci. USA 2018, 115, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; She, J.; Zeng, W.; Chen, Q.; Bai, X.; Jiang, Y. Structures of the calcium-activated, non-selective cation channel TRPM4. Nat. Cell Biol. 2017, 552, 205–209. [Google Scholar] [CrossRef]
- Winkler, P.A.; Huang, Y.; Sun, W.; Du, J.; Lü, W. Electron cryo-microscopy structure of a human TRPM4 channel. Nat. Cell Biol. 2017, 552, 200–204. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Tang, J.; Wang, C.; Owsianik, G.; Janssens, A.; Voets, T.; Zhu, M.X. Regulation of the Ca2+ Sensitivity of the Nonselective Cation Channel TRPM4. J. Biol. Chem. 2005, 280, 6423–6433. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Okawa, H.; Wang, Y.; Liman, E.R. Phosphatidylinositol 4,5-Bisphosphate Rescues TRPM4 Channels from Desensitization. J. Biol. Chem. 2005, 280, 39185–39192. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Prenen, J.; Droogmans, G.; Voets, T.; Vennekens, R.; Freichel, M.; Wissenbach, U.; Flockerzi, V. Voltage Dependence of the Ca2+-activated Cation Channel TRPM4. J. Biol. Chem. 2003, 278, 30813–30820. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.S.; Hildner, K.; Murphy, K.M.; Allen, P.M. Trpm4 differentially regulates Th1 and Th2 function by altering calcium signaling and NFAT localization. J. Immunol. 2010, 185, 2836–2846. [Google Scholar] [CrossRef]
- Barbet, G.; Demion, M.; Moura, I.C.; Serafini, N.; Léger, T.; Vrtovsnik, F.; Monteiro, R.C.; Guinamard, R.; Kinet, J.-P.; Launay, P. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat. Immunol. 2008, 9, 1148–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vennekens, R.; Olausson, J.; Meissner, M.; Bloch, W.; Mathar, I.; Philipp, E.S.; Schmitz, F.; Weissgerber, P.; Nilius, B.; Flockerzi, V.; et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat. Immunol. 2007, 8, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Earley, S.; Waldron, B.J.; Brayden, J.E. Critical Role for Transient Receptor Potential Channel TRPM4 in Myogenic Constriction of Cerebral Arteries. Circ. Res. 2004, 95, 922–929. [Google Scholar] [CrossRef] [Green Version]
- Kruse, M.; Schulze-Bahr, E.; Corfield, V.; Beckmann, A.; Stallmeyer, B.; Kurtbay, G.; Ohmert, I.; Schulze-Bahr, E.; Brink, P.; Pongs, O. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J. Clin. Investig. 2009, 119, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; El Zein, L.; Kruse, M.; Guinamard, R.; Beckmann, A.; Bozio, A.; Kurtbay, G.; Mégarbané, A.; Ohmert, I.; Blaysat, G.; et al. Gain-of-Function Mutations in TRPM4 Cause Autosomal Dominant Isolated Cardiac Conduction Disease. Circ. Cardiovasc. Genet. 2010, 3, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Mathar, I.; Kecskes, M.; Van Der Mieren, G.; Jacobs, G.; Londoño, J.E.C.; Uhl, S.; Flockerzi, V.; Voets, T.; Freichel, M.; Nilius, B.; et al. Increased β-Adrenergic Inotropy in Ventricular Myocardium From Trpm4 −/− Mice. Circ. Res. 2014, 114, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathar, I.; Vennekens, R.; Meissner, M.; Kees, F.; Van Der Mieren, G.; Londoño, J.E.C.; Uhl, S.; Voets, T.; Hummel, B.; Bergh, A.V.D.; et al. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J. Clin. Investig. 2010, 120, 3267–3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Chatel, S.; Simard, C.; Syam, N.; Salle, L.; Probst, V.; Morel, J.; Millat, G.; Lopez, M.; Abriel, H.; et al. Molecular Genetics and Functional Anomalies in a Series of 248 Brugada Cases with 11 Mutations in the TRPM4 Channel. PLoS ONE 2013, 8, e54131. [Google Scholar] [CrossRef] [Green Version]
- Stallmeyer, B.; Zumhagen, S.; Denjoy, I.; Duthoit, G.; Hébert, J.-L.; Ferrer, X.; Maugenre, S.; Schmitz, W.; Kirchhefer, U.; Schulze-Bahr, E.; et al. Mutational spectrum in the Ca2+-activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum. Mutat. 2011, 33, 109–117. [Google Scholar] [CrossRef]
- Abriel, H.; Syam, N.; Sottas, V.; Amarouch, M.Y.; Rougier, J.-S. TRPM4 channels in the cardiovascular system: Physiology, pathophysiology, and pharmacology. Biochem. Pharmacol. 2012, 84, 873–881. [Google Scholar] [CrossRef]
