Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Callus Induction
2.3. Preparation of Callus Aqueous Extract of S. incanum L.
2.4. Aqueous Callus Extract Mediated Green Synthesis of Ag-NPs
2.5. Analytical Methods for Characterization of Ag-NPs
2.5.1. UV-Visible Spectroscopy
2.5.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5.3. X-ray Diffraction (XRD)
2.5.4. Transmission Electron Microscopy (TEM)
2.5.5. Energy Dispersive Spectroscopic Analysis (SEM-EDX)
2.6. Biological Activities of Green Synthesized Ag-NPs
2.6.1. Antimicrobial Activities
2.6.2. Antifungal Activities against Phytopathogen Fungi
2.6.3. In Vitro Cytotoxic Efficacy of Ag-NPs against Cancerous Cells
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Plant Growth Regulators on Callus Initiation
3.2. Preparation of Aqueous Callus Extract and Phytochemical Screening
3.3. Callus Aqueous Extract Mediated Green Synthesis of Ag-Nps
3.3.1. Color Change and UV-Vis Spectroscopy
3.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3.3. X-ray Diffraction (XRD)
3.3.4. Transmission Electron Microscopy (TEM)
3.3.5. Energy Dispersive Spectroscopic Analysis (SEM-EDX)
3.4. Biological Activities of Green Synthesized Ag-NPs
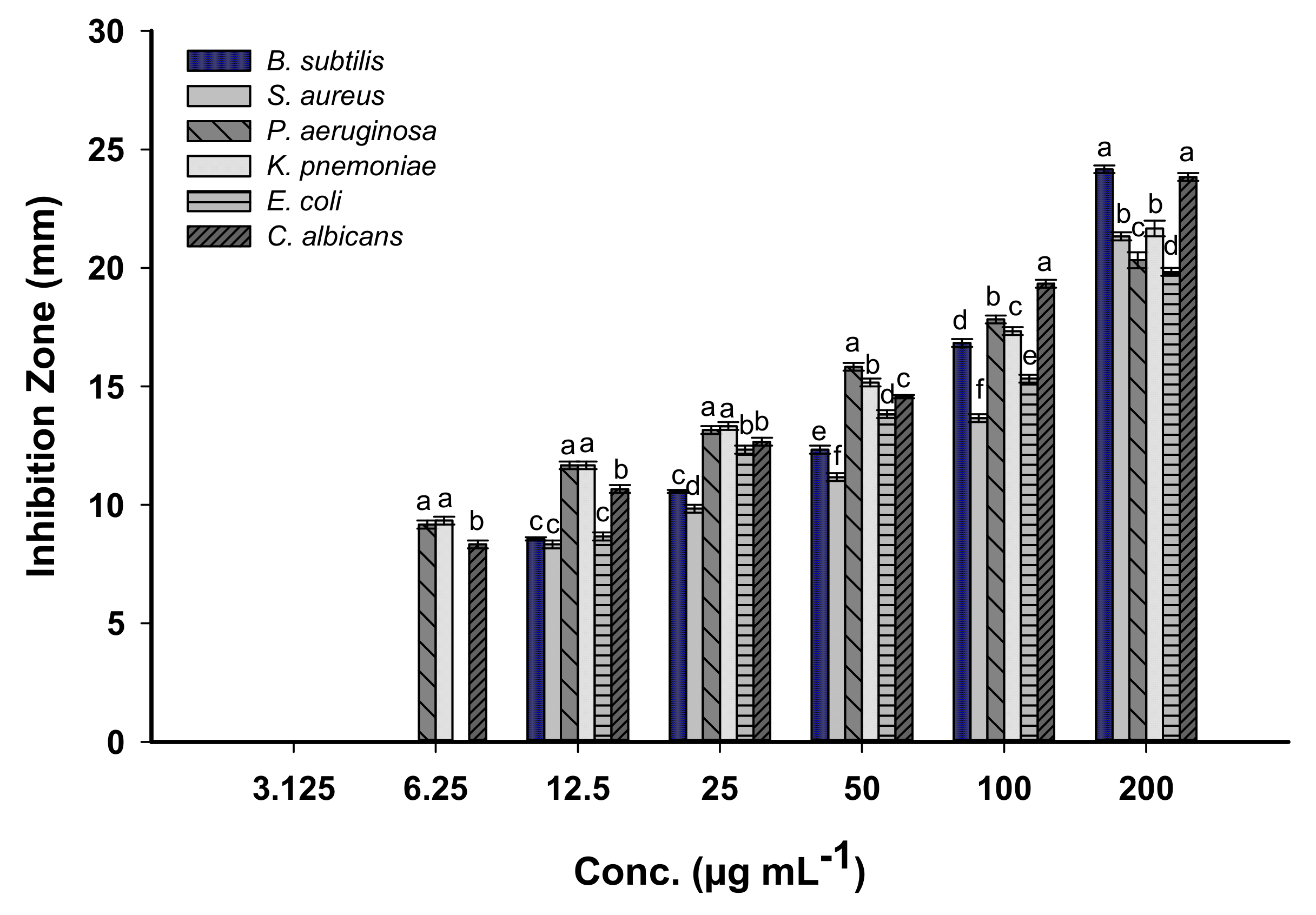
3.4.1. Antimicrobial Activities
3.4.2. Antifungal against Phytopathogen Fungi
3.4.3. In Vitro Cytotoxicity
3.5. Comparison Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Azeem, A.A.; Nada, A.; O’Donovan, A.; Kumar Thakur, V.; Elkelish, A. Mycogenic Silver Nanoparticles from Endophytic Trichoderma Atroviride with Antimicrobial Activity. J. Renew. Mater. 2019, 7, 171–185. [Google Scholar] [CrossRef]
- Saied, E.M.; Banhart, S.; Bürkle, S.E.; Heuer, D.; Arenz, C. A Series of Ceramide Analogs Modified at the 1-Position with Potent Activity against the Intracellular Growth of Chlamydia Trachomatis. Future Med. Chem. 2015, 7, 1971–1980. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.-D.; El-Sadany, M.A.-H. Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. Int. Biodeterior. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef]
- Soliman, M.; Qari, S.H.; Abu-Elsaoud, A.; El-Esawi, M.; Alhaithloul, H.; Elkelish, A. Rapid Green Synthesis of Silver Nanoparticles from Blue Gum Augment Growth and Performance of Maize, Fenugreek, and Onion by Modulating Plants Cellular Antioxidant Machinery and Genes Expression. Acta Physiol. Plant 2020, 42, 148. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2020, 9, 104693. [Google Scholar] [CrossRef]
- Banhart, S.; Saied, E.M.; Martini, A.; Koch, S.; Aeberhard, L.; Madela, K.; Arenz, C.; Heuer, D. Improved Plaque Assay Identifies a Novel Anti-Chlamydia Ceramide Derivative with Altered Intracellular Localization. J. Antimicrob. Chemother. 2014, 58, 5537–5546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samak, D.H.; El-Sayed, Y.S.; Shaheen, H.M.; El-Far, A.H.; Abd El-Hack, M.E.; Noreldin, A.E.; El-Naggar, K.; Abdelnour, S.A.; Saied, E.M.; El-Seedi, H.R.; et al. Developmental Toxicity of Carbon Nanoparticles during Embryogenesis in Chicken. Environ. Sci. Pollut. Res. Int. 2020, 27, 19058–19072. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shmgani, H.S.A.; Mohammed, W.H.; Sulaiman, G.M.; Saadoon, A.H. Biosynthesis of silver nanoparticles from Catharanthus roseus leaf extract and assessing their antioxidant, antimicrobial, and wound-healing activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1234–1240. [Google Scholar] [CrossRef]
- Botcha, S.; Prattipati, S.D. Callus Extract Mediated Green Synthesis of Silver Nanoparticles, Their Characterization and Cytotoxicity Evaluation Against MDA-MB-231 and PC-3 Cells. BioNanoScience 2020, 10, 11–22. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Fouda, A.; Salem, S.S. Integration of Cotton Fabrics with Biosynthesized CuO Nanoparticles for Bactericidal Activity in the Terms of Their Cytotoxicity Assessment. Ind. Eng. Chem. Res. 2021, 60, 1553–1563. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Veremeichik, G.N.; Kamenev, D.G.; Gorpenchenko, T.Y.; Yugay, Y.A.; Mashtalyar, D.V.; Nepomnyaschiy, A.V.; Avramenko, T.V.; Karabtsov, A.A.; Ivanov, V.V.; et al. Green synthesis of silver nanoparticles using transgenic Nicotiana tabacum callus culture expressing silicatein gene from marine sponge Latrunculia oparinae. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Eid, A.M.; Barghoth, M.G.; El-Sadany, M.A.-H. Monitoring the effect of biosynthesized nanoparticles against biodeterioration of cellulose-based materials by Aspergillus niger. Cellulose 2019, 26, 6583–6597. [Google Scholar] [CrossRef]
- Netala, V.R.; Kotakadi, V.S.; Domdi, L.; Gaddam, S.A.; Bobbu, P.; Venkata, S.K.; Ghosh, S.B.; Tartte, V. Biogenic silver nanoparticles: Efficient and effective antifungal agents. Appl. Nanosci. 2016, 6, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Fahimirad, S.; Ajalloueian, F.; Ghorbanpour, M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019, 168, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Selvakesavan, R.K.; Franklin, G.; Sarmento, B.; Dias, A.C. Antimicrobial activity of cream incorporated with silver nanoparticles biosynthesized from Withania somnifera. Int. J. Nanomed. 2015, 10, 5955–5963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of Silver Nanoparticles to Red Blood Cells: Size Dependent Adsorption, Uptake, and Hemolytic Activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Abdel-sattar, E.; Abou-Hussein, D.; Petereit, F. Chemical Constituents from the Leaves of Euphorbia ammak Growing in Saudi Arabia. Pharmacogn. Res. 2015, 7, 14–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelzer, G.; Gurib-Fakim, A.; Arroo, R.; Bosch, C.H.; de Ruijter, A.; Simmonds, M.S.J.; Lemmens, R.H.M.J.; Oyen, L.P.A. Plant Resources of Tropical Africa 11(1) Medicinal Plants 1; Backhuys Publishers/CTA: Wageningen, The Netherlands, 2008. [Google Scholar]
