“Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications
Abstract
:1. Introduction
2. Cell Membrane Theory of Aging
- The cell membrane is the physical and chemical barrier which separates the inside the cell from the outside environment.
- The structure of the cell membrane can be described as liquid bilayer of lipid embedded with proteins called as a “fluid mosaic model”.
- This bilayer of the cell membrane is formed by the amphipathic molecules (phosphate rich heads on the outside and hydrophobic lipid tails on the inside).
- The cell membrane is impermeable to water-soluble molecules but not to water, is soft and flexible. The flexibility of the membrane could be attributed to its lipid content. It has the unique property of being able to spontaneously repair pores.
- About the composition of the cell membrane: lipids form ~50% by weight, proteins another ~50% by weight and carbohydrate portions of glycolipids and glycoproteins form approximately about 10%.
- The outer membrane is mainly consisting of phosphatidylcholine and sphingomyelin and the inner membrane is composed of phosphatidylethanolamine, phosphatidylserine and phosphatidylinositol and variable amounts of cholesterol.
- The transmembrane proteins and lipid-anchored proteins are generally confined to one of the membranes. Most of the receptors for various proteins are located on the outer surface though some receptors are inside the membrane.
- Glycosylated components of glycolipids and glycoproteins form the carbohydrate component of the membrane and they form the cellular glycocalyx.
- In general, water is present between lipid molecules in a highly organized form and bulk of the water content is present in the pores and channels.
- Ions such as calcium, sodium, etc., are present in the membrane and are attracted to the membrane by the intrinsic negative charge of the phospholipid heads.
- Cholesterol is also a major membrane component and is present in a variable amount, depending on the cell and species.
3. Metabolism of Essential Fatty Acids and Factors That Influence Their Metabolism
4. Actions of GLA/DGLA/AA/EPA/DHA and Their Metabolites
5. Cross Talk between Pro- and Anti-Inflammatory Molecules
6. Pro- and Anti-Inflammatory Actions of PGE2
7. PGE1 and LXA4 Have Similar Actions
8. Bioactive Lipids and Immune Response
9. Anti-Microbial Action
10. Phospholipase A2 (PLA2) Has Antimicrobial Action
11. PUFAs Mediate the Microbicidal Action of Macrophages
12. Interaction between Microbes and Host Cells/Tissues
13. Immunoregulatory Actions of Bioactive Lipids
14. M1 and M2 Macrophages and Bioactive Lipids
15. PGE2 and LXA4 Interact to Induce Resolution of Inflammation
16. PGE2 Is Needed for Tissue Regeneration
17. LXA4 Is the Mediator of Beneficial Action of MSCs (Mesenchymal Stem Cells)
18. Low-Grade Systemic Inflammation Occurs in Aging and Aging Associated Disorders
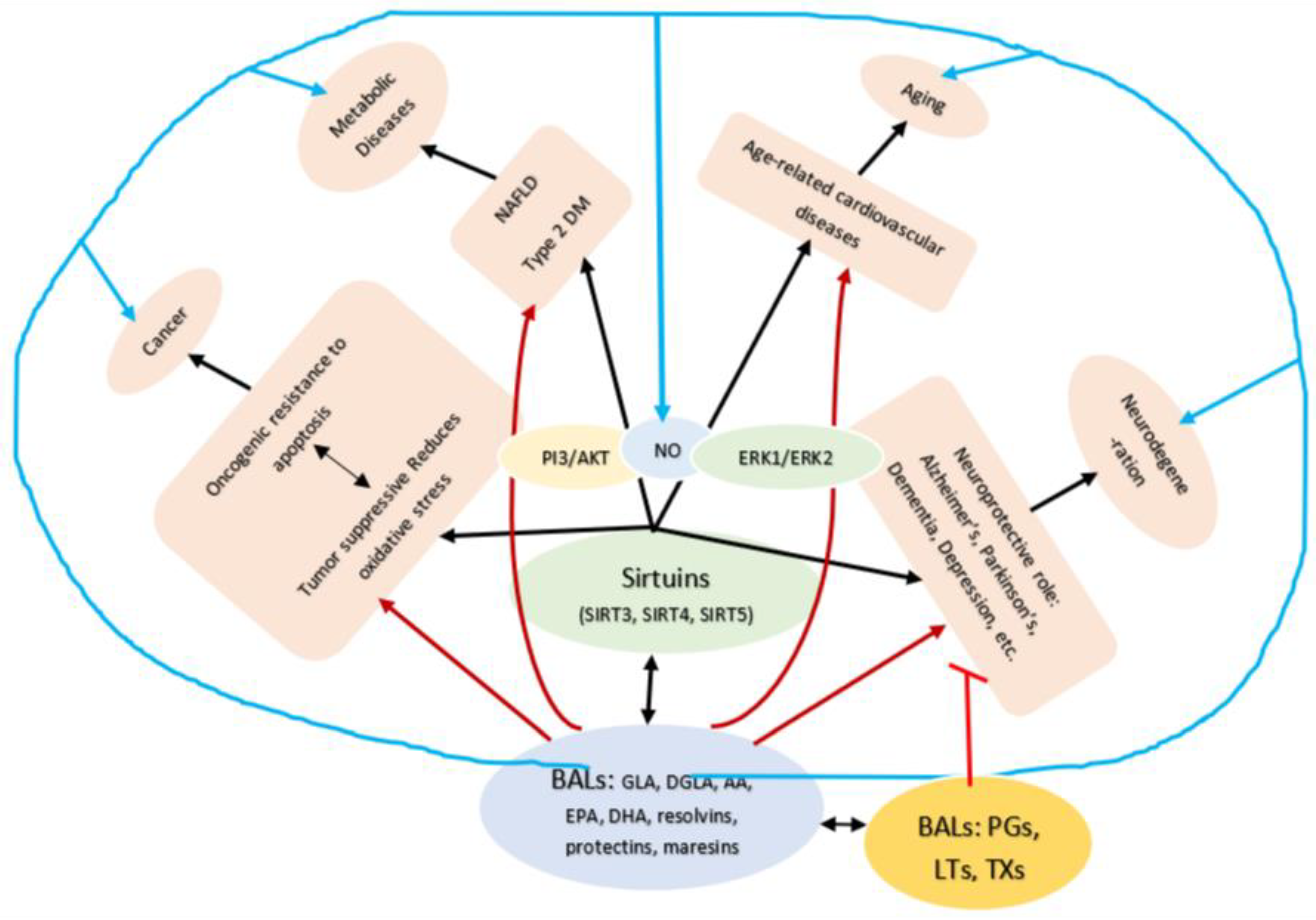
19. Regulatory Action of Bioactive Lipids on Sirtuins
20. Bioactive Lipids in Age-Associated Diseases
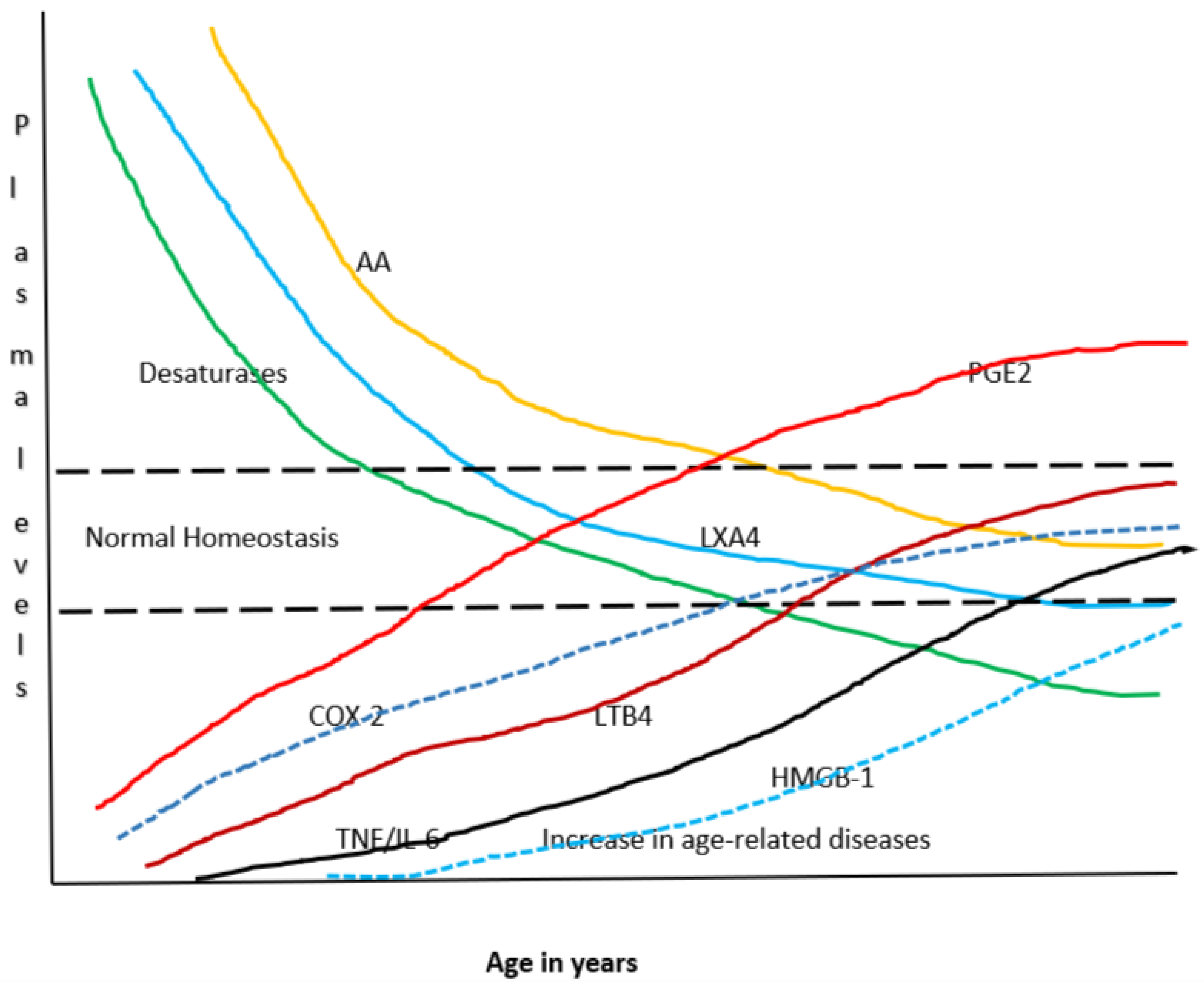
21. AA in Aging
22. AA in C elegans and Life Span
23. Alzheimer’s Disease and BALs
24. PLA2, COX-2, LOX Enzymes, Cytokines and Pro- and Anti-Inflammatory Eicosanoids and AD
25. Gangliosides, Sphingolipids, Cholesterol, and Plasmalogens and their Relationship to BALs in Ageing
26. BALs, Oxidative Stress and Longevity of Naked Mole Rat (NMR)
27. Conclusions and Therapeutic Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Essential fatty acids | Dietary linoleic acid (LA) and alpha-linolenic acid (ALA) |
| PUFAS | Polyunsaturated fatty acids: LA, ALA, GLA, DGLA, AA, EPA, DPA and DHA |
| Bioactive lipids (BALs) | All PUFAs and PGs, LTs, TXs, lipoxins, resolvins, protectins and maresins and cytochrome p450 metabolites of PUFAs |
| Pro-inflammatory cytokines | IL-6, TNF-α, HMGB1, IL-1, IL-2, IFN |
| Anti-inflammatory cytokines | IL-4, IL-10, etc., |
References
- Manjari, V.; Das, U. Effect of polyunsaturated fatty acids on dexamethasone induced gastric mucosal damage. Prostaglandins Leukot. Essent. Fat. Acids 2000, 62, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Mohan, I.K.; Das, U.N. Prevention of chemically induced diabetes mellitus in experimental animals by polyunsaturated fatty acids. Nutrition 2001, 17, 126–151. [Google Scholar] [CrossRef]
- Tamburini, I.; Quartacci, M.F.; Izzo, R.; Bergamini, E. Effects of dietary restriction on age-related changes in the phospholipid fatty acid composition of various rat tissues. Aging Clin. Exp. Res. 2004, 16, 425–431. [Google Scholar] [CrossRef]
- Jiang, J.C.; Jaruga, E.; Repnevskaya, M.V.; Jazwinski, S.M. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000, 14, 2135–2137. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.J.; Michan, S. Biology of Healthy Aging and Longevity. Rev. Invest. Clin 2016, 68, 7–16. [Google Scholar] [PubMed]
- Morselli, E.; Maiuri, M.C.; Markaki, M.; Megalou, E.; Pasparaki, A.; Palikaras, K.; Criollo, A.; Galluzzi, L.; Malik, S.A.; Vitale, I.; et al. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy 2010, 6, 186–188. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.; Das, S.K.; Golden, J.K.; Saltzman, E.; Roberts, S.B.; Meydani, S.N. Calorie Restriction Enhances T-Cell-Mediated Immune Response in Adult Overweight Men and Women. J. Gerontol. Ser. Biol. Sci. Med Sci. 2009, 64, 1107–1113. [Google Scholar] [CrossRef] [Green Version]
- Hegner, D. Age-dependence of molecular and functional changes in biological membrane properties. Mech. Ageing Dev. 1980, 14, 101–118. [Google Scholar] [CrossRef]
- Spector, A.; Yorek, M. Membrane lipid composition and cellular function. J. Lipid Res. 1985, 26, 1015–1035. [Google Scholar] [CrossRef]
- Das, U.N.; Begin, M.E.; Ells, G.; Horrobin, D.F. Clinical significance of essential fatty acids. Nutrition 1988, 4, 337–341. [Google Scholar]
- Das, U.N. Essential fatty acids in health and disease. J. Assoc. Physicians India 1999, 47, 906–911. [Google Scholar] [PubMed]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- Das, U.N. Biological significance of essential fatty acids. J. Assoc. Physicians India 2006, 54, 309–319. [Google Scholar]
- Nervi, A.M.; Peluffo, R.O.; Brenner, R.R.; Leikin, A.I. Effect of ethanol administration on fatty acid desaturation. Lipids 1980, 15, 263–268. [Google Scholar] [CrossRef]
- Das, U.N. Fetal Alcohol Syndrome and Essential Fatty Acids. PLoS Med. 2006, 3, e247. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, K.A.; George, F.; Brnouti, F.; Mooney, S.M. Docosahexaenoic acid partially ameliorates deficits in social behavior and ultrasonic vocalizations caused by prenatal ethanol exposure. Behav. Brain Res. 2015, 286, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Das, U. Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition 2003, 19, 62–65. [Google Scholar] [CrossRef]
- Robbez Masson, V.; Lucas, A.; Gueugneau, A.M.; Macaire, J.P.; Paul, J.L.; Grynberg, A.; Rousseau, D. Long-chain (n-3) poly-unsaturated fatty acids prevent metabolic and vascular disorders in fructose-fed rats. J. Nutr. 2008, 138, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, E.; Feskens, E.J.M.; Jansen, E.H.J.M.; Mensink, M.; Saris, W.H.M.; De Bruin, T.W.A.; Blaak, E.E. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: The SLIM study. Diabetology 2006, 49, 2392–2401. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.R.; Joshi, V.C. Regulation of rat hepatic stearoyl coenzyme A desaturase. The roles of insulin and carbo-hydrate. J. Biol. Chem. 1979, 254, 997–999. [Google Scholar] [CrossRef]