- Kecskés, M.; Jacobs, G.; Kerselaers, S.; Syam, N.; Menigoz, A.; Vangheluwe, P.; Freichel, M.; Flockerzi, V.; Voets, T.; Vennekens, R. The Ca2+-activated cation channel TRPM4 is a negative regulator of angiotensin II-induced cardiac hypertrophy. Basic Res. Cardiol. 2015, 110, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Piao, H.; Takahashi, K.; Yamaguchi, Y.; Wang, C.; Liu, K.; Naruse, K. Transient Receptor Potential Melastatin-4 Is Involved in Hypoxia-Reoxygenation Injury in the Cardiomyocytes. PLoS ONE 2015, 10, e0121703. [Google Scholar] [CrossRef] [PubMed]
- Launay, P.; Cheng, H.; Srivatsan, S.; Penner, R.; Fleig, A.; Kinet, J.-P. TRPM4 regulates calcium oscillations after T cell activation. Science 2004, 306, 1374–1377. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Owsianik, G.; Freichel, M.; Flockerzi, V.; Nilius, B.; Vennekens, R. TRPM4 regulates migration of mast cells in mice. Cell Calcium 2009, 45, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Schattling, B.; Steinbach, K.; Thies, E.; Kruse, M.; Menigoz, A.; Ufer, F.; Flockerzi, V.; Brück, W.; Pongs, O.; Vennekens, R.; et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 2012, 18, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Kahle, K.T.; Gerzanich, V. Molecular mechanisms of microvascular failure in central nervous system injury—synergistic roles of NKCC1 and SUR1/TRPM4. J. Neurosurg. 2010, 113, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Gerzanich, V.; Woo, S.K.; Vennekens, R.; Tsymbalyuk, O.; Ivanova, S.; Ivanov, A.R.; Geng, Z.; Chen, Z.; Nilius, B.; Flockerzi, V.; et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat. Med. 2009, 15, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Recasens, G.; Butnaru, C.M.; Brouwers, N.; Mitrovic, S.; Valverde, M.A.; Malhotra, V. Sodium channel TRPM4 and sodium/calcium exchangers (NCX) cooperate in the control of Ca2+-induced mucin secretion from goblet cells. J. Biol. Chem. 2019, 294, 816–826. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Beck, A.; Launay, P.; Gross, S.A.; Stokes, A.; Kinet, J.-P.; Fleig, A.; Penner, R. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium 2007, 41, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Kappel, S.; Stokłosa, P.; Hauert, B.; Kaschitza, D.R.; Borgström, A.; Baur, R.; Galván, J.A.; Zlobec, I.; Peinelt, C. TRPM4 is highly expressed in human colorectal tumor buds and contributes to proliferation, cell cycle, and invasion of colorectal cancer cells. Mol. Oncol. 2019, 13, 2393–2405. [Google Scholar] [CrossRef]
- Loo, S.K.; Ch’Ng, E.S.; Salleh, S.M.; Banham, A.H.; Pedersen, L.M.; Møller, M.B.; Green, T.M.; Wong, K.K. TRPM4 expression is associated with activated B cell subtype and poor survival in diffuse large B cell lymphoma. Histopathology 2017, 71, 98–111. [Google Scholar] [CrossRef]
- Sagredo, A.I.; Sagredo, E.; Pola, V.; Echeverría, C.; Andaur, R.; Michea, L.; Stutzin, A.; Simon, F.; Marcelain, K.; Armisén, R. TRPM4 channel is involved in regulating epithelial to mesenchymal transition, migration, and invasion of prostate cancer cell lines. J. Cell. Physiol. 2019, 234, 2037–2050. [Google Scholar] [CrossRef]
- Sagredo, A.I.; Sagredo, E.; Cappelli, C.; Báez, P.; Andaur, R.; Blanco, C.; Tapia, J.C.; Echeverría, C.; Cerda, O.; Stutzin, A.; et al. TRPM4 regulates Akt/GSK3-β activity and enhances β-catenin signaling and cell proliferation in prostate cancer cells. Mol. Oncol. 2017, 12, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Armisén, R.; Marcelain, K.; Simon, F.; Tapia, J.C.; Toro, J.; Quest, A.F.; Stutzin, A. TRPM4 enhances cell proliferation through up-regulation of the β-catenin signaling pathway. J. Cell. Physiol. 2010, 226, 103–109. [Google Scholar] [CrossRef]
- Hong, X.; Yu, J.-J. MicroRNA-150 suppresses epithelial-mesenchymal transition, invasion, and metastasis in prostate cancer through the TRPM4-mediated β-catenin signaling pathway. Am. J. Physiol. Physiol. 2019, 316, C463–C480. [Google Scholar] [CrossRef]