- Alamri, S.A.; Moustafa, M.F. Antimicrobial properties of 3 medicinal plants from Saudi Arabia against some clinical isolates of bacteria. Saudi Med. J. 2012, 33, 272–277. [Google Scholar] [PubMed]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I. Plant tissue culture: Current status and opportunities. Recent Adv. Plant Vitr. Cult. 2012, 1–28. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Sbhatu, D.B.; Abraha, H.B. Preliminary Antimicrobial Profile of Solanum incanum L.: A Common Medicinal Plant. Evid. Based Complement. Altern. Med. 2020. [Google Scholar] [CrossRef] [Green Version]
- Sofowora, A. Medicinal Plants and Traditional Medicine in Africa; Spectrum Books Ltd.: Ibadan, Nigeria, 1993; pp. 191–289. [Google Scholar]
- Fouda, A.; El-Din Hassan, S.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.; Abdel-Rahman, M.A.; Farag, M.M.; Shehbel-deen, A.M.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghannem, S.A.; Azab, M.S. Catalytic degradation of wastewater from textile and tannery industry by green synthesized hematite (α-Fe2O3)/magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Valgas, C.; Souza, S.M.D.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Mahdizadeh, V.; Safaie, N.; Khelghatibana, F. Evaluation of antifungal activity of silver nanoparticles against some phytopathogenic fungi and Trichoderma harzianum. J. Crop Prot. 2015, 4, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.-D.; Salem, S.S.; Abdo, A.M.; F Hetta, H.; Shaheen, T.I. Endophytic Streptomyces laurentii Mediated Green Synthesis of Ag-NPs with Antibacterial and Anticancer Properties for Developing Functional Textile Fabric Properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Liu, X.-Q.; Gituru, R.; Chen, L. Callus Induction and Plant Regeneration from In Vitro Cultured Leaves, Petioles and Scales of Lilium Leucanthum (Baker) Baker. Biotechnol. Biotechnol. Equip. 2014, 24, 2071–2076. [Google Scholar] [CrossRef]
- Rateb, M.E.; El-Hawary, S.S.; El-Shamy, A.M.; Yousef, E.M. Production of parthenolide in organ and callus cultures of Tanacetum parthenium (L.). Afr. J. Biotechnol. 2007, 6, 1306–1316. [Google Scholar]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, S.; Dhamodaran, M.; Prasad, R.; Ganesan, M. Synthesis and characterisation of zinc oxide nanoparticles using terpenoid fractions of Andrographis paniculata leaves. Int. Nano Lett. 2017, 7, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, M.; Sayed, M.; Alamri, S.; Alghamdii, H.; Shati, A.; Alrumman, S.; Al-Khatani, M.; Maghraby, T.; Temerk, H.; Khalaf, E. Pharmaceutical Properties of Synthesized Silver Nanoparticles from Aqueous Extract of Solanum incanum L. Fruits against Some Human Pathogenic Microbes. Int. J. Pharmacol. 2020, 16, 514–521. [Google Scholar] [CrossRef]
- Mude, N.; Ingle, A.; Gade, A.; Rai, M. Synthesis of Silver Nanoparticles Using Callus Extract of Carica papaya—A First Report. J. Plant Biochem. Biotechnol. 2009, 18, 83–86. [Google Scholar] [CrossRef]