- Das, U.N. Sucrose, fructose, glucose, and their link to metabolic syndrome and cancer. Nutrients 2015, 31, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Schmidt, R.; Muhly-Reinholz, M.; Bogeholz, T.; Gokorsch, S.; Grimminger, F.; Seeger, W. In vitro mimicry of essential fatty acid deficiency in human endothelial cells by TNFα impact of w-3 versus w-6 fatty acids. J. Lipid Res. 2002, 43, 944–951. [Google Scholar] [CrossRef]
- Das, U.N. Molecular Basis of Health and Disease; Springer: New York, NY, USA, 2011. [Google Scholar]
- Cao, D.; Luo, J.; Zang, W.; Chen, D.; Xu, H.; Shi, H. Gamma-linolenic acid suppresses NF-κΒ signaling via CD36 in the lipopolysaccharide induced inflammatory response in primary goat mammary gland epithelial cells. Inflammation 2016, 39, 1225–1237. [Google Scholar] [CrossRef]
- Dooper, M.M.; van Riel, B.; Graus, Y.M.; M’Rabet, L. Dihomo-gamma-linolenic acid inhibits tumour necrosis fac-tor-alpha production by human leucocytes independently of cyclooxygenase activity. Immunology 2003, 110, 348–357. [Google Scholar] [CrossRef]
- Khalfoun, B.; Thibault, F.; Watier, H.; Bardos, P.; Lebranchu, Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Single Mol. Single Cell Seq. 1997, 400, 589–597. [Google Scholar]
- Kumar, G.S.; Das, U. Effect of prostaglandins and their precursors on the proliferation of human lymphocytes and their secretion of tumor necrosis factor and various interleukins. Prostaglandins Leukot. Essent. Fat. Acids 1994, 50, 331–334. [Google Scholar] [CrossRef]
- Dooper, M.M.; Wassink, L.; M’Rabet, L.; Graus, Y.M.F. The modulatory effects of prostaglandin-E on cytokine production by human peripheral blood mononuclear cells are independent of the prostaglandin subtype. Immunology 2002, 107, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Wang, X.; Wang, S.; Wei, Y.; Feng, J.; Zhu, M. Maresin 1 Improves Cognitive Decline and Ameliorates In-flammation in a Mouse Model of Alzheimer’s Disease. Front. Cell Neurosci. 2019, 13, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N.; Devi, G.R.; Rao, K.P.; Rao, M.S. Prostaglandins and their precursors can modify genetic damage induced by benzo (a,) pyrene and gamma-radiation. Prostaglandins 1985, 29, 911–920. [Google Scholar] [CrossRef]
- Das, U.N.; Rao, K.P. Effect of γ-linolenic acid and prostaglandins E1 on gamma-radiation and chemical-induced genetic damage to the bone marrow cells of mice. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 165–173. [Google Scholar] [CrossRef]
- Suresh, Y.; Das, U.N. Differential effect of saturated, monounsaturated, and polyunsaturated fatty acids on alloxan-induced diabetes mellitus. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 199–213. [Google Scholar] [CrossRef]
- Suresh, Y.; Das, U.N. Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus: Effect of ω-6 fatty acids. Nutrition 2003, 19, 93–114. [Google Scholar] [CrossRef]
- Gundala, N.K.; Naidu, V.G.; Das, U.N. Arachidonic acid and lipoxin A4 attenuate alloxan-induced cytotoxicity to RIN5F cells in vitro and type 1 diabetes mellitus in vivo. BioFactors 2017, 43, 251–271. [Google Scholar] [CrossRef]
- Naveen, K.V.; Naidu, G.V.; Das, U.N. Streptozotocin-induced cytotoxicity to RIN5F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition 2017, 35, 61–80. [Google Scholar]
- Das, U.N. Current and emerging strategies for the treatment and management of systemic lupus erythematosus based on molecular signatures of acute and chronic inflammation. J. Inflamm. Res. 2010, 3, 143–170. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Clinical Laboratory Tools to Diagnose Inflammation. Adv. Virus Res. 2006, 41, 189–229. [Google Scholar] [CrossRef]
- Bonventre, J.V. Phospholipase A2 and signal transduction. J. Am. Soc. Nephrol. 1992, 3, 128–150. [Google Scholar] [PubMed]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H.R. Cutting Edge: Lipoxins Rapidly Stimulate Nonphlogistic Phagocytosis of Apoptotic Neutrophils by Monocyte-Derived Macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Colvillenash, P.R.; Willis, D.K.; Chivers, J.; Paulclark, M.J.; Willoughby, D. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999, 5, 698–701. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Newson, J.; Sawmynaden, P.; Willoughby, D.A.; Croxtall, J.D. A novel role for phospholipase A 2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004, 18, 489–498. [Google Scholar] [CrossRef]
- Cominelli, F.; Nast, C.C.; Llerena, R.; Dinarello, C.A.; Zipser, R.D. Interleukin 1 suppresses inflammation in rabbit colitis. Mediation by endogenous prostaglandins. J. Clin. Investig. 1990, 85, 582–586. [Google Scholar] [CrossRef] [Green Version]
- Schwab, J.H.; Anderle, S.K.; Brown, R.R.; Dalldorf, F.G.; Thompson, R.C. Pro-and anti-inflammatory roles of inter-leukin-1 in recurrence of bacterial cell wall-induced arthritis in rats. Infect. Immun. 1991, 59, 4436–4442. [Google Scholar] [CrossRef] [Green Version]
- Croxtall, J.D.; Choudhury, Q.; Tokumoto, H.; Flower, R.J. Lipocortin-1 and the control of arachidonic acid release in cell signalling. Glucocorticoids (changed from glucorticoids) inhibit G protein-dependent activation of cPLA2 activity. Biochem. Pharmacol. 1995, 50, 465–474. [Google Scholar] [CrossRef]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological Functions of Glucocorticoids in Stress and Their Relation to Pharmacological Actions*. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef] [Green Version]
- Rozenberg, A.; Rezk, A.; Boivin, M.-N.; Darlington, P.J.; Nyirenda, M.; Li, R.; Jalili, F.; Winer, R.; Artsy, E.A.; Uccelli, A.; et al. Human Mesenchymal Stem Cells Impact Th17 and Th1 Responses Through a Prostaglandin E2 and Myeloid-Dependent Mechanism. Stem Cells Transl. Med. 2016, 5, 1506–1514. [Google Scholar] [CrossRef] [Green Version]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2008, 15, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids are Self-activated to Produce Prostaglandin E2 that Directs Stimulated Macrophages into an Anti-inflammatory Phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [Green Version]
- Poloso, N.J.; Urquhart, P.; Nicolaou, A.; Wang, J.; Woodward, D.F. PGE2 differentially regulates monocyte-derived dendritic cell cytokine responses depending on receptor usage (EP2/EP4). Mol. Immunol. 2013, 54, 284–295. [Google Scholar] [CrossRef]
- Kalim, K.W.; Groettrup, M. Prostaglandin E2 inhibits IL-23 and IL-12 production by human monocytes through down-regulation of their common p40 subunit. Mol. Immunol. 2013, 53, 274–282. [Google Scholar] [CrossRef]
- Loynes, C.A.; Lee, J.A.; Robertson, A.L.; Steel, M.J.; Ellett, F.; Feng, Y.; Levy, B.D.; Whyte, M.K.; Renshaw, S.A. PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 2018, 4, eaar8320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, M.M.-Y.; Moore, A.R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxy-genase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 2010, 184, 6418–6426. [Google Scholar] [CrossRef] [Green Version]
- Heczko, P.B.; Lütticken, R.; Hryniewicz, W.; Neugebauer, M.; Pulverer, G. Susceptibility of Staphylococcus aureus and group A, B, C, and G streptococci to free fatty acids. J. Clin. Microbiol. 1979, 9, 333–335. [Google Scholar]
- Das, U.N. Can essential fatty acid deficiency predispose to AIDS? Can Med. Assoc. J. 1985, 132, 900–902. [Google Scholar]
- Kohn, A.; Gitelman, J.; Inbar, M. Unsaturated free fatty acids inactivate animal enveloped viruses. Arch. Virol. 1980, 66, 301–307. [Google Scholar] [CrossRef]
- Das, U.N. Anti-biotic-like action of essential fatty acids. Can. Med. Assoc. J. 1985, 132, 1350. [Google Scholar]
- Das, U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Bioactive lipids-based therapeutic approach to COVID-19 and other similar infection. Adv. Exp. Med. Biol. 2020, 1260, 33–83. [Google Scholar] [PubMed]
