Behavioral Effects of Developmental Exposure to JWH-018 in Wild-Type and Disrupted in Schizophrenia 1 (disc1) Mutant Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
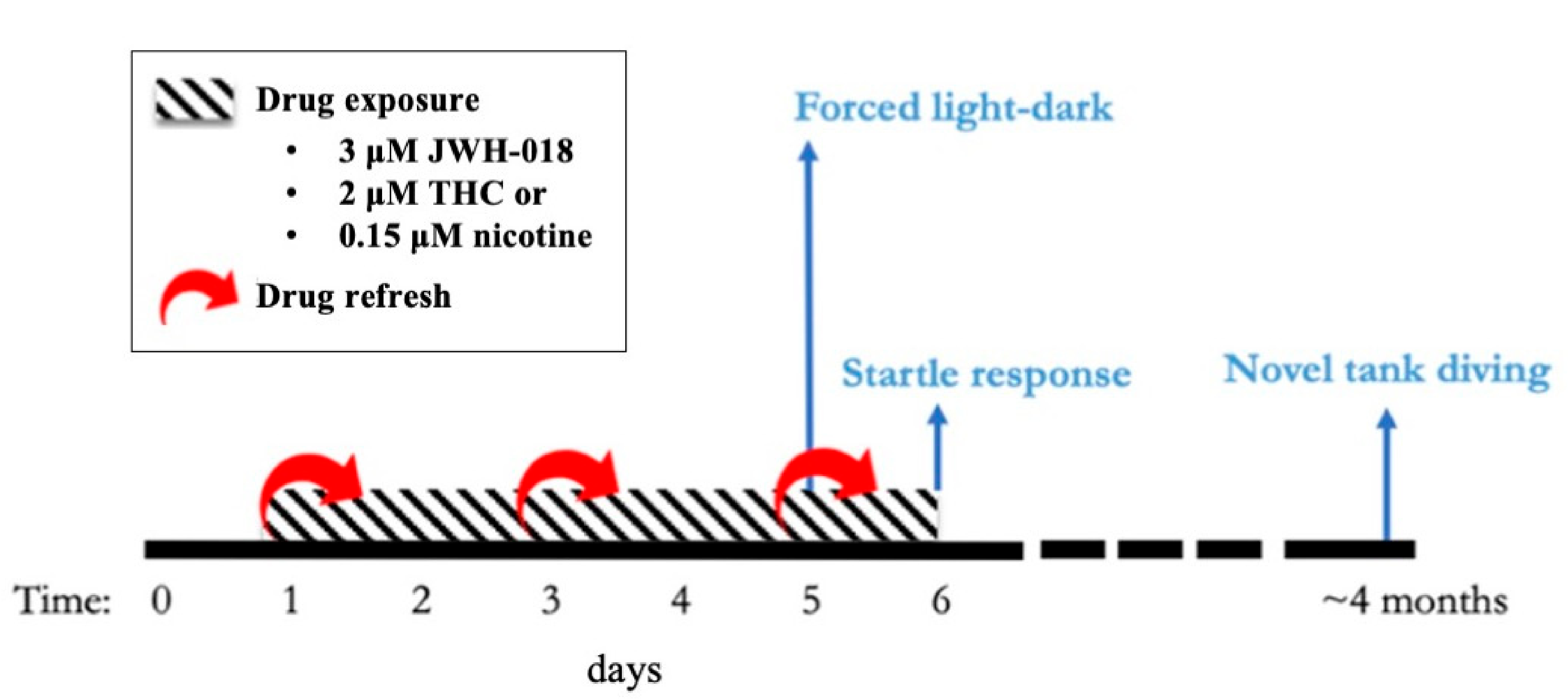
2.1. Experimental Design and Timeline
2.2. Animal Maintenance
2.3. Developmental Drug Exposure
2.3.1. Developmental Exposure to JWH-018, THC, and Nicotine in Wild-type Tübingen Larvae
2.3.2. Developmental Exposure to JWH-018 in disc1 Mutant Larvae
2.4. Behavioral Assays
2.4.1. Forced Light/Dark Test
2.4.2. Response and Habituation to Startle Stimuli Test
2.4.3. Novel Tank Diving Test
2.4.4. Code Availability
2.5. Competitive Allele-Specific PCR (KASP) disc1 Larvae Genotyping
3. Results
3.1. Larval Behavior during Developmental Exposure to JWH-018 in Wild-Type Zebrafish
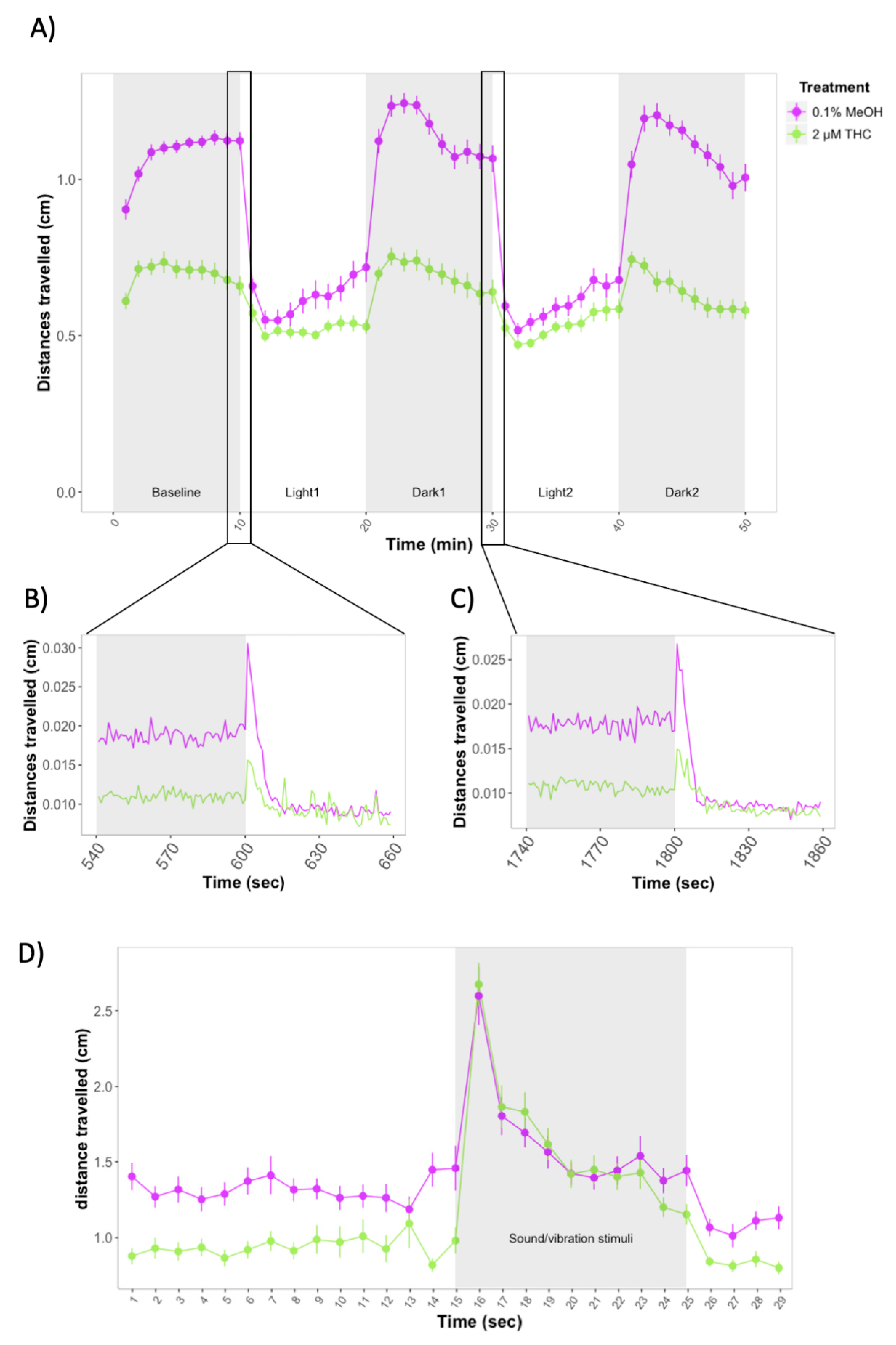
3.2. Larval Behavior during Developmental Exposure to THC and Nicotine in Wild-Type Zebrafish
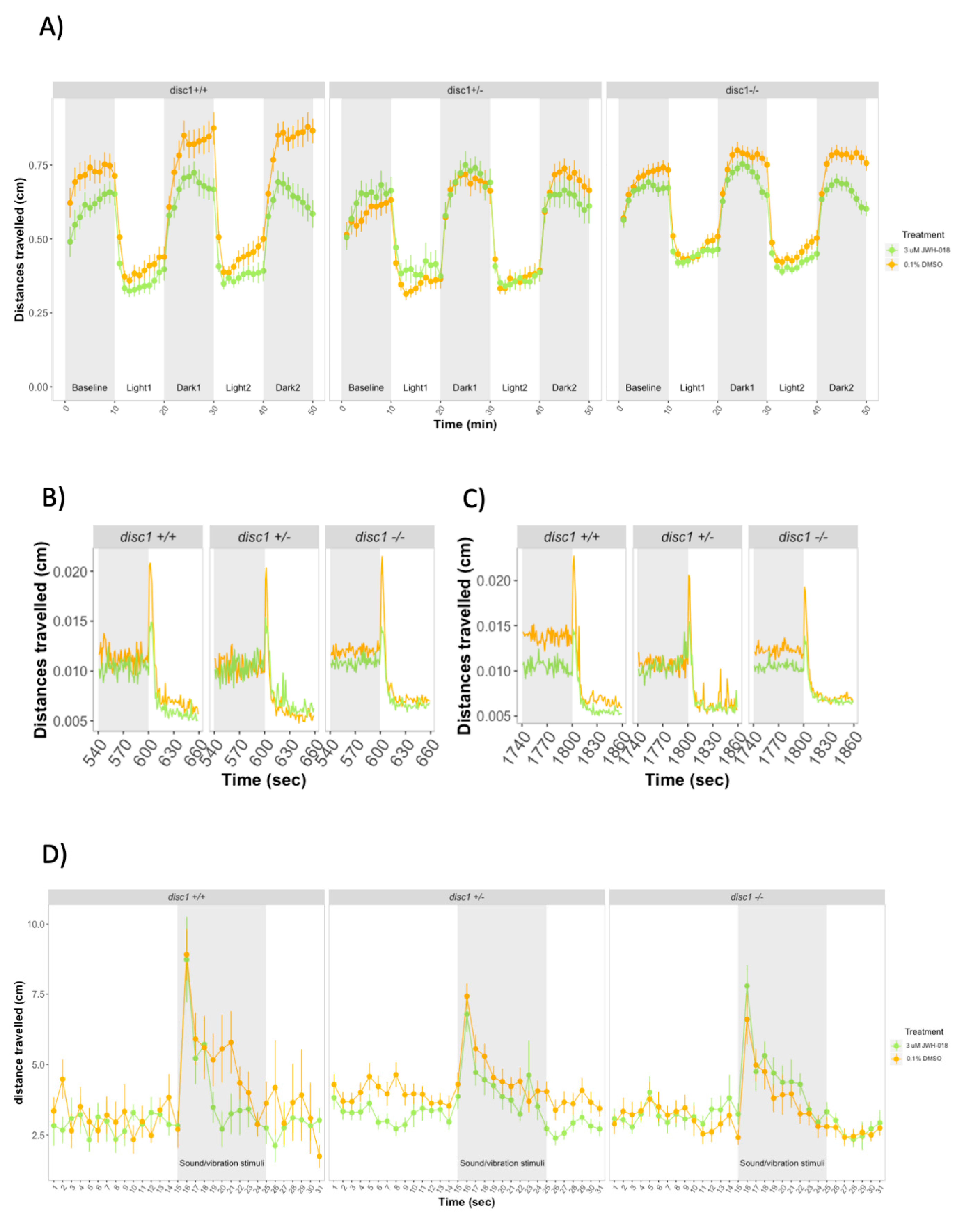
3.3. Larval Behavior during Developmental Exposure to JWH-018 in Wild-Type and Mutant disc1 Zebrafish
3.4. Larval Behavior during Developmental Exposure to THC in Wild-Type and Mutant disc1 Zebrafish
3.5. Adult Behavior after Developmental Exposure to JWH-018 in Wild-Type and Mutant disc1 Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic Cannabinoids: Epidemiology, Pharmacodynamics, and Clinical Implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeffler, G.; Delaney, E.; Hann, M. International Trends in Spice Use: Prevalence, Motivation for Use, Relationship to Other Substances, and Perception of Use and Safety for Synthetic Cannabinoids. Brain Res. Bull. 2016, 126, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Young-Wolff, K.C.; Tucker, L.-Y.; Alexeeff, S.; Armstrong, M.A.; Conway, A.; Weisner, C.; Goler, N. Trends in Self-Reported and Biochemically Tested Marijuana Use Among Pregnant Females in California From 2009–2016. JAMA 2017, 318, 2490–2491. [Google Scholar] [CrossRef]
- Fride, E. The Endocannabinoid-CB Receptor System: Importance for Development and in Pediatric Disease. Neuro Endocrinol. Lett. 2004, 25, 24–30. [Google Scholar]