- Narayan, G.; Bourdon, V.; Chaganti, S.; Arias-Pulido, H.; Nandula, S.V.; Rao, P.H.; Gissmann, L.; Dürst, M.; Schneider, A.; Pothuri, B.; et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: Identification of candidate amplified and overexpressed genes. Genes Chromosom. Cancer 2007, 46, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Rivas, J.; Díaz, N.; Silva, I.; Morales, D.; Lavanderos, B.; Álvarez, A.; Saldías, M.P.; Pulgar, E.; Cruz, P.; Maureira, D.; et al. KCTD5, a novel TRPM4-regulatory protein required for cell migration as a new predictor for breast cancer prognosis. FASEB J. 2020, 34, 7847–7865. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.K.; Hussain, F.A. TRPM4 is overexpressed in breast cancer associated with estrogen response and epithelial-mesenchymal transition gene sets. PLoS ONE 2020, 15, e0233884. [Google Scholar] [CrossRef]
- Holzmann, C.; Kappel, S.; Kilch, T.; Jochum, M.M.; Urban, S.K.; Jung, V.; Stöckle, M.; Rother, K.; Greiner, M.; Peinelt, C. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget 2015, 6, 41783–41793. [Google Scholar] [CrossRef]
- Fleig, A.; Penner, R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol. Sci. 2004, 25, 633–639. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Ouadid-Ahidouch, H.; Skryma, R.; Shuba, Y. Remodelling of Ca2+ transport in cancer: How it contributes to cancer hallmarks? Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S. The store-operated calcium channels in cancer metastasis from cell migration invasion to metastatic colonization. Front. Biosci. 2018, 23, 1241–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautier, M.; Trebak, M.; Fleig, A.; Vandier, C.; Ouadid-Ahidouch, H. Ca2+ channels in cancer. Cell Calcium 2019, 84, 102083. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Bakowski, D.; Mirams, G.R.; Parekh, A.B. Selective recruitment of different Ca2+-dependent transcription factors by STIM1-Orai1 channel clusters. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Lin, Y.-P.; Kramer, H.; Parekh, A.B. Single-nucleotide polymorphisms in Orai1 associated with atopic dermatitis inhibit protein turnover, decrease calcium entry and disrupt calcium-dependent gene expression. Hum. Mol. Genet. 2019, 29, 1808–1823. [Google Scholar] [CrossRef] [PubMed]
- Frischauf, I.; Zayats, V.; Deix, M.; Hochreiter, A.; Jardin, I.; Muik, M.; Lackner, B.; Svobodová, B.; Pammer, T.; Litviňuková, M.; et al. A calcium-accumulating region, CAR, in the channel Orai1 enhances Ca2+ permeation and SOCE-induced gene transcription. Sci. Signal. 2015, 8, ra131. [Google Scholar] [CrossRef] [Green Version]
- Frischauf, I.; Litviňuková, M.; Schober, R.; Zayats, V.; Svobodová, B.; Bonhenry, D.; Lunz, V.; Cappello, S.; Tociu, L.; Reha, D.; et al. Transmembrane helix connectivity in Orai1 controls two gates for calcium-dependent transcription. Sci. Signal. 2017, 10, eaao0358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samakai, E.; Hooper, R.; Martin, K.A.; Shmurak, M.; Zhang, Y.; Kappes, D.J.; Tempera, I.; Soboloff, J. Novel STIM1-dependent control of Ca2+ clearance regulates NFAT activity during T-cell activation. FASEB J. 2016, 30, 3878–3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flourakis, M.; Lehen’Kyi, V.; Beck, B.; Raphael, M.; Vandenberghe, M.; Van Denabeele, F.; Roudbaraki, M.; Lepage, G.; Mauroy, B.; Romanin, C.; et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010, 1, e75. [Google Scholar] [CrossRef] [Green Version]
- Schober, R.; Bonhenry, D.; Lunz, V.; Zhu, J.; Tiffner, A.; Frischauf, I.; Fahrner, M.; Zhang, M.; Waldherr, L.; Schmidt, T.; et al. Sequential activation of STIM1 links Ca2+ with luminal domain unfolding. Sci. Signal. 2019, 12, eaax3194. [Google Scholar] [CrossRef] [PubMed]
- Faris, P.; Ferulli, F.; Vismara, M.; Tanzi, M.; Negri, S.; Rumolo, A.; Lefkimmiatis, K.; Maestri, M.; Shekha, M.; Pedrazzoli, P.; et al. Hydrogen Sulfide-Evoked Intracellular Ca2+ Signals in Primary Cultures of Metastatic Colorectal Cancer Cells. Cancers 2020, 12, 3338. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef] [PubMed]
- Almasi, S.; El Hiani, Y. Exploring the Therapeutic Potential of Membrane Transport Proteins: Focus on Cancer and Chemoresistance. Cancers 2020, 12, 1624. [Google Scholar] [CrossRef]