- Das, U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch. Med. Res. 2020, 15, 282–286. [Google Scholar] [CrossRef]
- Das, U.N. Response to: Bioactive Lipids and Coronavirus (COVID-19)-further Discussion. Arch. Med Res. 2020, 51, 445–449. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipids as Mediators of the Beneficial Action(s) of Mesenchymal Stem Cells in COVID-19. Aging Dis. 2020, 11, 746–755. [Google Scholar] [CrossRef]
- Gimenez, A.P.; Wu, Y.Z.; Paya, M.; Delclaux, C.; Touqui, L.; Goossens, P.L. High bactericidal efficiency of type iia phospholipase A2 against Bacillus anthracis and inhibition of its secretion by the lethal toxin. J. Immunol. 2004, 173, 521–530. [Google Scholar] [CrossRef]
- Raymond, B.; LeDuc, D.; Ravaux, L.; Le Goffic, R.; Candela, T.; Raymondjean, M.; Goossens, P.L.; Touqui, L. Edema Toxin Impairs Anthracidal Phospholipase A2 Expression by Alveolar Macrophages. PLoS Pathog. 2007, 3, e187. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.; Ravaux, L.; Mémet, S.; Wu, Y.; Sturny-Leclère, A.; Leduc, D.; Touqui, L. Anthrax lethal toxin down-regulates type-IIA secreted phospholipase A(2) expression through MAPK/NF-kappaB inactivation. Biochem. Pharmacol. 2010, 79, 1149–1155. [Google Scholar] [CrossRef] [Green Version]
- Territo, M.C.; Golde, D.W. The function of human alveolar macrophages. J. Reticuloendothel. Soc. 1979, 25, 111–120. [Google Scholar]
- LaForce, F.M.; Kelly, W.J.; Huber, G.L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am. Rev. Respir. Dis. 1973, 108, 784–790. [Google Scholar] [PubMed]
- Juers, J.; Rogers, R.M.; McCurdy, J.B.; Cook, W.W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J. Clin. Investig. 1976, 58, 271–275. [Google Scholar] [CrossRef]
- Wyss, O.; Ludwig, B.J.; Joiner, R.R. The fungistatic and fungicidal action of fatty acids and related compounds. Arch. Biochem. 1945, 7, 415–424. [Google Scholar]
- Schlager, S.I.; Madden, L.D.; Meltzer, M.S.; Bara, S.; Mamula, M.J. Role of macrophage lipids in regulating tumoricidal activity. Cell. Immunol. 1983, 77, 52–68. [Google Scholar] [CrossRef]
- Baranov, V.; Nagaeva, O.; Hammarström, S.; Mincheva-Nilsson, L. Lipids are a constitutive component of cytolytic granules. Histochem. Cell Biol. 2000, 114, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.H.; Dalli, J.; Colas, R.A.; Shinohara, M.; Serhan, C.N. Aging Delays Resolution of Acute Inflammation in Mice: Reprogramming the Host Response with Novel Nano-Proresolving Medicines. J. Immunol. 2014, 193, 4235–4244. [Google Scholar] [CrossRef] [Green Version]
- Vinjé, S.; Stroes, E.; Nieuwdorp, M.; Hazen, S.L. The gut microbiome as novel cardio-metabolic target: The time has come! Eur. Hear. J. 2013, 35, 883–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, E.; Neves, J.S.; Ferreira-Magalhães, M.; Carvalho, D.; Freitas, P. Probiotic Ingestion, Obesity, and Metabolic-Related Disorders: Results from NHANES, 1999–2014. Nutrition 2019, 11, 1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmu, J.; Salosensaari, A.; Havulinna, A.S.; Cheng, S.; Inouye, M.; Jain, M.; Salido, R.A.; Sanders, K.; Brennan, C.; Humphrey, G.C.; et al. Association Between the Gut Microbiota and Blood Pressure in a Population Cohort of 6953 Individuals. J. Am. Hear. Assoc. 2020, 9. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.; Lu, A.; Liu, X.; Zhang, L.; Xu, C.; Liu, X.; Li, H.; Yang, T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin–angiotensin system. J. Hypertens. 2017, 35, 1899–1908. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Babbin, B.A.; Laukoetter, M.G.; Nava, P.; Koch, S.; Lee, W.Y.; Capaldo, C.T.; Peatman, E.; Severson, E.A.; Flower, R.J.; Perretti, M.; et al. Annexin A1 Regulates Intestinal Mucosal Injury, Inflammation, and Repair. J. Immunol. 2008, 181, 5035–5044. [Google Scholar] [CrossRef] [Green Version]
- Birkl, D.; O’Leary, M.N.; Quiros, M.; Azcutia, V.; Schaller, M.; Reed, M.; Nishio, H.; Keeney, J.; Neish, A.S.; Lukacs, N.W.; et al. Formyl peptide receptor 2 regulates monocyte recruitment to promote intestinal mucosal wound repair. FASEB J. 2019, 33, 13632–13643. [Google Scholar] [CrossRef] [Green Version]
- Barletta, A.B.F.; Trisnadi, N.; Ramirez, J.L.; Barillas-Mury, C. Mosquito Midgut Prostaglandin Release Establishes Systemic Immune Priming. iScience 2019, 19, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Cerquetti, M.C.; Hovsepian, E.; Sarnacki, S.H.; Goren, N.B. Salmonella enterica serovar enteritidis dam mutant induces low NOS-2 and COX-2 expression in macrophages via attenuation of MAPK and NF-kappaB pathways. Microbes. Infect. 2008, 10, 1431–1439. [Google Scholar] [CrossRef]
- Raisch, J.; Rolhion, N.; Dubois, A.; Darfeuille-Michaud, A.; Bringer, M.-A. Intracellular colon cancer-associated Esch-erichia coli promote protumoral activities of human macrophages by inducing sustained COX-2 expression. Lab. Invest. 2015, 95, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, R.K.; Dahiya, Y.; Sodhi, A. Mycobacterium indicus pranii downregulates MMP-9 and iNOS through COX-2 dependent and TNF-α independent pathway in mouse peritoneal macrophages in vitro. Microbes Infect. 2012, 14, 348–356. [Google Scholar] [CrossRef]
- Hooks, J.J.; Chin, S.M.; Srinivasan, K.; Momma, Y.; Hooper, L.C.; Nagineni, N.C.; Chan, C.-C.; Detrick, B. Human cy-tomegalovirus induced cyclooxygenase-2 in human retinal pigment epithelial cells augments viral replication through a prostaglandin pathway. Microbes. Infect. 2006, 8, 2236–2244. [Google Scholar] [CrossRef]
- Lim, S.M.; Jang, H.M.; Jang, S.E.; Han, M.J.; Kim, D.H. Lactobacillus fermentum IM12 attenuates inflammation in mice by inhibiting NF-κB-STAT3 signalling pathway. Benef. Microbes. 2017, 8, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Jenh, C.H. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef]
- Yu, H.N.; Zhu, J.; Pan, W.S.; Shen, S.R.; Wei-Guang, S.; Das, U.N. Effects of fish oil with high content of n-3 pol-yunsaturated fatty acids on mouse gut microbiota. Arch. Med. Res. 2014, 45, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.-I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Djuric, Z.; Bassis, C.M.; Plegue, M.A.; Sen, A.; Turgeon, D.K.; Herman, K.; Young, V.B.; Brenner, D.E.; Ruffin, M.T. Increases in Colonic Bacterial Diversity after ω-3 Fatty Acid Supplementation Predict Decreased Colonic Prostaglandin E2 Concentrations in Healthy Adults. J. Nutr. 2019, 149, 1170–1179. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids as possible enhancers of the beneficial actions of probiotics. Nutrients 2002, 18, 786–789. [Google Scholar] [CrossRef]
- Stolp, B.; Thelen, F.; Ficht, X.; Altenburger, L.M.; Ruef, N.; Inavalli, V.V.G.K.; Germann, P.; Page, N.; Moalli, F.; Raimondi, A.; et al. Salivary gland macrophages and tissue-resident CD8+ T cells cooperate for homeostatic organ surveillance. Sci. Immunol. 2020, 5, eaaz4371. [Google Scholar] [CrossRef] [PubMed]
- Coonrod, J.D. Rôle of surfactant free fatty acids in antimicrobial defenses. Eur. J. Respir. Dis. Suppl. 1987, 153, 209–214. [Google Scholar] [PubMed]
- Frizzell, H.; Fonseca, R.; Christo, S.N.; Evrard, M.; Cruz-Gomez, S.; Zanluqui, N.G.; Von Scheidt, B.; Freestone, D.; Park, S.L.; McWilliam, H.E.G.; et al. Organ-specific isoform selection of fatty acid–binding proteins in tissue-resident lymphocytes. Sci. Immunol. 2020, 5, eaay9283. [Google Scholar] [CrossRef] [PubMed]
