Small Molecule CCR4 Antagonists Protect Mice from Aspergillus Infection and Allergy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Vaccination Model
2.3. Aspergillus Infection and Allergy
2.4. Immunofluorescence
2.5. ELISA
2.6. RT-PCR
2.7. Statistical Analysis
3. Results
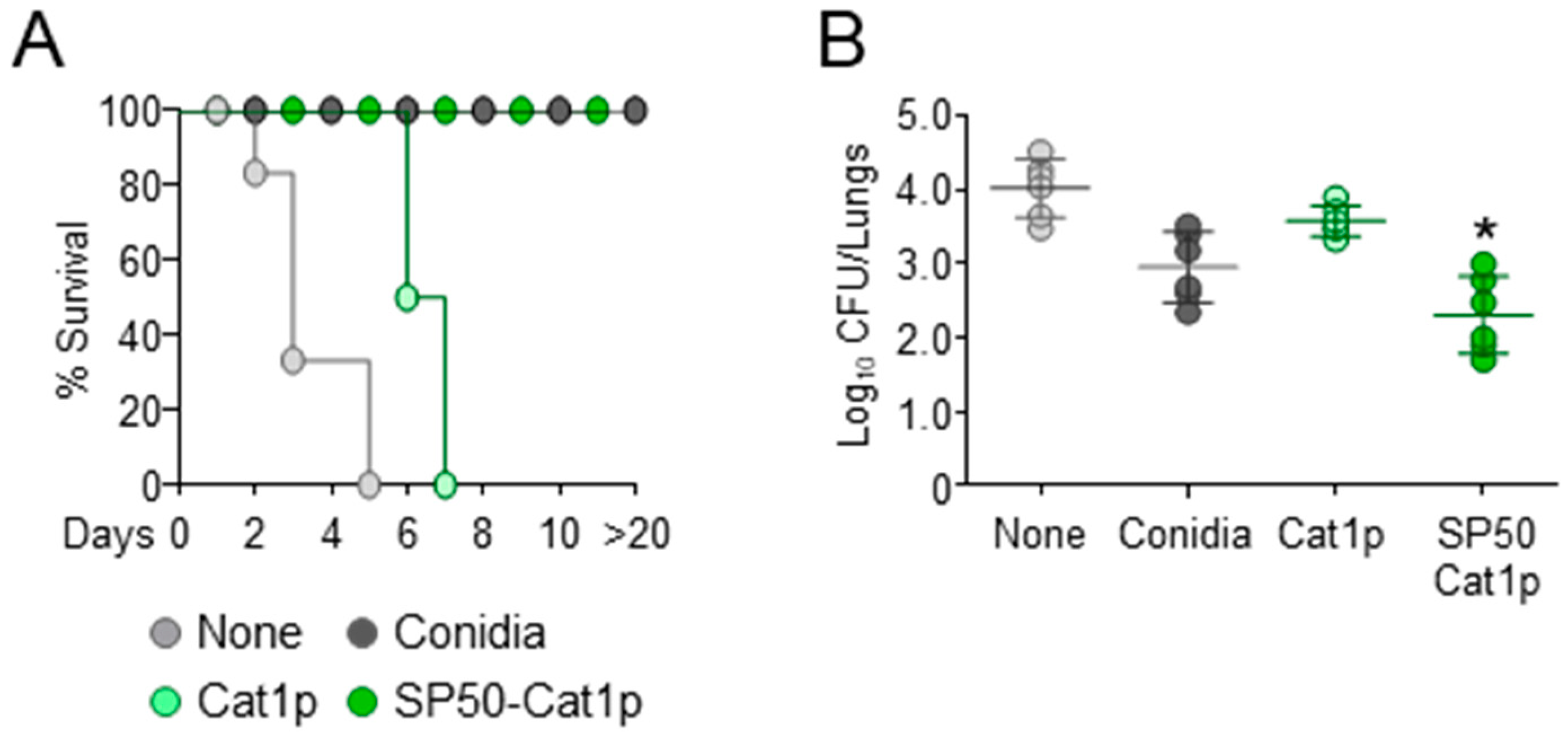
3.1. The CCR4 Antagonist SP50 Promotes Vaccine-Induced Resistance
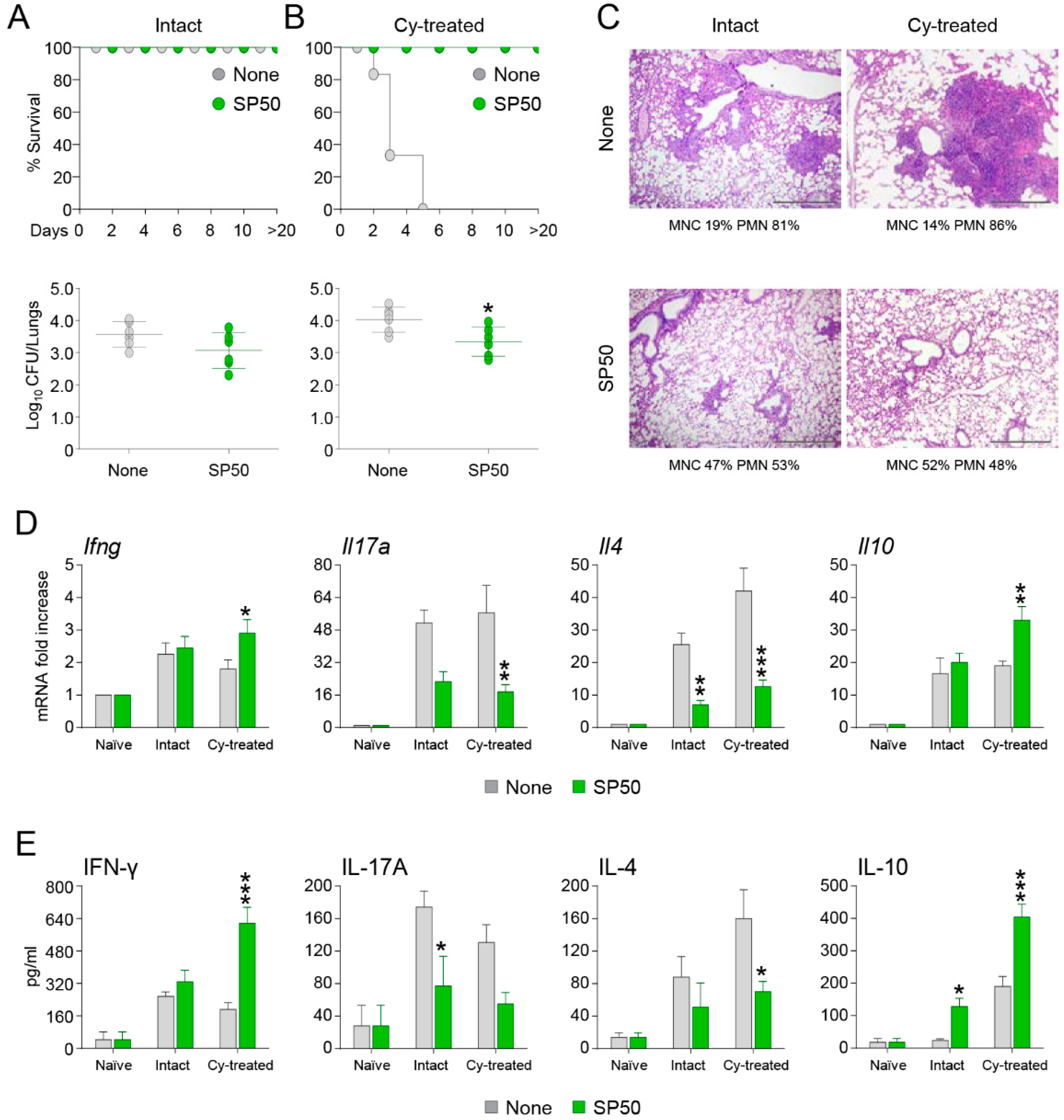
3.2. The CCR4 Antagonist SP50 Protects Immunosuppressed Mice from Invasive Aspergillosis
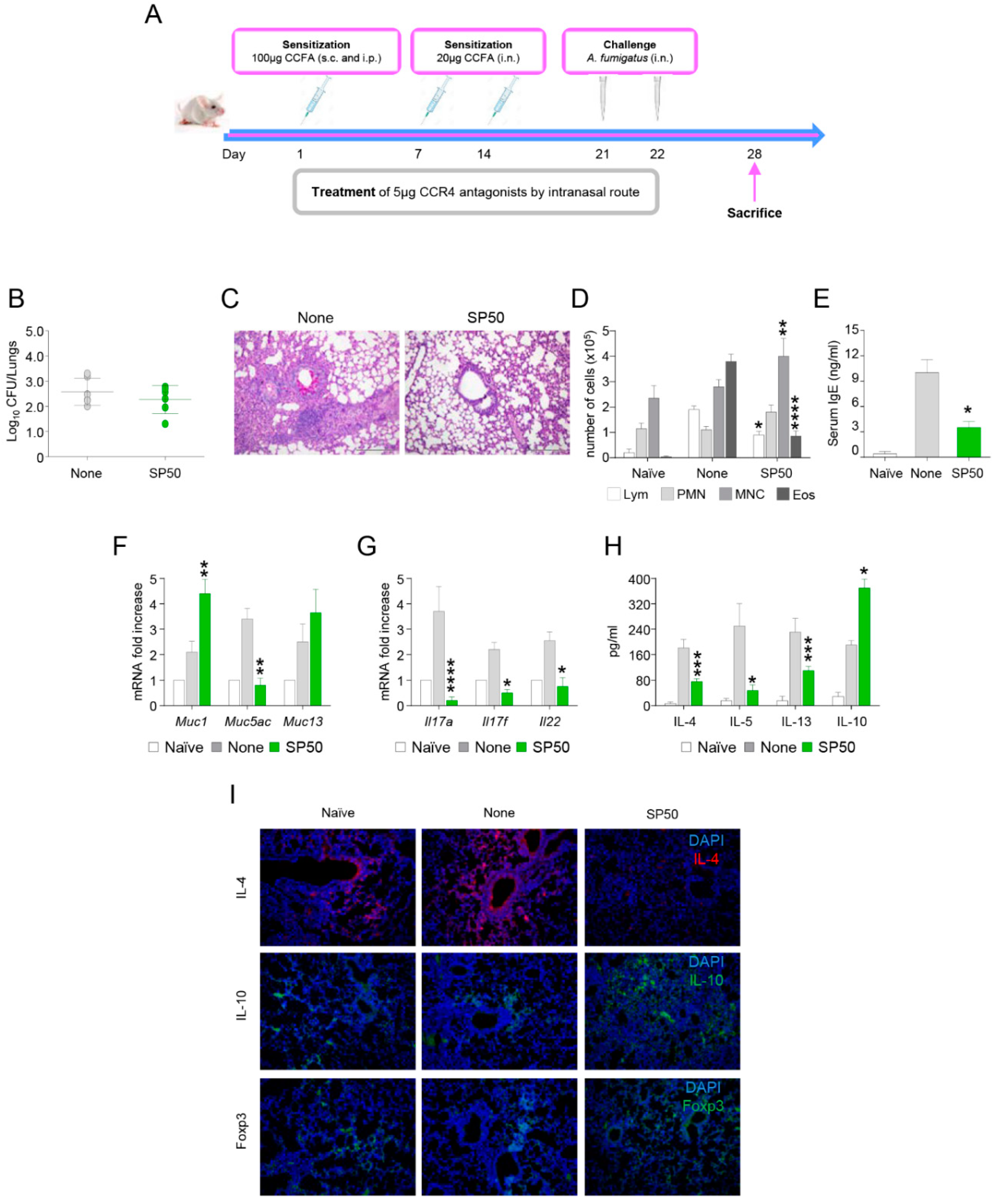
3.3. The CCR4 Antagonist SP50 Protects Mice from ABPA
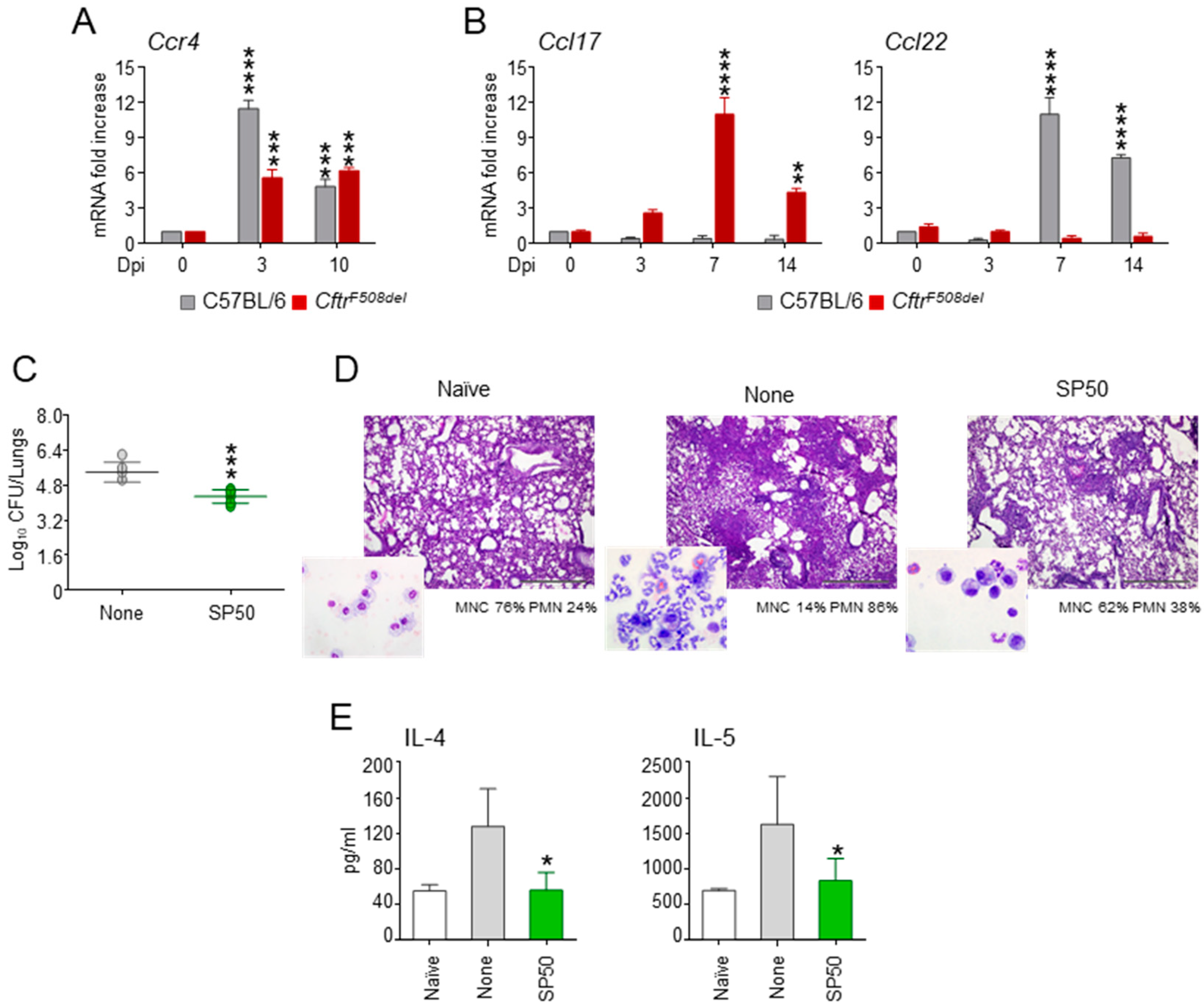
3.4. The CCR4 Antagonist SP50 Protects a Murine Model of Cystic Fibrosis from Aspergillosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Dyer, D.P. Understanding the mechanisms that facilitate specificity, not redundancy, of chemokine-mediated leukocyte recruitment. Immunology 2020, 160, 336–344. [Google Scholar] [CrossRef]
- Solari, R.; Pease, J.E.; Begg, M. Chemokine receptors as therapeutic targets: Why aren’t there more drugs? Eur. J. Pharmacol. 2015, 746, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Solari, R.; Pease, J.E. Targeting chemokine receptors in disease—A case study of CCR4. Eur. J. Pharmacol. 