- Jaques, S.C.; Kingsbury, A.; Henshcke, P.; Chomchai, C.; Clews, S.; Falconer, J.; Abdel-Latif, M.E.; Feller, J.M.; Oei, J.L. Cannabis, the Pregnant Woman and Her Child: Weeding out the Myths. J. Perinatol. 2014, 34, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Järbe, T.U.C.; Deng, H.; Vadivel, S.K.; Makriyannis, A. Cannabinergic Aminoalkylindoles, Including AM678=JWH018 Found in “Spice”, Examined Using Drug (Δ(9)-Tetrahydrocannabinol) Discrimination for Rats. Behav. Pharmacol. 2011, 22, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Ginsburg, B.C.; Schulze, D.R.; Hruba, L.; McMahon, L.R. JWH-018 and JWH-073: Δ9-Tetrahydrocannabinol-Like Discriminative Stimulus Effects in Monkeys. J. Pharmacol. Exp. Ther. 2012, 340, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, R.J.; Caldicott, D.; Mountain, D.; Hill, S.L.; Lenton, S. A Systematic Review of Adverse Events Arising from the Use of Synthetic Cannabinoids and Their Associated Treatment. Clin. Toxicol. 2016, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, E.L.; Hutten, N.R.P.W.; Mason, N.L.; Toennes, S.W.; Kuypers, K.P.C.; de Sousa Fernandes Perna, E.B.; Ramaekers, J.G. Neurocognition and Subjective Experience Following Acute Doses of the Synthetic Cannabinoid JWH-018: A Phase 1, Placebo-Controlled, Pilot Study. Br. J. Pharmacol. 2018, 175, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Carlier, J.; Huestis, M.A.; Zaami, S.; Pichini, S.; Busardò, F.P. Monitoring the Effects of Perinatal Cannabis and Synthetic Cannabinoid Exposure. Ther. Drug Monit. 2019. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Brandon, N.J.; Sawa, A. Linking Neurodevelopmental and Synaptic Theories of Mental Illness through DISC1. Nat. Rev. Neurosci. 2011, 12, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.A.; Brunton, M.; Frackiewicz, A.; Newton, M.; Cook, P.J.L.; Robson, E.B. Studies on a Family with Three Cytogenetic Markers. Ann. Hum. Genet. 1970, 33, 325–336. [Google Scholar] [CrossRef]
- Blackwood, D.H.R.; Fordyce, A.; Walker, M.T.; St’Clair, D.M.; Porteous, D.J.; Muir, W.J. Schizophrenia and Affective Disorders—Cosegregation with a Translocation at Chromosome 1q42 That Directly Disrupts Brain-Expressed Genes: Clinical and P300 Findings in a Family. Am. J. Hum. Genet. 2001, 69, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Ballinger, M.D.; Saito, A.; Abazyan, B.; Taniguchi, Y.; Huang, C.