- Ramon, S.; Dalli, J.; Sanger, J.M.; Winkler, J.W.; Aursnes, M.; Tungen, J.E.; Hansen, T.V.; Serhan, C.N. The Protectin PCTR1 Is Produced by Human M2 Macrophages and Enhances Resolution of Infectious Inflammation. Am. J. Pathol. 2016, 186, 962–973. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Bioactive lipids in intervertebral disc degeneration and its therapeutic implications. Biosci. Rep. 2019, 39, BSR20192117. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.; Dichter, E.; Lacorte, G.; Kerner, D.; Spur, B.; Rodriguez, A.; Yin, K. Lipoxin A4 Increases Survival by Decreasing Systemic Inflammation and Bacterial Load in Sepsis. Shock 2011, 36, 410–416. [Google Scholar] [CrossRef]
- Wu, B.; Capilato, J.; Pham, M.P.; Walker, J.; Spur, B.; Rodriguez, A.; Perez, L.J.; Yin, K. Lipoxin A 4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. FASEB J. 2016, 30, 2400–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. HLA-DR expression, cytokines and bioactive lipids in sepsis. Arch. Med. Sci. 2014, 10, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Is sepsis a pro-resolution deficiency disorder? Med. Hypotheses 2013, 80, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Das, U. Beneficial effect of eicosapentaenoic and docosahexaenoic acids in the management of systemic lupus erythematosus and its relationship to the cytokine network. Prostaglandins Leukot. Essent. Fat. Acids 1994, 51, 207–213. [Google Scholar] [CrossRef]
- Mohan, I.; Das, U. Oxidant stress, anti-oxidants and essential fatty acids in systemic lupus erythematosus. Prostaglandins Leukot. Essent. Fat. Acids 1997, 56, 193–198. [Google Scholar] [CrossRef]
- Suryaprabha, P.; Das, U.; Ramesh, G.; Kumar, K.; Kumar, G. Reactive oxygen species, lipid peroxides and essential fatty acids in patients with rheumatoid arthritis and systemic lupus erythematosus. Prostaglandins Leukot. Essent. Fat. Acids 1991, 43, 251–255. [Google Scholar] [CrossRef]
- Das, U.N.; Ramesh, G.; Kumar, G.S.; Madhavi, N.; Kumar, K.V.; Sagar, P.S.; Koratkar, R.; Padma, M. Free Radicals, Lipid Peroxidation and Essential Fatty Acids in Patients with Pneumonia, Septicemia and Collagen Vascular Diseases. J. Nutr. Med. 1992, 3, 117–127. [Google Scholar] [CrossRef]
- Ishizuka, K.; Kon, K.; Lee-Okada, H.C.; Arai, K.; Uchiyama, A.; Yamashina, S.; Yokomizo, T.; Ikejima, K. Aging exac-erbates high-fat diet-induced steatohepatitis through alteration in hepatic lipid metabolism in mice. J. Gastroenterol. Hepatol. 2020, 35, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Delachambre, M.-C.; Narce, M.; Asdrubal, P.; Poisson, J.-P. Changes in tissue polyunsaturated fatty acids with age, in spontaneously hypertensive rats. Lipids 1998, 33, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.A.L.; Bordoni, A.; Lorenzini, A.; Rossi, C.; Biagi, P.; Hrelia, S. Linoleic acid metabolism in primary cultures of adult rat cardiomyocytes is impaired by aging. Biochem. Biophys. Res. Commun. 1997, 237, 142–145. [Google Scholar] [CrossRef]
- Dakin, S.G.; Dudhia, J.; Werling, N.J.; Werling, D.; Abayasekara, D.R.; Smith, R.K. Inflamm-aging and arachidonic acid metabolite differences with stage of tendon disease. PLoS ONE 2012, 7, e48978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Kim, J.; Saima, F.T.; Rhee, K.-J.; Hwang, S.; Kim, M.Y.; Baik, S.K.; Eom, Y.W.; Kim, H.-S. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem. Biophys. Res. Commun. 2018, 498, 988–995. [Google Scholar] [CrossRef]
- An, J.-H.; Song, W.-J.; Li, Q.; Kim, S.-M.; Yang, J.-I.; Ryu, M.-O.; Nam, A.R.; Bhang, D.H.; Jung, Y.-C.; Youn, H.-Y. Prostaglandin E2 secreted from feline adipose tissue-derived mesenchymal stem cells alleviate DSS-induced colitis by increasing regulatory T cells in mice. BMC Veter. Res. 2018, 14, 354. [Google Scholar] [CrossRef]
- Terraza-Aguirre, C.; Campos-Mora, M.; Elizondo-Vega, R.; Contreras-López, R.A.; Luz-Crawford, P.; Jorgensen, C.; Djouad, F. Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells. Cells 2020, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Qu, X.; Chen, Y.; Liao, L.; Cheng, K.; Shao, C.; Zenke, M.; Keating, A.; Zhao, R.C.H. Mesenchymal Stem/Stromal Cells Induce the Generation of Novel IL-10–Dependent Regulatory Dendritic Cells by SOCS3 Activation. J. Immunol. 2012, 189, 1182–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, H.; Sugimoto, Y.; Tanaka, S.; Ichikawa, A. Prostaglandin E(2) receptors, EP2 and EP4, differentially modulate TNF-alpha and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem. Biophys. Res. Commun. 2000, 278, 224–228. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipids in Age-Related Disorders. Adv. Exp. Med. Biol. 2020, 1260, 33–83. [Google Scholar] [CrossRef]
- Schroder, R.; Xue, L.; Konya, V.; Martini, L.; Kampitsch, N.; Whistler, J.L.; Ulven, T.; Heinemann, A.; Pettipher, R.; Kostenis, E. PGH1, the Precursor for the Anti-Inflammatory Prostaglandins of the 1-series, Is a Potent Activator of the Pro-Inflammatory Receptor CRTH2/DP2. PLoS ONE 2012, 7, e33329. [Google Scholar] [CrossRef]
- Zhang, Y.; Desai, A.; Yang, S.Y.; Bae, K.B.; Antczak, M.I.; Fink, S.P.; Tiwari, S.; Willis, J.E.; Williams, N.S.; Dawson, D.M.; et al. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 2015, 348, aaa2340. [Google Scholar] [CrossRef] [Green Version]
- North, T.E.; Goessling, W.; Walkley, C.R.; Lengerke, C.; Kopani, K.R.; Lord, A.M.; Weber, G.J.; Bowman, T.V.; Jang, I.-H.; Grosser, T.; et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nat. Cell Biol. 2007, 447, 1007–1011. [Google Scholar] [CrossRef]
- Li, F.; Huang, Q.; Chen, J.; Peng, Y.; Roop, D.R.; Bedford, J.S.; Li, C.-Y. Apoptotic Cells Activate the Phoenix Rising Pathway to Promote Wound Healing and Tissue Regeneration. Sci. Signal. 2010, 3, ra13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoggatt, J.; Mohammad, K.S.; Singh, P.; Hoggatt, A.F.; Chitteti, B.R.; Speth, J.M.; Hu, P.; Poteat, B.A.; Stilger, K.N.; Ferraro, F.; et al. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nat. Cell Biol. 2013, 495, 365–369. [Google Scholar] [CrossRef]
- Diaz, M.F.; Li, N.; Lee, H.J.; Adamo, L.; Evans, S.M.; Willey, H.E.; Arora, N.; Torisawa, Y.-S.; Vickers, D.A.; Morris, S.A.; et al. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP–PKA signaling axis. J. Exp. Med. 2015, 212, 665–680. [Google Scholar] [CrossRef]
- Fang, X.; Abbott, J.; Cheng, L.; Colby, J.K.; Lee, J.W.; Levy, B.D.; Matthay, M.A. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J. Immunol. 2015, 195, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; He, S.; Yuan, J.; Miao, S.; Gao, H.; Zhang, J.; Li, Y.; Peng, W.; Wu, P. Lipoxin A4 attenuates LPS-induced mouse acute lung injury via Nrf2-mediated E-cadherin expression in airway epithelial cells. Free Radic. Biol. Med. 2016, 93, 52–66. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, J.; He, Z.; Yang, M.; Li, L.; Jiang, H. Mesenchymal Stem Cells Reverse Diabetic Nephropathy Disease via Lipoxin A4 by Targeting Transforming Growth Factor β (TGF-β)/smad Pathway and Pro-Inflammatory Cytokines. Med. Sci. Monit. 2019, 25, 3069–3076. [Google Scholar] [CrossRef]
- Coulombe, F.; Jaworska, J.; Verway, M.; Tzelepis, F.; Massoud, A.; Gillard, J.; Wong, G.; Kobinger, G.; Xing, Z.; Couture, C.; et al. Targeted Prostaglandin E2 Inhibition Enhances Antiviral Immunity through Induction of Type I Interferon and Apoptosis in Macrophages. Immunology 2014, 40, 554–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuchiwal, S.K.; Balestrieri, B.; Raff, H.; Boyce, J.A. Endogenous prostaglandin E2 amplifies IL-33 production by macrophages through an E prostanoid (EP)2/EP4-cAMP-EPAC-dependent pathway. J. Biol. Chem. 2017, 292, 8195–8206. [Google Scholar] [CrossRef] [Green Version]