2015, 763, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, C.J.; Koglin, M.; Olson, W.C.; Marshall, F.H. Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat. Rev. Drug Discov. 2017, 16, 787–810. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santulli-Marotto, S.; Boakye, K.; Lacy, E.; Wu, S.J.; Luongo, J.; Kavalkovich, K.; Coelho, A.; Hogaboam, C.M.; Ryan, M. Engagement of two distinct binding domains on CCL17 is required for signaling through CCR4 and establishment of localized inflammatory conditions in the lung. PLoS ONE 2013, 8, e81465. [Google Scholar] [CrossRef]
- Marshall, L.A.; Marubayashi, S.; Jorapur, A.; Jacobson, S.; Zibinsky, M.; Robles, O.; Hu, D.X.; Jackson, J.J.; Pookot, D.; Sanchez, J.; et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Ketcham, J.M.; Marshall, L.A.; Talay, O. CCR4 Antagonists Inhibit Treg Trafficking into the Tumor Microenvironment. ACS Med. Chem. Lett. 2018, 9, 953–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Barnes, P.D.; Marr, K.A. Aspergillosis: Spectrum of disease, diagnosis, and treatment. Infect. Dis. Clin. N. Am. 2006, 20, 545–561. [Google Scholar] [CrossRef]
- Felton, I.C.; Simmonds, N.J. Aspergillus and cystic fibrosis: Old disease—New classifications. Curr. Opin. Pulm. Med. 2014, 20, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ralhan, A.; Schwarz, C.; Hartl, D.; Hector, A. Fungal Pathogens in CF Airways: Leave or Treat? Mycopathologia 2018, 183, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Paugam, A.; Hubert, D.; Martin, C. Aspergillus fumigatus in the cystic fibrosis lung: Pros and cons of azole therapy. Infect. Drug Resist. 2016, 9, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castanos, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [Green Version]
- Costantini, C.; Puccetti, M.; Pariano, M.; Renga, G.; Stincardini, C.; D’Onofrio, F.; Bellet, M.M.; Cellini, B.; Giovagnoli, S.; Romani, L. Selectively targeting key inflammatory pathways in cystic fibrosis. Eur. J. Med. Chem. 2020, 206, 112717. [Google Scholar] [CrossRef]
- van Doorninck, J.H.; French, P.J.; Verbeek, E.; Peters, R.H.; Morreau, H.; Bijman, J.; Scholte, B.J. A mouse model for the cystic fibrosis delta F508 mutation. EMBO J. 1995, 14, 4403–4411. [Google Scholar] [CrossRef] [Green Version]