- Tran, T.N.; Stovall, K.; Suantawee, T.; Hu, Y.; Yao, S.; Yang, L.-J.; Adisakwattana, S.; Cheng, H. Transient receptor potential melastatin 4 channel is required for rat dental pulp stem cell proliferation and survival. Cell Prolif. 2017, 50, e12360. [Google Scholar] [CrossRef]
- Serafini, N.; Dahdah, A.; Barbet, G.; Demion, M.; Attout, T.; Gautier, G.; Arcos-Fajardo, M.; Souchet, H.; Jouvin, M.-H.; Vrtovsnik, F.; et al. The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J. Immunol. 2012, 189, 3689–3699. [Google Scholar] [CrossRef]
- Fliegert, R.; Glassmeier, G.; Schmid, F.; Cornils, K.; Genisyuerek, S.; Harneit, A.; Schwarz, J.R.; Guse, A.H. Modulation of Ca2+ entry and plasma membrane potential by human TRPM4b. FEBS J. 2007, 274, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, D.; Montorfano, I.; Cerda, O.; Cáceres, M.; Becerra, A.; Cabello-Verrugio, C.; Elorza, A.A.; Riedel, C.; Tapia, P.; Velasquez, L.; et al. Increases in reactive oxygen species enhance vascular endothelial cell migration through a mechanism dependent on the transient receptor potential melastatin 4 ion channel. Microvasc. Res. 2015, 98, 187–196. [Google Scholar] [CrossRef]
- Cáceres, M.; Ortiz, L.; Recabarren, T.; Romero, A.; Colombo, A.; Leiva-Salcedo, E.; Varela, D.; Rivas, J.; Silva, I.; Morales, D.; et al. TRPM4 Is a Novel Component of the Adhesome Required for Focal Adhesion Disassembly, Migration and Contractility. PLoS ONE 2015, 10, e0130540. [Google Scholar] [CrossRef]
- Blanco, C.; Morales, D.; Mogollones, I.; Vergara-Jaque, A.; Vargas, C.; Álvarez, A.; Riquelme, D.; Leiva-Salcedo, E.; González, W.; Morales, D.; et al. EB1- and EB2-dependent anterograde trafficking of TRPM4 regulates focal adhesion turnover and cell invasion. FASEB J. 2019, 33, 9434–9452. [Google Scholar] [CrossRef] [Green Version]
- Hempel, N.; Trebak, M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium 2017, 63, 70–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Gibhardt, C.S.; Will, T.; Stanisz, H.; Körbel, C.; Mitkovski, M.; Stejerean, I.; Cappello, S.; Pacheu-Grau, D.; Dudek, J.; et al. Redox signals at theER–mitochondria interface control melanoma progression. EMBO J. 2019, 38, e100871. [Google Scholar] [CrossRef]
- Gibhardt, C.S.; Cappello, S.; Bhardwaj, R.; Schober, R.; Kirsch, S.A.; Del Rio, Z.B.; Gahbauer, S.; Bochicchio, A.; Sumanska, M.; Ickes, C.; et al. Oxidative Stress-Induced STIM2 Cysteine Modifications Suppress Store-Operated Calcium Entry. Cell Rep. 2020, 33, 108292. [Google Scholar] [CrossRef] [PubMed]
- Bogeski, I.; Kummerow, C.; Al-Ansary, D.; Schwarz, E.C.; Koehler, R.; Kozai, D.; Takahashi, N.; Peinelt, C.; Griesemer, D.; Bozem, M.; et al. Differential Redox Regulation of ORAI Ion Channels: A Mechanism to Tune Cellular Calcium Signaling. Sci. Signal. 2010, 3, ra24. [Google Scholar] [CrossRef] [Green Version]
- Holzmann, C.; Kilch, T.; Kappel, S.; Armbrüster, A.; Jung, V.; Stöckle, M.; Bogeski, I.; Schwarz, E.C.; Peinelt, C. ICRAC controls the rapid androgen response in human primary prostate epithelial cells and is altered in prostate cancer. Oncotarget 2013, 4, 2096–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzmann, C.; Kilch, T.; Kappel, S.; Dörr, K.; Jung, V.; Stöckle, M.; Bogeski, I.; Peinelt, C. Differential Redox Regulation of Ca2+ Signaling and Viability in Normal and Malignant Prostate Cells. Biophys. J. 2015, 109, 1410–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saul, S.; Gibhardt, C.S.; Schmidt, B.; Lis, A.; Pasieka, B.; Conrad, D.; Jung, P.; Gaupp, R.; Wonnenberg, B.; Diler, E.; et al. A calcium-redox feedback loop controls human monocyte immune responses: The role of ORAI Ca2+ channels. Sci. Signal. 2016, 9, ra26. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, H.; Saul, S.; Müller, C.S.L.; Kappl, R.; Niemeyer, B.A.; Vogt, T.; Hoth, M.; Roesch, A.; Bogeski, I. Inverse regulation of melanoma growth and migration by Orai1/STIM2-dependent calcium entry. Pigment. Cell Melanoma Res. 2014, 27, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Benzerdjeb, N.; Sevestre, H.; Ahidouch, A.; Ouadid-Ahidouch, H. Orai3 is a predictive marker of metastasis and survival in resectable lung adenocarcinoma. Oncotarget 2016, 7, 81588–81597. [Google Scholar] [CrossRef] [Green Version]