- Sander, W.J.; O’Neill, H.G.; Ezeokoli, O.T. Prostaglandin E2 as a Modulator of Viral Infections. Front. Physiol. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, E.; Dutile, S.; Kazani, S.; Wechsler, M.E.; Yang, J.; Hammock, B.D.; Douda, D.N.; Tabet, Y.; Khaddaj-Mallat, R.; Sirois, M.; et al. Lipoxin Generation Is Related to Soluble Epoxide Hydrolase Activity in Severe Asthma. Am. J. Respir. Crit. Care Med. 2014, 190, 886–897. [Google Scholar] [CrossRef]
- Caligiuri, S.P.; Aukema, H.M.; Ravandi, A.; Guzman, R.; Dibrov, E.; Pierce, G.N.; Ishigami, T.; Kino, T.; Chen, L.; Minegishi, S.; et al. Flaxseed Consumption Reduces Blood Pressure in Patients With Hypertension by Altering Circulating Oxylipins via an α-Linolenic Acid–Induced Inhibition of Soluble Epoxide Hydrolase. Hypertension 2014, 64, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Guarner, V.; Rubio-Ruiz, M.E. Low-Grade Systemic Inflammation Connects Aging, Metabolic Syndrome and Cardiovascular Disease. In Aging and Health—A Systems Biology Perspective; Yashin, A.I., Jazwinski, S.M., Eds.; Interdiscipl Top Gerontol: Basel, Karger, 2015; Volume 40, pp. 99–106. [Google Scholar]
- Franceschi, C. Inflammaging as a Major Characteristic of Old People: Can It Be Prevented or Cured? Nutr. Rev. 2007, 65, 173–176. [Google Scholar] [CrossRef]
- Bernal, G.M.; Wahlstrom, J.S.; Crawley, C.D.; Cahill, K.E.; Pytel, P.; Liang, H.; Kang, S.; Weichselbaum, R.R.; Yamini, B. Loss of Nfkb1 leads to early onset aging. Aging 2014, 6, 931–942. [Google Scholar] [CrossRef] [Green Version]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014, 5, 4172. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Ageing: Is there a role for arachidonic acid and other bioactive lipids? A review. J. Adv. Res. 2018, 11, 67–79. [Google Scholar] [CrossRef]
- Limbkar, K.; Dhenge, A.; Jadhav, D.D.; Thulasiram, H.V.; Kale, V.; Limaye, L. Data on the effect of oral feeding of arachidonic acid or docosahexaenoic acid on haematopoiesis in mice. Data Brief. 2017, 14, 551–557. [Google Scholar] [CrossRef]
- Feldman, J.L.; Baeza, J.; Denu, J.M. Activation of the Protein Deacetylase SIRT6 by Long-chain Fatty Acids and Widespread Deacylation by Mammalian Sirtuins*. J. Biol. Chem. 2013, 288, 31350–31356. [Google Scholar] [CrossRef] [Green Version]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic Instability and Aging-like Phenotype in the Absence of Mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastián, C.; Zwaans, B.M.M.; Silberman, D.M.; Gymrek, M.; Goren, A.; Zhong, L.; Ram, O.; Truelove, J.; Guimaraes, A.R.; Toiber, D.; et al. The Histone Deacetylase SIRT6 Is a Tumor Suppressor that Controls Cancer Metabolism. Cell 2012, 151, 1185–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanfi, Y.; Peshti, V.; Gil, R.; Naiman, S.; Nahum, L.; Levin, E.; Kronfeld-Schor, N.; Cohen, H.Y. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell 2010, 9, 162–173. [Google Scholar] [CrossRef]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nat. Cell Biol. 2012, 483, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Preyat, N.; Leo, O. Sirtuin deacylases: A molecular link between metabolism and immunity. J. Leukoc. Biol. 2013, 93, 669–680. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, A.; Yang, D.; Cui, T.; Yang, H.; Zhang, H.; Wang, C. CYP2J2-produced epoxyeicosatrienoic acids attenuate ischemia/reperfusion-induced acute kidney injury by activating the SIRT1-FoxO3a pathway. Life Sci. 2020, 246, 117327. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; You, Y.; Zhu, H. 15-HETE protects pulmonary artery smooth muscle cells against apoptosis via SIRT1 regulation during hypoxia. Biomed. Pharmacother. 2018, 108, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Song, S.; Zhang, L.; Qiu, Z.; Shi, S.; Liu, Y.; Yao, L.; Zhu, D. 15-Hydroxyeicosatetraenoic acid (15-HETE) protects pulmonary artery smooth muscle cells from apoptosis via inducible nitric oxide synthase (iNOS) pathway. Prostaglandins Other Lipid Mediat. 2012, 97, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Jiang, J.; Wang, R.; Li, L.; Qiu, Z.; Wu, H.; Zhu, D. 15-HETE protects rat pulmonary arterial smooth muscle cells from apoptosis via the PI3K/Akt pathway. Prostaglandins Other Lipid Mediat. 2010, 91, 51–60. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, S.; Wang, Z.; Ma, J.; Liu, S.; Li, W.; Zhu, D. The role of ERK1/2 in 15-HETE-inhibited apoptosis in pulmonary arterial smooth muscle cells. J. Recept. Signal Transduct. Res. 2011, 31, 45–52. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Endo, A.; Tsujikado, K.; Inukai, T. Involvement of Resveratrol and ω-3 Polyunsaturated Fatty Acids on Sirtuin 1 Gene Expression in THP1 Cells. Am. J. Med. Sci. 2017, 354, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; et al. Omega-3 polyunsaturated fatty acids suppress the inflammatory responses of lipopolysaccharide-stimulated mouse microglia by activating SIRT1 pathways. Biochim. et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jia, R.; Yao, Q.; Xu, Y.; Luo, Z.; Luo, X.; Wang, N. Docosahexaenoic acid attenuates adipose tissue angiogenesis and insulin resistance in high fat diet-fed middle-aged mice via a sirt1-dependent mechanism. Mol. Nutr. Food Res. 2016, 60, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Iyengar, N.M.; Morrow, M.; Elemento, O.; Zhou, X.K.; Dannenberg, A.J. Prostaglandin E2 down-regulates sirtuin 1 (SIRT1), leading to elevated levels of aromatase, providing insights into the obesity–breast cancer connection. J. Biol. Chem. 2019, 294, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, Y.; Zhang, S.; Li, C.; Yang, L.; Gao, H.; Wang, X. Resolvin D1 Promotes SIRT1 Expression to Counteract the Activation of STAT3 and NF-κB in Mice with Septic-Associated Lung Injury. Inflammation 2018, 41, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Clara, D.F.; Gesualdo, C.; Testa, F.; Trotta, M.C.; Maisto, R.; Ferraro, B.; Ferraraccio, F.; Accardo, M.; Simonelli, F.; et al. Interplay between Intravitreal RvD1 and Local Endogenous Sirtuin-1 in the Protection from Endotoxin-Induced Uveitis in Rats. Mediat. Inflamm. 2015, 2015, 126408. [Google Scholar] [CrossRef]
- Das, U. Essential fatty acid metabolism in patients with essential hypertension, diabetes mellitus and coronary heart disease. Prostaglandins Leukot. Essent. Fat. Acids 1995, 52, 387–391. [Google Scholar] [CrossRef]
- Sasaki, H.; Sueyasu, T.; Tokuda, H.; Ito, M.; Kaneda, Y.; Rogi, T.; Kawashima, H.; Horiguchi, S.; Kawabata, T.; Shibata, H. Aging and FADS1 polymorphisms decrease the biosynthetic capacity of long-chain PUFAs: A human trial using [U-13C]linoleic acid. Prostaglandins Leukot. Essent. Fat. Acids 2019, 148, 1–8. [Google Scholar] [CrossRef]
- Horiguchi, S.; Nakayama, K.; Iwamoto, S.; Ishijima, A.; Minezaki, T.; Baba, M.; Kontai, Y.; Horikawa, C.; Kawashima, H.; Shibata, H.; et al. Associations between a fatty acid desaturase gene polymorphism and blood arachidonic acid compositions in Japanese elderly. Prostaglandins Leukot. Essent. Fat. Acids 2016, 105, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Mašek, T.; Filipović, N.; Hamzić, L.F.; Puljak, L.; Starčević, K. Long-term streptozotocin diabetes impairs arachidonic and docosahexaenoic acid metabolism and ∆5 desaturation indices in aged rats. Exp. Gerontol. 2014, 60, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, S.; Rabinovitz, S.; Carasso, R.L.; Mostofsky, D.I. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol. Aging 2002, 23, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Foucher, C.; Narce, M.; Nasr, L.; Delachambre, M.C.; Poisson, J.P. Liver microsomal membrane fluidity and microsomal desaturase activities in adult spontaneously hypertensive rats. J. Hypertens. 1997, 15, 863–869. [Google Scholar] [CrossRef]