- Bozza, S.; Clavaud, C.; Giovannini, G.; Fontaine, T.; Beauvais, A.; Sarfati, J.; D’Angelo, C.; Perruccio, K.; Bonifazi, P.; Zagarella, S.; et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J. Immunol. 2009, 183, 2407–2414. [Google Scholar] [CrossRef] [Green Version]
- Bayry, J.; Tchilian, E.Z.; Davies, M.N.; Forbes, E.K.; Draper, S.J.; Kaveri, S.V.; Hill, A.V.; Kazatchkine, M.D.; Beverley, P.C.; Flower, D.R.; et al. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc. Natl. Acad. Sci. USA 2008, 105, 10221–10226. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.N.; Bayry, J.; Tchilian, E.Z.; Vani, J.; Shaila, M.S.; Forbes, E.K.; Draper, S.J.; Beverley, P.C.; Tough, D.F.; Flower, D.R. Toward the discovery of vaccine adjuvants: Coupling in silico screening and in vitro analysis of antagonist binding to human and mouse CCR4 receptors. PLoS ONE 2009, 4, e8084. [Google Scholar] [CrossRef]
- Montagnoli, C.; Fallarino, F.; Gaziano, R.; Bozza, S.; Bellocchio, S.; Zelante, T.; Kurup, W.P.; Pitzurra, L.; Puccetti, P.; Romani, L. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J. Immunol. 2006, 176, 1712–1723. [Google Scholar] [CrossRef] [Green Version]