-H.; Ito, K.; Zhu, X.; Segal, H.; Jaaro-Peled, H.; Sawa, A.; et al. Adolescent Cannabis Exposure Interacts with Mutant DISC1 to Produce Impaired Adult Emotional Memory. Neurobiol. Dis. 2015, 82, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Jouroukhin, Y.; Zhu, X.; Shevelkin, A.V.; Hasegawa, Y.; Abazyan, B.; Saito, A.; Pevsner, J.; Kamiya, A.; Pletnikov, M.V. Adolescent Δ9-Tetrahydrocannabinol Exposure and Astrocyte-Specific Genetic Vulnerability Converge on Nuclear Factor-KB-Cyclooxygenase-2 Signaling to Impair Memory in Adulthood. Biol. Psychiatry 2019, 85, 891–903. [Google Scholar] [CrossRef]
- Clark, K.J.; Boczek, N.J.; Ekker, S.C. Stressing Zebrafish for Behavioral Genetics. Rev. Neurosci 2011, 22, 49–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Bossé, G.D.; Peterson, R.T. Development of an Opioid Self-Administration Assay to Study Drug Seeking in Zebrafish. Behav. Brain Res. 2017, 335, 158–166. [Google Scholar] [CrossRef]
- Iacono, W.G. Endophenotypes in Psychiatric Disease: Prospects and Challenges. Genome Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irons, T.D.; MacPhail, R.C.; Hunter, D.L.; Padilla, S. Acute Neuroactive Drug Exposures Alter Locomotor Activity in Larval Zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Sensorimotor Gating in Larval Zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef] [Green Version]
- Rankin, C.H.; Abrams, T.; Barry, R.J.; Bhatnagar, S.; Clayton, D.; Colombo, J.; Coppola, G.; Geyer, M.A.; Glanzman, D.L.; Marsland, S.; et al. Habituation Revisited: An Updated and Revised Description of the Behavioral Characteristics of Habituation. Neurobiol. Learn. Mem. 2009, 92, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Quednow, B.B.; Wagner, M.; Westheide, J.; Beckmann, K.; Bliesener, N.; Maier, W.; Kühn, K.-U. Sensorimotor Gating and Habituation of the Startle Response in Schizophrenic Patients Randomly Treated with Amisulpride or Olanzapine. Biol. Psychiatry 2006, 59, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Best, J.D.; Berghmans, S.; Hunt, J.J.F.G.; Clarke, S.C.; Fleming, A.; Goldsmith, P.; Roach, A.G. Non-Associative Learning in Larval Zebrafish. Neuropsychopharmacology 2008, 33, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- García-González, J.; Brock, A.J.; Parker, M.O.; Riley, R.J.; Joliffe, D.; Sudwarts, A.; Teh, M.