- Hasna, J.; Hague, F.; Rodat-Despoix, L.; Geerts, D.; Leroy, C.; Tulasne, D.; Ouadid-Ahidouch, H.; Kischel, P. Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: The p53 connection. Cell Death Differ. 2018, 25, 693–707. [Google Scholar] [CrossRef]
- Yoast, R.E.; Emrich, S.M.; Zhang, X.; Xin, P.; Johnson, M.T.; Fike, A.J.; Walter, V.; Hempel, N.; Yule, D.I.; Sneyd, J.; et al. The native ORAI channel trio underlies the diversity of Ca2+ signaling events. Nat. Commun. 2020, 11, 2444. [Google Scholar] [CrossRef] [PubMed]
- Motiani, R.K.; Zhang, X.; Harmon, K.E.; Keller, R.S.; Matrougui, K.; Bennett, J.A.; Trebak, M. Orai3 is an estrogen receptor α-regulated Ca2+ channel that promotes tumorigenesis. FASEB J. 2013, 27, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobradillo, D.; Hernández-Morales, M.; Ubierna, D.; Moyer, M.P.; Núñez, L.; Villalobos, C. A Reciprocal Shift in Transient Receptor Potential Channel 1 (TRPC1) and Stromal Interaction Molecule 2 (STIM2) Contributes to Ca2+ Remodeling and Cancer Hallmarks in Colorectal Carcinoma Cells. J. Biol. Chem. 2014, 289, 28765–28782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Shen, Q.; Zhang, S.; Huang, H.; Meng, X.; Zheng, X.; Yao, Z.; He, Z.; Lu, S.; Cai, C.; et al. Calcium-sensing stromal interaction molecule 2 upregulates nuclear factor of activated T cells 1 and transforming growth factor-β signaling to promote breast cancer metastasis. Breast Cancer Res. 2019, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Miao, Y.; Zheng, X.; Gong, Y.; Zhang, J.; Zou, F.; Cai, C. STIM1 and STIM2 differently regulate endogenous Ca2+ entry and promote TGF-β-induced EMT in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 488, 74–80. [Google Scholar] [CrossRef]
- Sanchez-Collado, J.; Lopez, J.J.; Gonzalez-Gutierrez, L.; Cantonero, C.; Jardin, I.; Salido, G.M.; Rosado, J.A. Functional role of TRPC6 and STIM2 in cytosolic and endoplasmic reticulum Ca2+ content in resting estrogen receptor-positive breast cancer cells. Biochem. J. 2020, 477, 3183–3197. [Google Scholar] [CrossRef]
- Faouzi, M.; Kischel, P.; Hague, F.; Ahidouch, A.; Benzerdjeb, N.; Sevestre, H.; Penner, R.; Ouadid-Ahidouch, H. ORAI3 silencing alters cell proliferation and cell cycle progression via c-myc pathway in breast cancer cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1833, 752–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faouzi, M.; Hague, F.; Potier, M.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J. Cell. Physiol. 2010, 226, 542–551. [Google Scholar] [CrossRef]
- Staff, T.P.O. Correction: Orai3 Constitutes a Native Store-Operated Calcium Entry That Regulates Non Small Cell Lung Adenocarcinoma Cell Proliferation. PLoS ONE 2015, 10, e0124201. [Google Scholar] [CrossRef] [Green Version]
- Simon, F.; Leiva-Salcedo, E.; Armisén, R.; Riveros, A.; Cerda, O.; Varela, D.; Eguiguren, A.L.; Olivero, P.; Stutzin, A. Hydrogen Peroxide Removes TRPM4 Current Desensitization Conferring Increased Vulnerability to Necrotic Cell Death. J. Biol. Chem. 2010, 285, 37150–37158. [Google Scholar] [CrossRef] [Green Version]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Wiedmann, F.; Schlund, D.; Voigt, N.; Ratte, A.; Kraft, M.; Katus, H.A.; Schmidt, C. N-glycosylation-dependent regulation of hK2P17.1 currents. Mol. Biol. Cell 2019, 30, 1425–1436. [Google Scholar] [CrossRef]
- Wiedmann, F.; Schlund, D.; Faustino, F.; Kraft, M.; Ratte, A.; Thomas, D.; Katus, H.A.; Schmidt, C. N-Glycosylation of TREK-1/hK2P2.1 Two-Pore-Domain Potassium (K2P) Channels. Int. J. Mol. Sci. 2019, 20, 5193. [Google Scholar] [CrossRef] [Green Version]