- Aref, M.; Salah Salem, S. Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal. Agric. Biotechnol. 2020, 27, 101689. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Narsing Rao, M.P.; Xiao, M.; Wang, H.F.; Hozzein, W.N.; Chen, W.; Li, W.J. Antibacterial Activity of Silver Nanoparticles against Staphylococcus warneri Synthesized Using Endophytic Bacteria by Photo-irradiation. Front. Microbiol. 2017, 8, 1090. [Google Scholar] [CrossRef] [Green Version]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J. Microbiol. Biotechnol. 2018, 34, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsharif, S.M.; Salem, S.S.; Abdel-Rahman, M.A.; Fouda, A.; Eid, A.M.; El-Din Hassan, S.; Awad, M.A.; Mohamed, A.A. Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 2020, 6, e03943. [Google Scholar] [CrossRef] [PubMed]
- Ardila, N.; Daigle, F.; Heuzey, M.C.; Ajji, A. Antibacterial Activity of Neat Chitosan Powder and Flakes. Molecules 2017, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Trivedi, P.; Dutta Gupta, S. Surface Plasmon Resonance (SPR) Based Optimization of Biosynthesis of Silver Nanoparticles from Rhizome Extract of Curculigo orchioides Gaertn. and Its Antioxidant Potential. J. Clust. Sci. 2016, 27, 1893–1912. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Karthik, C.; Suresh, S.; Kavitha, S. A FTIR approach of green synthesized silver nanoparticles by Ocimum sanctum and Ocimum gratissimum on mung bean seeds. Inorg. Nano-Met. Chem. 2020, 50, 606–612. [Google Scholar] [CrossRef]
- Litvin, V.A.; Minaev, B.F. Spectroscopy study of silver nanoparticles fabrication using synthetic humic substances and their antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 108, 115–122. [Google Scholar] [CrossRef]
- Biresaw, S.S.; Damte, S.M.; Taneja, P. Green Synthesized Silver Nanoparticles: A Promising Anticancer Agent. Int. J. Nanosci. 2020, 19, 1950027. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Elgamal, M.S.; EL-Din Hassan, S.; Shaheen, T.I.; Salem, S.S. Enhancing of Cotton Fabric Antibacterial Properties by Silver Nanoparticles Synthesized by New Egyptian Strain Fusarium keratoplasticum A1-3. Egypt. J. Chem. 2017, 60, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Gnanajobitha, G.; Rajeshkumar, S.; Kannan, C.; Annadurai, G. Preparation and characterization of fruit-mediated silver nanoparticles using pomegranate extract and assessment of its antimicrobial activity. J. Environ. Nanotechnol. 2013, 2, 4–10. [Google Scholar]
- Thomas, B.; Vithiya, B.S.M.; Prasad, T.A.A.; Mohamed, S.B.; Magdalane, C.M.; Kaviyarasu, K.; Maaza, M. Antioxidant and Photocatalytic Activity of Aqueous Leaf Extract Mediated Green Synthesis of Silver Nanoparticles Using Passiflora edulis f. flavicarpa. J. Nanosci. Nanotechnol. 2019, 19, 2640–2648. [Google Scholar] [CrossRef]