- Raederstorff, D.; Loechleiter, V.; Moser, U. Polyunsaturated fatty acid metabolism of human skin fibroblasts during cellular aging. Int. J. Vitam. Nutr. Res. 1995, 65, 51–55. [Google Scholar]
- Bordoni, A.; Biagi, P.L.; Turchetto, E.; Hrelia, S. Aging influence on delta-6-desaturase activity and fatty acid compo-sition of rat liver microsomes. Biochem. Int. 1988, 17, 1001–1009. [Google Scholar] [PubMed]
- Hrelia, S.; Bordoni, A.; Celadon, M.; Turchetto, E.; Biagi, P.L.; Rossi, C.A. Age-related changes in linoleate and al-pha-linolenate desaturation by rat liver microsomes. Biochem. Biophys. Res. Commun. 1989, 163, 348–355. [Google Scholar] [CrossRef]
- Chen, H.; Ma, F.; Hu, X.; Jin, T.; Xiong, C.; Teng, X. Elevated COX2 expression and PGE2 production by downregulation of RXRα in senescent macrophages. Biochem. Biophys. Res. Commun. 2013, 440, 157–162. [Google Scholar] [CrossRef]
- Heymes, C.; Habib, A.; Yang, D.; Mathieu, E.; Marotte, F.; Samuel, J.; Boulanger, C.M. Cyclo-oxygenase-1 and −2 contribution to endothelial dysfunction in ageing. Br. J. Pharmacol. 2000, 131, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Gangemi, S.; Pescara, L.; D’Urbano, E.; Basile, G.; Nicita-Mauro, V.; Davì, G.; Romano, M. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp. Gerontol. 2005, 40, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Kinder, M.; Wei, C.; Shelat, S.G.; Kundu, M.; Zhao, L.; Blair, I.A.; Puré, E. Hematopoietic stem cell function requires 12/15-lipoxygenase-dependent fatty acid metabolism. Blood 2010, 115, 5012–5022. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Aggarwal, N.; Holmes, B.B.; Kuhn, H.; Campbell, W.B. Age-related decrease in 15-lipoxygenase contributes to reduced vasorelaxation in rabbit aorta. Am. J. Physiol. Circ. Physiol. 2008, 294, H679–H687. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Luciotti, G.; D’Urbano, E.; Mallamace, A.; Santoro, D.; Bellinghieri, G.; Davi, G.; Romano, M. Physical exercise increases urinary excretion of lipoxin A4 and related compounds. J. Appl. Physiol. 2003, 94, 2237–2240. [Google Scholar] [CrossRef] [Green Version]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Cameron-Smith, D. Human inflammatory and resolving lipid mediator responses to re-sistance exercise and ibuprofen treatment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 1281–1296. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Kwon, S.; Ham, S.; Lee, D.; Park, H.H.; Yamaoka, Y.; Jeong, D.; Artan, M.; Altintas, O.; Park, S.; et al. Caenorhabditis elegans Lipin 1 moderates the lifespan-shortening effects of dietary glucose by maintaining ω-6 polyunsaturated fatty acids. Aging Cell 2020, 19, 13150. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, E.J.; Kuballa, P.; Xavier, R.; Ruvkun, G. ω-6 Polyunsaturated fatty acids extend life span through the acti-vation of autophagy. Genes Dev. 2013, 27, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Watts, J.L.; Phillips, E.; Griffing, K.R.; Browse, J. Deficiencies in C20 polyunsaturated fatty acids cause behavioral and developmental defects in Caenorhabditis elegans fat-3 mutants. Genetics 2003, 163, 581–589. [Google Scholar]
- Lesa, G.M.; Palfreyman, M.; Hall, D.H.; Clandinin, M.T.; Rudolph, C.; Jorgensen, E.M.; Schiavo, G. Long chain polyunsaturated fatty acids are required for efficient neu-rotransmission in C. elegans. J. Cell Sci. 2003, 116, 4965–4975. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Molecular Biochemical Aspects of Cancer; Springer Nature: New York, NY, USA, 2020. [Google Scholar]
- Das, U.N. A Perinatal Strategy for Preventing Adult Diseases: The Role of Long-Chain Polyunsaturated Fatty Acids; Kluwer Academic Publishers: Boston, MA, USA, 2002. [Google Scholar]
- Das, U.N. Metabolic Syndrome Pathophysiology: The Role of Lipoxins and Their Metabolites; Wiley-Blackwell Publishers: Ames, IA, USA, 2010. [Google Scholar]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [Green Version]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.C.; Santos, R.X.; Perry, G.; Zhu, X.; Moreira, P.I.; Smith, M.A. Insulin-resistant brain state: The culprit in sporadic Alzheimer’s disease? Ageing Res. Rev. 2011, 10, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 19, 152–169. [Google Scholar] [CrossRef]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; De La Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s Disease is this type 3 diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šmidák, R.; Koefeler, H.; Hoeger, H.; Lubec, G. Comprehensive identification of age-related lipidome changes in rat amygdala during normal aging. PLoS ONE 2017, 12, e0180675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, S.E.; Friedrich, M.G.; Mitchell, T.W.; Truscott, R.J.W.; Else, P.L. Decreases in Phospholipids Containing Adrenic and Arachidonic Acids Occur in the Human Hippocampus over the Adult Lifespan. Lipids 2015, 50, 861–872. [Google Scholar] [CrossRef]
- Weir, J.M.; Wong, G.; Barlow, C.K.; Greeve, M.A.; Kowalczyk, A.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Jowett, J.B.M.; Shaw, J.; et al. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 2013, 54, 2898–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.L.F.; Huynh, K.; Chatterjee, P.; Martins, I.; Jayawardana, K.S.; Giles, C.; Mellett, N.A.; Laws, S.M.; Bush, A.I.; Rowe, C.C.; et al. Relationships Between Plasma Lipids Species, Gender, Risk Factors, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 303–315. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Can memory be improved? A discussion on the role of ras, GABA, acetylcholine, NO, insulin, TNF-α, and long-chain polyunsaturated fatty acids in memory formation and consolidation. Brain Dev. 2003, 25, 251–261. [Google Scholar] [CrossRef]
- Das, U.N. Long-chain polyunsaturated fatty acids in memory formation and consolidation: Further evidence and discussion. Nutrition 2003, 19, 988–993. [Google Scholar] [CrossRef]
- Das, U.N. Nutritional factors in the pathobiology of autism. Nutrition 2013, 29, 1066–1069. [Google Scholar] [CrossRef]
- Das, U.N. Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of poly-unsaturated fatty acids. Nutrition 2013, 29, 1175–1185. [Google Scholar] [CrossRef]
- Janssen, C.I.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Conquer, J.A.; Tierney, M.C.; Zecevic, J.; Bettger, W.J.; Fisher, R.H. Fatty acid analysis of blood plasma of patients with alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 2000, 35, 1305–1312. [Google Scholar] [CrossRef]
- Stephenson, D.T.; Lemere, C.A.; Selkoe, D.J.; Clemens, J.A. Cytosolic Phospholipase A2(cPLA2) Immunoreactivity Is Elevated in Alzheimer’s Disease Brain. Neurobiol. Dis. 1996, 3, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Kudo, I.; Murakami, M. Phospholipase A2 Enzymes. Prostaglandins Other Lipid Mediat. 2002, 68–69, 3–58. [Google Scholar] [CrossRef]
- Balboa, M.A.; Varela-Nieto, I.; Lucas, K.K.; Dennis, E.A. Expression and function of phospholipase A2 in brain. FEBS Lett. 2002, 531, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Holscher, C.; Canevari, L.; Richter-Levin, G. Inhibitors of PLA2 and NO synthase cooperate in producing amnesia of a spatial task. NeuroReport 1995, 6, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Holscher, C.; Rose, S.P. Inhibitors of phospholipase A2 produce amnesia for a passive avoidance task in the chick. Behav. Neural Biol. 1994, 61, 225–232. [Google Scholar] [CrossRef]
- Schaeffer, E.L.; Gattaz, W.F. Requirement of hippocampal phospholipase A2 activity for long-term memory retrieval in rats. J. Neural Transm. 2006, 114, 379–385. [Google Scholar] [CrossRef]