- Ondacova, K.; Karmažínová, M.; Lazniewska, J.; Weiss, N.; Lacinova, L. Modulation of Cav3.2 T-type calcium channel permeability by asparagine-linked glycosylation. Channels 2016, 10, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Thayer, D.A.; Yang, S.; Jan, Y.N.; Jan, L.Y. N-linked glycosylation of Kv1.2 voltage-gated potassium channel facilitates cell surface expression and enhances the stability of internalized channels. J. Physiol. 2016, 594, 6701–6713. [Google Scholar] [CrossRef] [Green Version]
- Dörr, K.; Kilch, T.; Kappel, S.; AlAnsary, D.; Schwär, G.; Niemeyer, B.A.; Peinelt, C. Cell type–specific glycosylation of Orai1 modulates store-operated Ca2+ entry. Sci. Signal. 2016, 9, ra25. [Google Scholar] [CrossRef]
- Erler, I.; Al-Ansary, D.M.; Wissenbach, U.; Wagner, T.F.; Flockerzi, V.; Niemeyer, B.A. Trafficking and Assembly of the Cold-sensitive TRPM8 Channel*. J. Biol. Chem. 2006, 281, 38396–38404. [Google Scholar] [CrossRef] [Green Version]
- Lehen′kyi, V.; Prevarskaya, N. Study of TRP Channels in Cancer Cells; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Woo, S.K.; Kwon, M.S.; Ivanov, A.; Geng, Z.; Gerzanich, V.; Simard, J.M. ComplexN-Glycosylation Stabilizes Surface Expression of Transient Receptor Potential Melastatin 4b Protein. J. Biol. Chem. 2013, 288, 36409–36417. [Google Scholar] [CrossRef] [Green Version]
- Syam, N.; Rougier, J.-S.; Abriel, H. Glycosylation of TRPM4 and TRPM5 channels: Molecular determinants and functional aspects. Front. Cell. Neurosci. 2014, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Xian, W.; Hui, X.; Tian, Q.; Wang, H.; Moretti, A.; Laugwitz, K.-L.; Flockerzi, V.; Ruppenthal, S.; Lipp, P. Aberrant Deactivation-Induced Gain of Function in TRPM4 Mutant Is Associated with Human Cardiac Conduction Block. Cell Rep. 2018, 24, 724–731. [Google Scholar] [CrossRef]
- Syam, N.; Chatel, S.; Ozhathil, L.C.; Sottas, V.; Rougier, J.-S.; Baruteau, A.-E.; Baron, E.; Amarouch, M.; Daumy, X.; Probst, V.; et al. Variants of Transient Receptor Potential Melastatin Member 4 in Childhood Atrioventricular Block. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Cerda, O.; Cáceres, M.; Park, K.-S.; Leiva-Salcedo, E.; Romero, A.; Varela, D.; Trimmer, J.S.; Stutzin, A. Casein kinase-mediated phosphorylation of serine 839 is necessary for basolateral localization of the Ca2+-activated non-selective cation channel TRPM4. Pflüg. Arch.-Eur. J. Physiol. 2015, 467, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Gerzanich, V.; Kwon, M.S.; Woo, S.K.; Ivanov, A.; Simard, J.M. SUR1-TRPM4 channel activation and phasic secretion of MMP-9 induced by tPA in brain endothelial cells. PLoS ONE 2018, 13, e0195526. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Choi, H.Y.; Na, W.H.; Ju, B.G.; Yune, T.Y. 17β-Estradiol Inhibits MMP-9 and SUR1/TrpM4 Expression and Activation and Thereby Attenuates BSCB Disruption/Hemorrhage After Spinal Cord Injury in Male Rats. Endocrinology 2015, 156, 1838–1850. [Google Scholar] [CrossRef]
- Cho, C.-H.; Kim, E.; Lee, Y.-S.; Yarishkin, O.; Yoo, J.C.; Park, J.-Y.; Hong, S.-G.; Hwang, E.M. Depletion of 14-3-3γ reduces the surface expression of Transient Receptor Potential Melastatin 4b (TRPM4b) Channels and attenuates TRPM4b-mediated glutamate-induced neuronal cell death. Mol. Brain 2014, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-Y.; Hwang, E.M.; Yarishkin, O.V.; Seo, J.-H.; Kim, E.; Yoo, J.; Yi, G.-S.; Kim, D.-G.; Park, N.; Ha, C.M.; et al. TRPM4b channel suppresses store-operated Ca2+ entry by a novel protein–protein interaction with the TRPC3 channel. Biochem. Biophys. Res. Commun. 2008, 368, 677–683. [Google Scholar] [CrossRef]
- Lee, N.K.; Park, J.Y.; Yoo, J.C.; Byun, E.H.; Bae, Y.; Lee, Y.-S.; Park, N.; Kang, D.; Han, J.; Park, J.-Y.; et al. PTPN6 regulates the cell-surface expression of TRPM4 channels in HEK293 cells. Pflüg. Arch.-Eur. J. Physiol. 2018, 470, 1449–1458. [Google Scholar] [CrossRef]
- Yan, J.; Bengtson, C.P.; Buchthal, B.; Hagenston, A.M.; Bading, H. Coupling of NMDA receptors and TRPM4 guides discovery of unconventional neuroprotectants. Science 2020, 370, eaay3302. [Google Scholar] [CrossRef]
- Expression of PTPN6 in Cancer—Summary—The Human Protein Atlas. n.d. Available online: https://www.proteinatlas.org/ENSG00000111679-PTPN6/pathology (accessed on 4 December 2020).