- Jemal, K.; Sandeep, B.V.; Pola, S. Synthesis, Characterization, and Evaluation of the Antibacterial Activity of Allophylus serratus Leaf and Leaf Derived Callus Extracts Mediated Silver Nanoparticles. J. Nanomater. 2017. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, T.I.; Fouda, A. Green approach for one-pot synthesis of silver nanorod using cellulose nanocrystal and their cytotoxicity and antibacterial assessment. Int. J. Biol. Macromol. 2018, 106, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Kalia, A.; Manchanda, P.; Bhardwaj, S.; Singh, G. Biosynthesized silver nanoparticles from aqueous extracts of sweet lime fruit and callus tissues possess variable antioxidant and antimicrobial potentials. Inorg. Nano-Met. Chem. 2020, 50, 1053–1062. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vijayabharathi, R.; Sathya, A.; Gopalakrishnan, S. Extracellular biosynthesis of silver nanoparticles using Streptomyces griseoplanus SAI-25 and its antifungal activity against Macrophomina phaseolina, the charcoal rot pathogen of sorghum. Biocatal. Agric. Biotechnol. 2018, 14, 166–171. [Google Scholar] [CrossRef]
- Magudapathy, P.; Gangopadhyay, P.; Panigrahi, B.K.; Nair, K.G.M.; Dhara, S. Electrical transport studies of Ag nanoclusters embedded in glass matrix. Phys. B Condens. Matter 2001, 299, 142–146. [Google Scholar] [CrossRef]
- Khan, F.A.; Zahoor, M.; Jalal, A.; Rahman, A.U. Green Synthesis of Silver Nanoparticles by Using Ziziphus nummularia Leaves Aqueous Extract and Their Biological Activities. J. Nanomater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Yourick, J.J.; Topping, V.D.; Black, T.; Olejnik, N.; Keltner, Z.; Sprando, R.L. Toxicogenomic study in rat thymus of F1 generation offspring following maternal exposure to silver ion. Toxicol. Rep. 2015, 2, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Barkat, M.A.; Mujeeb, M.; Samim, M.; Verma, S. Biosynthesis of silver nanoparticles using callus extract of Catharanthus roseus var. alba and assessment of its antimicrobial activity. J. Pharm. Res. Int. 2014, 1591–1603. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Abalkhil, T.A.; Alharbi, S.A.; Salmen, S.H.; Wainwright, M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 2017, 31, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Kanwal, Z.; Raza, M.A.; Riaz, S.; Manzoor, S.; Tayyeb, A.; Sajid, I.; Naseem, S. Synthesis and characterization of silver nanoparticle-decorated cobalt nanocomposites (Co@AgNPs) and their density-dependent antibacterial activity. R. Soc. Open Sci. 2019, 6, 182135. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.E.L.D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Thenmozhi, M.; Kannabiran, K.; Kumar, R.; Gopiesh Khanna, V. Antifungal activity of Streptomyces sp. VITSTK7 and its synthesized Ag2O/Ag nanoparticles against medically important Aspergillus pathogens. J. Mycol. Med. 2013, 23, 97–103. [Google Scholar] [CrossRef]
- Vivek, M.; Kumar, P.S.; Steffi, S.; Sudha, S. Biogenic Silver Nanoparticles by Gelidiella acerosa Extract and their Antifungal Effects. Avicenna J. Med. Biotechnol. 2011, 3, 143–148. [Google Scholar] [PubMed]