-T.; Busch-Nentwich, E.M.; Stemple, D.L.; Martineau, A.R.; et al. Identification of Slit3 as a Locus Affecting Nicotine Preference in Zebrafish and Human Smoking Behaviour. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- De Rienzo, G.; Bishop, J.A.; Mao, Y.; Pan, L.; Ma, T.P.; Moens, C.B.; Tsai, L.-H.; Sive, H. Disc1 Regulates Both β-Catenin-Mediated and Noncanonical Wnt Signaling during Vertebrate Embryogenesis. FASEB J. 2011, 25, 4184–4197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henn, K.; Braunbeck, T. Dechorionation as a Tool to Improve the Fish Embryo Toxicity Test (FET) with the Zebrafish (Danio Rerio). Comp. Biochem. Physiol. C 2011, 153, 91–98. [Google Scholar] [CrossRef]
- Kais, B.; Schneider, K.E.; Keiter, S.; Henn, K.; Ackermann, C.; Braunbeck, T. DMSO Modifies the Permeability of the Zebrafish (Danio Rerio) Chorion-Implications for the Fish Embryo Test (FET). Aquatic Toxicol. 2013, 140–141, 229–238. [Google Scholar] [CrossRef]
- Achenbach, J.C.; Hill, J.; Hui, J.P.M.; Morash, M.G.; Berrue, F.; Ellis, L.D. Analysis of the Uptake, Metabolism, and Behavioral Effects of Cannabinoids on Zebrafish Larvae. Zebrafish 2018, 15, 349–360. [Google Scholar] [CrossRef]
- De Luca, M.A.; Bimpisidis, Z.; Melis, M.; Marti, M.; Caboni, P.; Valentini, V.; Margiani, G.; Pintori, N.; Polis, I.; Marsicano, G.; et al. Stimulation of in Vivo Dopamine Transmission and Intravenous Self-Administration in Rats and Mice by JWH-018, a Spice Cannabinoid. Neuropharmacology 2015, 99, 705–714. [Google Scholar] [CrossRef]
- Elmore, J.S.; Baumann, M.H. Repeated Exposure to the “Spice” Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazer, L.; Hawkey, A.B.; Wells, C.N.; Drastal, M.; Odamah, K.-A.; Behl, M.; Levin, E.D. Developmental Exposure to Low Concentrations of Organophosphate Flame Retardants Causes Life-Long Behavioral Alterations in Zebrafish. Toxicol. Sci. 2018, 165, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Parker, M.O.; Millington, M.E.; Combe, F.J.; Brennan, C.H. Housing Conditions Differentially Affect Physiological and Behavioural Stress Responses of Zebrafish, as Well as the Response to Anxiolytics. PLoS ONE 2012, 7, e34992. [Google Scholar] [CrossRef] [Green Version]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Petzold, A.M.; Balciunas, D.; Sivasubbu, S.; Clark, K.J.; Bedell, V.M.; Westcot, S.E.; Myers, S.R.; Moulder, G.L.; Thomas, M.J.; Ekker, S.C. Nicotine Response Genetics in the Zebrafish. Proc. Natl. Am. Sci. USA 2009. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.T.; Amin, M.R.; Shah, P.; Ali, D.W. Motor Neuron Development in Zebrafish Is Altered by Brief (5-Hr) Exposures to THC (∆ 9 -Tetrahydrocannabinol) or CBD (Cannabidiol) during Gastrulation. Sci. Rep. 2018, 8, 10518. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.B.; Schwab, T.L.; Sigafoos, A.N.; Gauerke, J.L.; Krug, R.G.; Serres, M.R.; Jacobs, D.C.; Cotter, R.P.; Das, B.; Petersen, M.O.; et al. Novel Zebrafish Behavioral Assay to Identify Modifiers of the Rapid, Nongenomic Stress Response. Genes Brain Behav. 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Am, S.; Av, K. The Behavioral Effects of Acute Δ9-Tetrahydrocannabinol and Heroin (Diacetylmorphine) Exposure in Adult Zebrafish. Brain Res. 2013, 1543, 109–119. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic Effects of Nicotine in Zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef] [PubMed]
- López-Patiño, M.A.; Yu, L.; Cabral, H.; Zhdanova, I.V. Anxiogenic Effects of Cocaine Withdrawal in Zebrafish. Physiol. Behav. 2008, 93, 160–171. [Google Scholar] [CrossRef]
- Krook, J.T.; Duperreault, E.; Newton, D.; Ross, M.S.; Hamilton, T.J. Repeated Ethanol Exposure Increases Anxiety-like Behaviour in Zebrafish during Withdrawal. PeerJ 2019, 7, e6551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.Z.; Liu, X.; Griffith, W.H.; Winzer-Serhan, U.H. Chronic Neonatal Nicotine Increases Anxiety but Does Not Impair Cognition in Adult Rats. Behav. Neurosci 2007, 121, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Eddins, D.; Cerutti, D.; Williams, P.; Linney, E.; Levin, E.D. Zebrafish Provide a Sensitive Model of Persisting Neurobehavioral Effects of Developmental Chlorpyrifos Exposure: Comparison with Nicotine and Pilocarpine Effects and Relationship to Dopamine Deficits. Neurotoxicol. Teratol 2010, 32, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Eachus, H.; Bright, C.; Cunliffe, V.T.; Placzek, M.; Wood, J.D.; Watt, P.J. Disrupted-in-Schizophrenia-1 Is Essential for Normal Hypothalamic-Pituitary-Interrenal (HPI) Axis Function. Hum. Mol. Genet. 