- Cho, C.-H.; Lee, Y.-S.; Kim, E.; Hwang, E.M.; Park, J.-Y. Physiological functions of the TRPM4 channels via protein interactions. BMB Rep. 2015, 48, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.D.; Soldini, D.; Jung, M.; Dietrich, D.; Stephan, C.; Jung, K.; Dietel, M.; Vainer, B.; Kristiansen, G. TRPM4 protein expression in prostate cancer: A novel tissue biomarker associated with risk of biochemical recurrence following radical prostatectomy. Virchows Arch. 2015, 468, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Sozucan, Y.; Kalender, M.E.; Sari, I.; Suner, A.; Oztuzcu, S.; Arman, K.; Yumrutas, O.; Bozgeyik, I.; Cengiz, B.; Igci, Y.Z.; et al. Trp Genes Family Expression in Colorectal Cancer. Exp. Oncol. 2015, 37, 208–212. [Google Scholar] [CrossRef]
- Ceylan, G.G.; Önalan, E.E.; Kuloğlu, T.; Aydoğ, G.; Keleş, I.; Tonyali, Ş.; Ceylan, C. Potential role of melastatin-related transient receptor potential cation channel subfamily M gene expression in the pathogenesis of urinary bladder cancer. Oncol. Lett. 2016, 12, 5235–5239. [Google Scholar] [CrossRef]
- Liu, L.; Lin, J.; He, H. Identification of Potential Crucial Genes Associated with the Pathogenesis and Prognosis of Endometrial Cancer. Front. Genet. 2019, 10, 373. [Google Scholar] [CrossRef]
- Pérez-Riesgo, E.; Gutiérrez, L.G.; Ubierna, D.; Acedo, A.; Moyer, M.P.; Núñez, L.; Villalobos, C. Transcriptomic Analysis of Calcium Remodeling in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 922. [Google Scholar] [CrossRef]
- Bianchi, B.; Ozhathil, L.C.; Medeiros-Domingo, A.; Gollob, M.H.; Abriel, H. Four TRPM4 Cation Channel Mutations Found in Cardiac Conduction Diseases Lead to Altered Protein Stability. Front. Physiol. 2018, 9, 177. [Google Scholar] [CrossRef] [Green Version]
- SMART—Servier Medical ART n.d. Available online: https://smart.servier.com/ (accessed on 22 January 2021).
- Borgström, A.; Hauert, B.; Kappel, S.; Zoni, E.; Kiener, M.; Stokłosa, P.; Baur, R.; Spahn, M.; Julio, M.K.-D.; Peinelt, C. Small Molecular Inhibitors Block TRPM4 Currents in Prostate Cancer Cells, with Limited Impact on Cancer Hallmark Functions. J. Mol. Biol. 2020, 12. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Han, B.-K.; Kim, E.-K.; Choi, W.J.; Choi, Y.; Kim, H.H.; Moon, W.K. Breast Cancer Detected at Screening US: Survival Rates and Clinical-Pathologic and Imaging Factors Associated with Recurrence. Radiology 2017, 284, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Mc Georges, L.; Verset, L.; Zlobec, I.; Demetter, P.; De Wever, O. Impact of the Microenvironment on Tumour Budding in Colorectal Cancer. Adv. Exp. Med. Biol. 2018, 1110, 101–111. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Zlobec, I.; Lugli, A. Tumor budding in colorectal cancer—ready for diagnostic practice? Hum. Pathol. 2016, 47, 4–19. [Google Scholar] [CrossRef]
- Karamitopoulou, E.; Zlobec, I.; Koelzer, V.H.; Langer, R.; Dawson, H.; Lugli, A. Tumour border configuration in colorectal cancer: Proposal for an alternative scoring system based on the percentage of infiltrating margin. Histopathology 2015, 67, 464–473. [Google Scholar] [CrossRef]
- Li, X.-C.; Cheng, Y.; Yang, X.; Zhou, J.-Y.; Dong, Y.-Y.; Shen, B.-Q.; Wang, J.-Q.; Zhao, L.-J.; Wang, Z.Q.; Li, X.P.; et al. Decreased expression of TRPM4 is associated with unfavora-ble prognosis and aggressive progression of endometrial carcinoma. Am. J. Trans. Res. 2020, 12, 3926–3939. [Google Scholar]
- El Boustany, C.; Bidaux, G.; Enfissi, A.; Delcourt, P.; Prevarskaya, N.; Capiod, T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology 2008, 47, 2068–2077. [Google Scholar] [CrossRef]
- Schinke, E.N.; Bii, V.; Nalla, A.; Rae, D.T.; Tedrick, L.; Meadows, G.G.; Trobridge, G.D. A novel approach to identify driver genes involved in androgen-independent prostate cancer. Mol. Cancer 2014, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tsai, F.C.; Kuo, G.-H.; Chang, S.-W.; Tsai, P.-J. Ca2+ Signaling in Cytoskeletal Reorganization, Cell Migration, and Cancer Metastasis. Biomed. Res. Int. 2015, 2015, 409245. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.C.; Hung, M.-C. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef]