- Hassan, S.E.D.; Fouda, A.; Radwan, A.A.; Salem, S.S.; Barghoth, M.G.; Awad, M.A.; Abdo, A.M.; El-Gamal, M.S. Endophytic actinomycetes Streptomyces spp. mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J. Biol. Inorg. Chem. 2019, 24, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Choi, J.; Chung, K.H.; Park, K.; Yi, J.; Ryu, D.Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. In Vitro 2009, 23, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Sriram, M.I.; Kanth, S.B.M.; Kalishwaralal, K.; Gurunathan, S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 2010, 5, 753–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.H.; Ma, Y.J.; Wang, J.W. Biosynthesis of Silver Nanoparticles Using Taxus yunnanensis Callus and Their Antibacterial Activity and Cytotoxicity in Human Cancer Cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivek, R.; Thangam, R.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and Its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Wang, J.; Yang, Q.; Yao, B.; Zhao, Y.; Zhao, A.; Sun, W.; Zhang, Q. Synthesis, characterization and evaluation cytotoxic activity of silver nanoparticles synthesized by Chinese herbal Cornus officinalis via environment friendly approach. Environ. Toxicol. Pharmacol. 2017, 56, 56–60. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; Wang, K.; Wang, Z.; Duan, J.; Cui, L.; Zheng, H.; Wang, Y.; Wang, S. Evaluation of biosynthesis parameters, stability and biological activities of silver nanoparticles synthesized by Cornus Officinalis extract under 365 nm UV radiation. RSC Adv. 2020, 10, 27173–27182. [Google Scholar] [CrossRef]
- Kawata, K.; Osawa, M.; Okabe, S. In Vitro Toxicity of Silver Nanoparticles at Noncytotoxic Doses to HepG2 Human Hepatoma Cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Park, J.H.; Gurunathan, S.; Choi, Y.-J.; Han, J.W.; Song, H.; Kim, J.-H. Silver nanoparticles suppresses brain-derived neurotrophic factor-induced cell survival in the human neuroblastoma cell line SH-SY5Y. J. Ind. Eng. Chem. 2017, 47, 62–73. [Google Scholar] [CrossRef]
- Satyavani, K.; Ramanathan, T.; Gurudeeban, S. Green synthesis of silver nanoparticles by using stem derived callus extract of bitter apple (Citrullus colocynthis). Dig. J. Nanomater. Biostruct. 2011, 6, 1019–1024. [Google Scholar] [CrossRef] [Green Version]
- Netala, V.R.; Kotakadi, V.S.; Nagam, V.; Bobbu, P.; Ghosh, S.B.; Tartte, V. First report of biomimetic synthesis of silver nanoparticles using aqueous callus extract of Centella asiatica and their antimicrobial activity. Appl. Nanosci. 2015, 5, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N.M. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf. B Biointerfaces 2010, 79, 488–493. [Google Scholar] [CrossRef] [PubMed]








| MS Supplemented with mg/L | Callus Induction Percentages (%) | ||
|---|---|---|---|
| BA | 2,4-D | Petioles | Leaves |
| 0 | 0 | 0% | 0% |