- Gregg, K.S.; Kauffman, C.A. Invasive Aspergillosis: Epidemiology, Clinical Aspects, and Treatment. Semin. Respir. Crit. Care Med. 2015, 36, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Ridley, C.; Thornton, D.J. Mucins: The frontline defence of the lung. Biochem. Soc. Trans. 2018, 46, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Mariani, M.; Lang, R.; Binda, E.; Panina-Bordignon, P.; D’Ambrosio, D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur. J. Immunol. 2004, 34, 231–240. [Google Scholar] [CrossRef]
- Hartl, D. Immunological mechanisms behind the cystic fibrosis-ABPA link. Med. Mycol. 2009, 47 (Suppl. 1), S183–S191. [Google Scholar] [CrossRef] [Green Version]
- Schuh, J.M.; Power, C.; Proudfoot, A.E.; Kunkel, S.L.; Lukacs, N.W.; Hogaboam, C.M. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4-/- mice. FASEB J. 2002, 16, 1313–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lloyd, C.M.; Noble, A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013, 6, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannitti, R.G.; Carvalho, A.; Cunha, C.; De Luca, A.; Giovannini, G.; Casagrande, A.; Zelante, T.; Vacca, C.; Fallarino, F.; Puccetti, P.; et al. Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines. Am. J. Respir. Crit. Care Med. 2013, 187, 609–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, K.J.; Hogaboam, C.M. Immunosuppressive effects of CCL17 on pulmonary antifungal responses during pulmonary invasive aspergillosis. Infect. Immun. 2005, 73, 7198–7207. [Google Scholar] [CrossRef] [Green Version]
- Pere, H.; Montier, Y.; Bayry, J.; Quintin-Colonna, F.; Merillon, N.; Dransart, E.; Badoual, C.; Gey, A.; Ravel, P.; Marcheteau, E.; et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood 2011, 118, 4853–4862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozza, S.; Iannitti, R.G.; Pariano, M.; Renga, G.; Costantini, C.; Romani, L.; Bayry, J. Small Molecule CCR4 Antagonists Protect Mice from Aspergillus Infection and Allergy. Biomolecules 2021, 11, 351. https://doi.org/10.3390/biom11030351
Bozza S, Iannitti RG, Pariano M, Renga G, Costantini C, Romani L, Bayry J. Small Molecule CCR4 Antagonists Protect Mice from Aspergillus Infection and Allergy. Biomolecules. 2021; 11(3):351. https://doi.org/10.3390/biom11030351
Chicago/Turabian StyleBozza, Silvia, Rossana Giulietta Iannitti, Marilena Pariano, Giorgia Renga, Claudio Costantini, Luigina Romani, and Jagadeesh Bayry. 2021. "Small Molecule CCR4 Antagonists Protect Mice from Aspergillus Infection and Allergy" Biomolecules 11, no. 3: 351. https://doi.org/10.3390/biom11030351