- Liu, J.J.; Chao, J.R.; Jiang, M.C.; Ng, S.Y.; Yen, J.J.; Yang-Yen, H.F. Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol. Cell. Biol. 1995, 15, 3654–3663. [Google Scholar] [CrossRef] [Green Version]
- Canales, J.; Cruz, P.; Díaz, N.; Riquelme, D.; Leiva-Salcedo, E.; Cerda, O. K+ Channel Tetramerization Domain 5 (KCTD5) Protein Regulates Cell Migration, Focal Adhesion Dynamics and Spreading through Modulation of Ca2+ Signaling and Rac1 Activity. Cells 2020, 9, 2273. [Google Scholar] [CrossRef]
- Chinigò, G.; Pla, A.F.; Gkika, D. TRP Channels and Small GTPases Interplay in the Main Hallmarks of Metastatic Cancer. Front. Pharmacol. 2020, 11, 581455. [Google Scholar] [CrossRef]
- Wang, F.; Wu, P.; Gong, S.; Chen, Y.; Gao, J.; Wang, S.; Shen, Q.; Tao, H.; Hua, F.; Zhou, Z.; et al. Aberrant TRPM4 expression in MLL-rearranged acute myeloid leukemia and its blockade induces cell cycle arrest via AKT/GLI1/Cyclin D1 pathway. Cell. Signal. 2020, 72, 109643. [Google Scholar] [CrossRef]
- Gardam, K.E.; Geiger, J.E.; Hickey, C.M.; Hung, A.Y.; Magoski, N.S. Flufenamic Acid Affects Multiple Currents and Causes Intracellular Ca2+ Release in Aplysia Bag Cell Neurons. J. Neurophysiol. 2008, 100, 38–49. [Google Scholar] [CrossRef]
- Burris, S.K.; Wang, Q.; Bulley, S.; Neeb, Z.P.; Jaggar, J.H. 9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels. Br. J. Pharmacol. 2015, 172, 2459–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garland, C.J.; Smirnov, S.; Bagher, P.; Lim, C.S.; Huang, C.Y.; Mitchell, R.; Stanley, C.; Pinkney, A.; Dora, K. TRPM4 inhibitor 9-phenanthrol activates endothelial cell intermediate conductance calcium-activated potassium channels in rat isolated mesenteric artery. Br. J. Pharmacol. 2014, 172, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.P.H.; Striessnig, J.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The Concise Guide to Pharmacology 2017/18: Voltage-gated ion channels. Br. J. Pharmacol. 2017, 174, S160–S194. [Google Scholar] [CrossRef] [Green Version]
- Demion, M.; Bois, P.; Launay, P.; Guinamard, R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc. Res. 2007, 73, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Gao, Y.; Wei, S.; Low, S.W.; Ng, G.; Yu, D.; Tu, T.M.; Soong, T.W.; Nilius, B.; Liao, P. TRPM4-specific blocking antibody attenuates reperfusion injury in a rat model of stroke. Pflüg. Arch.-Eur. J. Physiol. 2019, 471, 1455–1466. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Low, S.W.; Poore, C.P.; Chen, B.; Gao, Y.; Nilius, B.; Liao, P. Comparison of Anti-oncotic Effect of TRPM4 Blocking Antibody in Neuron, Astrocyte and Vascular Endothelial Cell Under Hypoxia. Front. Cell Dev. Biol. 2020, 8, 1175. [Google Scholar] [CrossRef]
- Delalande, C.; Awale, M.; Rubin, M.; Probst, D.; Ozhathil, L.C.; Gertsch, J.; Abriel, H.; Reymond, J.-L. Optimizing TRPM4 inhibitors in the MHFP6 chemical space. Eur. J. Med. Chem. 2019, 166, 167–177. [Google Scholar] [CrossRef]
- Ozhathil, L.C.; Delalande, C.; Bianchi, B.; Németh, G.; Kappel, S.; Thomet, U.; Kaschitza, D.R.; Simonin, C.; Rubin, M.; Gertsch, J.; et al. Identification of potent and selective small molecule inhibitors of the cation channel TRPM4. Br. J. Pharmacol. 2018, 175, 2504–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgström, A.; Peinelt, C.; Stokłosa, P. TRPM4 in Cancer—A New Potential Drug Target. Biomolecules 2021, 11, 229. https://doi.org/10.3390/biom11020229
Borgström A, Peinelt C, Stokłosa P. TRPM4 in Cancer—A New Potential Drug Target. Biomolecules. 2021; 11(2):229. https://doi.org/10.3390/biom11020229
Chicago/Turabian StyleBorgström, Anna, Christine Peinelt, and Paulina Stokłosa. 2021. "TRPM4 in Cancer—A New Potential Drug Target" Biomolecules 11, no. 2: 229. https://doi.org/10.3390/biom11020229
APA StyleBorgström, A., Peinelt, C., & Stokłosa, P. (2021). TRPM4 in Cancer—A New Potential Drug Target. Biomolecules, 11(2), 229. https://doi.org/10.3390/biom11020229