| 0.5 | 1.0 | 80% | 76.66% |
| 0.5 | 1.5 | 60% | 56.66% |
| 0.5 | 2.0 | 83.33% | 73.33% |
| 1.0 | 1.0 | 86.66% | 90% |
| 1.0 | 1.5 | 46.66% | 36.66% |
| 1.0 | 2.0 | 33.33% | 26.66% |
| 1.5 | 1.0 | 53.33% | 50% |
| 1.5 | 1.5 | 26.66% | 20% |
| 1.5 | 2.0 | 16.66% | 13.33% |
| MS Supplementedwith mg L−1 | Explants | ||||||
|---|---|---|---|---|---|---|---|
| Petioles | Leaves | ||||||
| BA | 2,4-D | Fresh Weight (g/jar) | Dry Weight (g/jar) | Moisture Content (%) | Fresh Weight (g/jar) | Dry Weight (g/jar) | Moisture Content (%) |
| 0 | 0 | 0 | 0 | 0 % | 0 | 0 % | |
| 0.5 | 1.0 | 4.08 | 0.30 | 92.64% | 4.18 | 0.29 | 93.06% |
| 0.5 | 1.5 | 2.63 | 0.23 | 91.25% | 2.77 | 0.26 | 90.61% |
| 0.5 | 2.0 | 3.11 | 0.29 | 90.67% | 3.15 | 0.28 | 91.11% |
| 1.0 | 1.0 | 4.68 | 0.32 | 93.16% | 5.13 | 0.37 | 92.78% |
| 1.0 | 1.5 | 1.86 | 0.11 | 94.08% | 2.08 | 0.20 | 90.38% |
| 1.0 | 2.0 | 1.33 | 0.10 | 92.48% | 1.41 | 0.12 | 91.48% |
| 1.5 | 1.0 | 2.16 | 0.19 | 91.20% | 2.43 | 0.25 | 89.71% |
| 1.5 | 1.5 | 1.08 | 0.09 | 91.66% | 1.16 | 0.10 | 91.37% |
| 1.5 | 2.0 | 0.9 | 0.08 | 91.11% | 1.10 | 0.09 | 91.81% |
| Callus Aqueous Extract Mediated Biosynthesis of Ag-NPs | Shape | Size | Applications | Reference |
|---|---|---|---|---|
| Citrullus colocynthis (L.) | Spherical | 75 nm | Antibacterial activity | [86] |
| Allophylus serratus | Spherical | 42 to 50 nm | Antibacterial activity | [56] |
| Centella asiatica | Spherical | 5–40 nm | Antibacterial activity | [87] |
| Hyptis suaveolens | Spherical | 12 to 25 nm | In vitro cytotoxicity against cancer cells | [13] |
| Gymnema sylvestre | Spherical | 3–30 nm | Antifungal activity against Candida spp. | [18] |
| Taxus yunnanensis | Spherical | 6.4 to 27.2 nm | Antibacterial activityIn vitro cytotoxicity | [79] |
| Sesuvium portulacastrum L. | Spherical | 5 to 20 nm | Antibacterial and antifungal activities | [88] |
| Cinnamomum camphora | Spherical | 5.47–9.48 nm | Antibacterial activity | [42] |
| Solanum incanum L. | Spherical | 15–60 nm | Antibacterial and anticandidalAnti-phytopathogenic fungiIn vitro cytotoxicity | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L. Biomolecules 2021, 11, 341. https://doi.org/10.3390/biom11030341
Lashin I, Fouda A, Gobouri AA, Azab E, Mohammedsaleh ZM, Makharita RR. Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L. Biomolecules. 2021; 11(3):341. https://doi.org/10.3390/biom11030341
Chicago/Turabian StyleLashin, Islam, Amr Fouda, Adil A. Gobouri, Ehab Azab, Zuhair M. Mohammedsaleh, and Rabab R. Makharita. 2021. "Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L." Biomolecules 11, no. 3: 341. https://doi.org/10.3390/biom11030341
APA StyleLashin, I., Fouda, A., Gobouri, A. A., Azab, E., Mohammedsaleh, Z. M., & Makharita, R. R. (2021). Antimicrobial and In Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-NPs) Fabricated by Callus Extract of Solanum incanum L. Biomolecules, 11(3), 341. https://doi.org/10.3390/biom11030341