- Fujita, S.; Ikegaya, Y.; Nishiyama, N.; Matsuki, N. Ca2+-Independent Phospholipase A2 Inhibitor Impairs Spatial Memory of Mice. Jpn. J. Pharmacol. 2000, 83, 277–278. [Google Scholar] [CrossRef]
- Strokin, M.; Sergeeva, M.; Reiser, G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+. Br. J. Pharmacol. 2003, 139, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Mury, F.B.; da Silva, W.C.; Barbosa, N.R.; Mendes, C.T.; Bonini, J.S.; Sarkis, J.E.; Cammarota, M.; Izquierdo, I.; Gattaz, W.F.; Dias-Neto, E. Lithium activates brain phospholipase A2 and improves memory in rats: Implications for Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 607–618. [Google Scholar] [CrossRef]
- Nomura, T.; Nishizaki, T.; Enomoto, T.; Itoh, H. A long-lasting facilitation of hippocampal neurotransmission via a phospholipase A2 signaling pathway. Life Sci. 2001, 68, 2885–2891. [Google Scholar] [CrossRef]
- Williams, J.H.; Errington, M.L.; Lynch, M.A.; Bliss, T.V.P. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nat. Cell Biol. 1989, 341, 739–742. [Google Scholar] [CrossRef]
- Lynch, M.; Errington, M.; Bliss, T. Nordihydroguaiaretic acid blocks the synaptic component of long-term potentiation and the associated increases in release of glutamate and arachidonate: An in vivo study in the dentate gyrus of the rat. Neuroscience. 1989, 30, 693–701. [Google Scholar] [CrossRef]
- Dumuis, A.; Sebben, M.; Haynes, L.P.; Pin, J.-P.; Bockaert, J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nat. Cell Biol. 1988, 336, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Yamada, K.A.; Finn, M.B.; Sloviter, R.S.; Bales, K.R.; May, P.C.; Schoepp, D.D.; Paul, S.M.; Mennerick, S.; Holtzman, D.M. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuronology 2005, 48, 913–922. [Google Scholar]
- Ho, C.F.-Y.; Ismail, N.B.; Koh, J.K.-Z.; Gunaseelan, S.; Low, Y.-H.; Ng, Y.-K.; Chua, J.J.-E.; Ong, W.-Y. Localisation of Formyl-Peptide Receptor 2 in the Rat Central Nervous System and Its Role in Axonal and Dendritic Outgrowth. Neurochem. Res. 2018, 43, 1587–1598. [Google Scholar] [CrossRef] [Green Version]
- Shalini, S.-M.; Ho, C.F.-Y.; Ng, Y.-K.; Tong, J.-X.; Ong, E.-S.; Herr, D.R.; Dawe, G.S.; Ong, W.-Y. Distribution of Alox15 in the Rat Brain and Its Role in Prefrontal Cortical Resolvin D1 Formation and Spatial Working Memory. Mol. Neurobiol. 2018, 55, 1537–1550. [Google Scholar] [CrossRef] [Green Version]
- Bazan, N.G. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Lukiw, W.J.; Cui, J.-G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Molecular pathobiology of scleritis and its therapeutic implications. Int. J. Ophthalmol. 2020, 13, 163–175. [Google Scholar] [CrossRef]
- Ferguson, A.C.; Tank, R.; Lyall, L.M.; Ward, J.; Celis-Morales, C.; Strawbridge, R.; Ho, F.; Whelan, C.D.; Gill, J.; Welsh, P.; et al. Alzheimer’s Disease Susceptibility Gene Apolipoprotein E (APOE) and Blood Biomarkers in UK Biobank (N = 395,769). J. Alzheimer’s Dis. 2020, 76, 1–11. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, N.; Kakutani, S.; Kawashima, H.; Shibata, H.; Morita, I. Dietary supplementation of arachidonic acid increases arachidonic acid and lipoxin A4 contents in colon, but does not affect severity or prostaglandin E2 content in murine colitis model. Lipids Health. Dis. 2014, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Tateishi, N.; Kaneda, Y.; Kakutani, S.; Kawashima, H.; Shibata, H.; Morita, I. Dietary supplementation with arachidonic acid increases arachidonic acid content in paw, but does not affect arthritis severity or prostaglandin E2 content in rat adjuvant-induced arthritis model. Lipids Health. Dis. 2015, 14, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakutani, S.; Ishikura, Y.; Tateishi, N.; Horikawa, C.; Tokuda, H.; Kontani, M.; Kawashima, H.; Sakakibara, Y.; Kiso, Y.; Shibata, H.; et al. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: A randomized controlled study. Lipids Health. Dis. 2011, 10, 241. [Google Scholar] [CrossRef] [Green Version]
- Rotstein, N.P.; De Boschero, M.G.I.; Giusto, N.M.; Aveldaño, M.I. Effects of aging on the composition and metabolism of docosahexaenoate-containing lipids of retina. Lipids 1987, 22, 253–260. [Google Scholar] [CrossRef]
- Giusto, N.M.; Salvador, G.A.; Castagnet, P.I.; Pasquaré, S.J.; Ilincheta de Boschero, M.G. Age-associated changes in central nervous system glycerolipid composition and metabolism. Neurochem. Res. 2002, 27, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Ferchaud-Roucher, V.; Zair, Y.; Aguesse, A.; Krempf, M.; Ouguerram, K. Omega 3 Improves Both apoB100-containing Lipoprotein Turnover and their Sphingolipid Profile in Hypertriglyceridemia. J. Clin. Endocrinol. Metab. 2020, 105, 3152–3164. [Google Scholar] [CrossRef]
- Mitchell, T.W.; Buffenstein, R.; Hulbert, A.J. Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): A comparative study using shotgun lipidomics. Exp. Gerontol. 2007, 42, 1053–1062. [Google Scholar] [CrossRef]
- Andziak, B.; O’Connor, T.P.; Qi, W.; DeWaal, E.M.; Pierce, A.; Chaudhuri, A.R.; Van Remmen, H.; Buffenstein, R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell 2006, 5, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. PUFAs, BDNF and lipoxin A4 inhibit chemical-induced cytotoxicity of RIN5F cells in vitro and streptozotocin-induced type 2 diabetes mellitus in vivo. Lipids Heal. Dis. 2019, 18, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Siresha, B.; Naveen, K.V.G.; Poorani, R.; Sailaja, P.; Devi, H.D.; Monika, S.; Das, U.N. Resolvin D1 ameliorates nico-tinamide-streptozotocin-induced type 2 diabetes mellitus by its anti-inflammatory action and modulating PI3K/Akt/mTOR pathway in the brain. Arch. Med. Res. 2020, 51, 492–503. [Google Scholar]
- Das, U.N. Essential fatty acids, lipid peroxidation and apoptosis. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Das, U. Tumoricidal action of cis-unsaturated fatty acids and their relationship to free radicals and lipid peroxidation. Cancer Lett. 1991, 56, 235–243. [Google Scholar] [CrossRef]
- Das, U.N.; Huang, Y.-S.; Bēgin, M.E.; Ells, G.; Horrobin, D.F. Uptake and distribution of cis-unsaturated fatty acids and their effect on free radical generation in normal and tumor cells in vitro. Free Radic. Biol. Med. 1987, 3, 9–14. [Google Scholar] [CrossRef]
- Das, U.; Begin, M.; Ells, G.; Huang, Y.; Horrobin, D. Polyunsaturated fatty acids augment free radical generation in tumor cells in vitro. Biochem. Biophys. Res. Commun. 1987, 145, 15–24. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids enhance free radical generation and lipid peroxidation to induce apoptosis of tumor cells. Clin. Lipidol. 2011, 6, 463–489. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive lipids as modulators of immune check point inhibitors. Med. Hypotheses. 2020, 135, 109473. [Google Scholar] [CrossRef]
- Viswanathan, V.S.; Ryan, M.J.; Dhruv, H.D.; Gill, S.; Eichhoff, O.M.; Seashore-Ludlow, B.; Kaffenberger, S.D.; Eaton, J.K.; Shimada, K.; Aguirre, A.J.; et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nat. Cell Biol. 2017, 547, 453–457. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nat. Cell Biol. 2017, 551, 247–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, G.R.; Das, U.N.; Rao, K.P.; Rao, M.S. Prostaglandins and mutagenesis: Prevention and/or reversibility of genetic damage induced by benzo (a) pyrene in the bone marrow cells of mice by prostaglandins El. Prostaglandins Leukotrienes Med. 1984, 15, 287–292. [Google Scholar]
- Ponnala, S.; Rao, K.P.; Chaudhury, J.R.; Ahmed, J.; Rao, B.R.; Kanjilal, S.; Hasan, Q.; Das, U.N. Effect of polyunsaturated fatty acids on diphenyl hydantoin-induced genetic damage in vitro and in vivo. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 43–50. [Google Scholar] [CrossRef]