2017, 26, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Bonath, F.; Kumar, S.; Ross, C.A.; Cunliffe, V.T. Disrupted-in-Schizophrenia 1 and Neuregulin 1 Are Required for the Specification of Oligodendrocytes and Neurones in the Zebrafish Brain. Hum. Mol. Genet. 2009, 18, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Drerup, C.M.; Wiora, H.M.; Topczewski, J.; Morris, J.A. Disc1 Regulates Foxd3 and Sox10 Expression, Affecting Neural Crest Migration and Differentiation. Development 2009, 136, 2623–2632. [Google Scholar] [CrossRef] [Green Version]
- Craft, R.M. Sex Differences in Behavioral Effects of Cannabinoids. Life Sci. 2005, 77, 2471–2478. [Google Scholar] [CrossRef]
- Kaminitz, A.; Barzilay, R.; Segal, H.; Taler, M.; Offen, D.; Gil-Ad, I.; Mechoulam, R.; Weizman, A. Dominant Negative DISC1 Mutant Mice Display Specific Social Behaviour Deficits and Aberration in BDNF and Cannabinoid Receptor Expression. World J. Biol. Psychiatry 2014, 15, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Christou, M.; Kavaliauskis, A.; Ropstad, E.; Fraser, T.W.K. DMSO Effects Larval Zebrafish (Danio Rerio) Behavior, with Additive and Interaction Effects When Combined with Positive Controls. Sci. Total. Environ. 2020, 709, 134490. [Google Scholar] [CrossRef]
- Ogungbemi, A.; Leuthold, D.; Scholz, S.; Küster, E. Hypo- or Hyperactivity of Zebrafish Embryos Provoked by Neuroactive Substances: A Review on How Experimental Parameters Impact the Predictability of Behavior Changes. Environ. Sci. Eu. 2019, 31, 88. [Google Scholar] [CrossRef]
- Ponzoni, L.; Braida, D.; Pucci, L.; Andrea, D.; Fasoli, F.; Manfredi, I.; Papke, R.L.; Stokes, C.; Cannazza, G.; Clementi, F.; et al. The Cytisine Derivatives, CC4 and CC26, Reduce Nicotine-Induced Conditioned Place Preference in Zebrafish by Acting on Heteromeric Neuronal Nicotinic Acetylcholine Receptors. Psychopharmacology 2014, 231, 4681–4693. [Google Scholar] [CrossRef] [PubMed]