- Das, U.N. Minerals, trace elements and vitamins interact with essential fatty acids and prostaglandins to prevent hypertension, thrombosis, hypercholesterolemia and atherosclerosis and their attendant complications. IRCS J. Med. Sci. 1985, 13, 684–687. [Google Scholar]
- Sandhya, P.; Das, U.N. Vitamin C therapy for maturity onset diabetes mellitus: Relevance to prostaglandin in-volvement. IRCS Med. Sci. 1981, 9, 618. [Google Scholar]
- Das, U.N. Vitamin C for Type 2 Diabetes Mellitus and Hypertension. Arch. Med. Res. 2019, 50, 11–14. [Google Scholar] [CrossRef]
- Das, U.N. Magnesium and cardiovascular diseases. J. Cardiovasc. Pharmacol. 2019, 74, 508–510. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Giamarellos-Bourboulis, E.J.; Skiathitis, S.; Demetzos, C.; Donta, I.; Papaioannou, G.T.; Dionyssiou-Asteriou, A.; Karayannacos, P.E.; Giamarellou, H. Rapid alterations of serum fatty acids with the intravenous administration of an arachidonate solution. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Sacks, F. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabo-lism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907. [Google Scholar] [PubMed] [Green Version]
- Nelson, G.J.; Schmidt, P.C.; Bartolini, G.; Kelley, D.S.; Kyle, D. The effect of dietary arachidonic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids 1997, 32, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Wilborn, C.M.; Roberts, C.; Kerksick, M.I.; Taylor, L.; Campbell, B.; Kreider, R. Changes in whole blood and clinical safety markers over 50 days of concomitant arachidonic acid supplementation and resistance training. In Proceedings of the International Society of Sports Nutrition Archived 2011-07-07 at the Wayback Machine, (ISSN) Conference, Portland, OR, USA, 15–17 June 2006. [Google Scholar]
- De Souza, E.O.; Lowery, R.P.; Wilson, J.M.; Sharp, M.H.; Mobley, C.B.; Fox, C.D.; Lopez, H.L.; Shields, K.A.; Rauch, J.T.; Healy, J.C.; et al. Effects of Arachidonic Acid Supplementation on Acute Anabolic Signaling and Chronic Functional Performance and Body Composition Adaptations. PLoS ONE 2016, 11, e0155153. [Google Scholar] [CrossRef] [Green Version]









| Fatty Acid | Plasma | Liver | Stomach | Duodenum | Skeletal Muscle |
|---|---|---|---|---|---|
| 14:0 (MA) | 0.39 ± 0.1 | 0.14 ± 0.04 | 0.49 ± 0.08 | 1.26 ± 0.37 | - |
| 16:0 (PA) | 36.84 ± 1.85 | 27.6 ± 0.49 | 27.16 ± 4.59 | 27.59 ± 3.9 | 23.66 ± 2.19 |
| 18:0 (SA) | 14.55 ± 3.52 | 11.67 ± 1.75 | 18.22 ± 2.93 | 8.17 ± 1.24 | 15.54 ± 1.35 |
| 18:1 n−9 (OA) | 3.74 ± 0.53 | 2.48 ± 0.37 | 14.09 ± 2.51 | 7.66 ± 1.86 | 9.79 ± 1.43 |
| 18:2 n−6 (LA) | 15.44 ± 1.44 | 11.27 ± 1.76 | 14.51 ± 3.41 | 8.54 ± 1.22 | 19.66 ± 1.79 |
| 18:3 n−6 (GLA) | 0.22 ± 0.07 | 0.24 ± 0.02 | - | - | 0.42 ± 0.06 |
| 20:3 n−6 (DGLA) | - | 0.24 ± 0.02 | - | - | 0.42 ± 0.05 |
| 20:4 n−6 (AA) | 22.83 ± 2.3 | 32.6 ± 1.70 | 14.12 ± 1.82 | 16.09 ± 1.04 | 20.09 ± 2.42 |
| 18:3 n−3 (ALA) | 0.22 ± 0.07 | 0.33 ± 0.09 | 0.42 ± 0.25 | 0.51 ± 0.07 | 0.50 ± 0.08 |
| 20:5 n−3 (EPA) | 0.33 ± 0.08 | 0.59 ± 0.08 | 0.32 ± 0.02 | 0.35 ± 0.02 | 0.49 ± 0.05 |
| 22:6 n−3 (DHA) | 1.94 ± 0.24 | 1.76 ± 0.41 | 1.0 ± 0.08 | 1.08 ± 0.2 | 4.14 ± 0.49 |
| Property/Action | LXA4 | PGE1 |
|---|---|---|
| Derived from | Arachidonic acid (AA) | Di-homo-gamma-linolenic acid (DGLA) |
| Rate limiting step in AA/DGLA synthesis | Delta-6- and delta-5-desaturases | Delta-6-desaturase |
| Platelet anti-aggregator | ++ | + |
| Vasodilator | ++ | + |
| Anti-inflammatory action | ++ | + |
| Suppresses IL-6 and TNF-α | ++ | + |
| Cytoprotective action | +++ | ++ |
| Geno-protective action | +/− | ++ |
| Anti-diabetic action | ++ | + |
| Suppresses ROS generation | ++ | + |
| Suppression of PGE2 production | ++ | Not known |
| Inflammation resolution action | ++ | + |
| Wound healing action | ++ | ++ |
| Blood pressure lowering action | + | + |
| Anti-arrhythmic action | +/− | +/− |
| Protects endothelium | ++ | ++ |
| PGE2 can trigger synthesis | Yes | Not known |
| Anti-microbial action | ++ | + |
| Has a specific receptor | Yes-ALX | Yes-EP1 and EP3 |
| Half-life | Few seconds | 5–30 min |
| Fatty Acid | Control (n = 10) | Pneumonia (n = 12) | Septicemia (n = 14) | RA (n = 12) | SLE (Lupus) (n = 5) |
|---|---|---|---|---|---|
| 16:0 | 24.8 ± 3.4 | 32.5 ± 3.6 | 26.95 ± 4.1 | 30.2 ± 3.0 | 32.0 ± 3.75 |
| 18:0 | 23.3 ± 4.1 | 21.4 ± 7.1 | 24.58 ± 6.0 | 19.0 ±6.1 | 14.6 ± 5.82 |
| 18:1 n−9 | 13.1 ± 2.3 | 15.6 ± 3.2 | 16.5 ± 3.3 * | 14.8 ± 2.1 | 16.0 ± 2.78 |
| 18:2 n−6 | 17.7 ± 3.1 | 14.2 ± 0.3 * | 16.3 ± 2.4 | 17.5 ± 2.7 | 20.8 ± 2.2 |
| 18:3 n−6 | 0.13 ± 0.09 | 0.13 ± 0.08 | 0.04 ± 0.05 * | 0.02 ± 0.04 ** | 0.01 ± 0.01 ** |
| 20:3 n−6 | 3.2 ± 0.79 | 1.5 ± 0.4 * | 0.46 ± 0.54 * | 2.5 ± 0.58 | 2.12 ± 0.52 |
| 20:4 n−6 | 8.8 ± 2.0 | 5.1 ±0.4 * | 5.8 ± 1.6 * | 9.5 ± 2.2 | 8.93 ± 2.0 |
| 22:4 n−6 | 0.42 ± 0.23 | 0.8 ± 0.9 | 0.34 ± 0.28 | 0.26 ± 0.37 ** | 0.18 ± 0.18 ** |
| 22:5 n−6 | 0.73 ± 0.55 | 0.45 ± 0.63 | 1.5 ± 1.02 * | 0.6 ± 0.7 | 0.8 ± 1.0 |
| 18:3 n−3 | 0.27 ± 0.12 | 0.09 ± 0.04 * | 0.16 ± 0.11 * | 0.12 ± 0.16 * | 0.1 ± 0.1 * |
| 20:5 n−3 | 0.25 ± 0.26 | 0.23 ± 0.24 | 0.01 ± 0.01 * | 0.05 ± 0.14 ** | 0.04 ± 0.04 ** |
| 22:6 n−3 | 1.43 ± 0.43 | 0.54 ± 0.43 * | 1.2 ± 1.14 | 0.62 ± 0.56 * | 0.88 ± 0.75 * |
| Measurement (Fatty Acid) | Normal Intact Liver | Intact Yoshida Cells | Normal Liver Microsomes | Yoshida Microsomes |
|---|---|---|---|---|
| 16:0 | 18.5 ± 0.2 | 18.7 ± 2.0 | 18.9 ± 1.1 | 18.5 ± 0.5 |
| 18:0 | 17.5 ± 0.5 | 13.3 ± 1.1 | 22.0 ± 3.0 | 13.7 ± 0.2 |
| 18:1, n−9 (oleic acid) | 12.1 ± 1.0 | 21.5 ± 0.8 | 8.6 ± 1.0 | 18.1 ± 0.3 |
| 20:4 (AA) | 16.7 ± 2.4 | 8.7 ± 0.7 | 19.1 ± 2.4 | 9.6 ± 0.8 |
| 22:5 | - | 2.9 ± 0.1 | - | 2.4 ± 0.3 |
| 22:6 (DHA) | 6.3 ± 0.2 | 5.2 ± 0.6 | 6.1 ± 0.3 | 5.3 ± 0.4 |
| Fatty Acid | Control | HTN | CHD | Type 2 DM | Diabetic Nephropathy |
|---|---|---|---|---|---|
| 16:0 | 25.9 ± 3.0 | 29.3 ± 2.7 * | 27.8 ± 3.5 | 26.6 ± 5.2 | 26.8 ± 2.7 |
| 18:0 | 20.9 ± 3.6 | 23.2 ± 4.9 * | 18:0 ± 10.7 | 14.6 ± 4.1 | 11.6 ± 3.6 * |
| 18:1 n−9 | 13.0 ± 2.3 | 12.1 ± 1.5 | 11.5 ± 3.1 | 12.0 ± 2.6 | 14.5 ± 3.1 |
| 18:2 n−6 (LA) | 18.6 ± 3.1 | 14.5 ± 3.1 * | 17.8 ± 5.0 | 13.9 ± 5.3 | 15.1 ± 3.1 |
| 18:3 n−6 (GLA) | 0.14 ± 0.1 | 0.4 ± 0.3 * | 0.1 ± 0.1 * | 0.2 ± 0.3 | 0.1 ± 0.2 |
| 20:3 n−6 (DGLA) | 3.4 ± 1.0 | 3.1 ± 0.9 | 2.7 ± 1.1 | 1.7 ± 1.0 * | 2.0 ± 0.8 * |
| 20:4 n−6 (AA) | 9.4 ± 1.8 | 7.8 ± 2.0 * | 7.0 ± 2.1 * | 4.6 ± 1.8 * | 6.6 ± 2.6 * |
| 22:5 n−6 | 0.7 ± 0.4 | 0.4 ± 0.4 * | 1.0 ± 0.9 | 2.1 ± 0.6 * | 1.3 ± 0.5 * |
| 18:3 n−6/18:2 n−6 | 0.008 | 0.026 | 0.005 | 0.017 | 0.008 |
| 20:4 n−6/18:2 n−6 | 0.51 | 0.54 | 0.39 | 0.33 | 0.43 |
| 20:4 n−6/20:3−6 | 2.8 | 2.53 | 2.59 | 2.8 | 3.3 |
| 18:3 n−3 (ALA) | 0.2 ± 0.1 | 0.4 ± 0.2 * | 0.3 ± 0.5 | 0.1 ± 0.2 * | 0.1 ± 0.1 * |
| 20:5 n−3 (EPA) | 0.4 ± 0.4 | 0.6 ± 0.6 | 0.1 ± 0.2 * | 0.3 ± 0.3 | 0.2 ± 0.3 |
| 22:5 n−3 | 0.5 ± 0.2 | 0.4 ± 0.5 | 0.3 ± 0.3 * | 1.6 ± 1.3 | 1.7 ± 1.1 |
| 22:6 n−3 (DHA) | 1.4 ± 0.5 | 1.2 ± 0.6 | 0.8 ± 0.4 * | 0.5 ± 0.4 * | 0.5 ± 0.3 * |
| 20:5 n−3/18:3 n−3 | 1.8 | 1.39 | 0.41 | 3.2 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, U.N. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. https://doi.org/10.3390/biom11020241
Das UN. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules. 2021; 11(2):241. https://doi.org/10.3390/biom11020241
Chicago/Turabian StyleDas, Undurti N. 2021. "“Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications" Biomolecules 11, no. 2: 241. https://doi.org/10.3390/biom11020241
APA StyleDas, U. N. (2021). “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules, 11(2), 241. https://doi.org/10.3390/biom11020241