- Kily, L.J.M.; Cowe, Y.C.M.; Hussain, O.; Patel, S.; McElwaine, S.; Cotter, F.E.; Brennan, C.H. Gene Expression Changes in a Zebrafish Model of Drug Dependency Suggest Conservation of Neuro-Adaptation Pathways. J. Exp. Biol. 2008, 211, 1623–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Position | Genomic Sequence Surrounding the SNP Polymorphism |
|---|---|
| 13:49125537- 49125647 | AGAGGGTTTCGAGAGAGACAACTCATCAAAGTC TTCAAATAAACACCATT[T/A]GCATGATGAGGAG GACAATTTACCAGTGCAATCACGTGATGTTTTCAATT |
| Behavioral Assay | JWH-018 | THC | disc1 | disc1 × JWH-018 Interaction | disc1 × THC Interaction |
|---|---|---|---|---|---|
| Forced Light/Dark assay at 5 dpf | |||||
| Basal locomotion | (− −) | (− −) | No effect | No | Yes |
| Light period locomotion | No change | (− −) | No effect | No | Yes |
| Light period slope | No change | (−) | No effect | No | No |
| Dark period locomotion | (− −) | (– –) | No effect | No | Yes |
| Dark period slope | (− −) | No change | (−) | No | Yes |
| Acoustic startle at 6 dpf | |||||
| Basal locomotion | No change | (− −) | No effect | No | Yes |
| Across taps | No change | No change | No effect | No | No |
| Adult tank diving—time on bottom | (− −) | No change | (+ +) | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-González, J.; de Quadros, B.; Havelange, W.; Brock, A.J.; Brennan, C.H. Behavioral Effects of Developmental Exposure to JWH-018 in Wild-Type and Disrupted in Schizophrenia 1 (disc1) Mutant Zebrafish. Biomolecules 2021, 11, 319. https://doi.org/10.3390/biom11020319
García-González J, de Quadros B, Havelange W, Brock AJ, Brennan CH. Behavioral Effects of Developmental Exposure to JWH-018 in Wild-Type and Disrupted in Schizophrenia 1 (disc1) Mutant Zebrafish. Biomolecules. 2021; 11(2):319. https://doi.org/10.3390/biom11020319
Chicago/Turabian StyleGarcía-González, Judit, Bruno de Quadros, William Havelange, Alistair J. Brock, and Caroline H. Brennan. 2021. "Behavioral Effects of Developmental Exposure to JWH-018 in Wild-Type and Disrupted in Schizophrenia 1 (disc1) Mutant Zebrafish" Biomolecules 11, no. 2: 319. https://doi.org/10.3390/biom11020319
APA StyleGarcía-González, J., de Quadros, B., Havelange, W., Brock, A. J., & Brennan, C. H. (2021). Behavioral Effects of Developmental Exposure to JWH-018 in Wild-Type and Disrupted in Schizophrenia 1 (disc1) Mutant Zebrafish. Biomolecules, 11(2), 319. https://doi.org/10.3390/biom11020319







