Plant Prebiotics and Their Role in the Amelioration of Diseases
Abstract
:1. Introduction
2. What Are Prebiotics?
3. Prebiotics and Dietary Fiber
4. Types of Prebiotics
4.1. Synthetic Prebiotics
4.1.1. Fructooligosaccharides (FOS)
4.1.2. Galactooligosaccharides (GOS)
4.1.3. Xylooligosaccharides (XOS)
4.1.4. Fructans
4.1.5. Isomaltooligosaccharides (IMO)
4.1.6. Soybean Oligosaccharides (SOS)
4.1.7. Guar Gum
4.1.8. Pectin Oligosaccharides
4.1.9. Other Polysaccharides
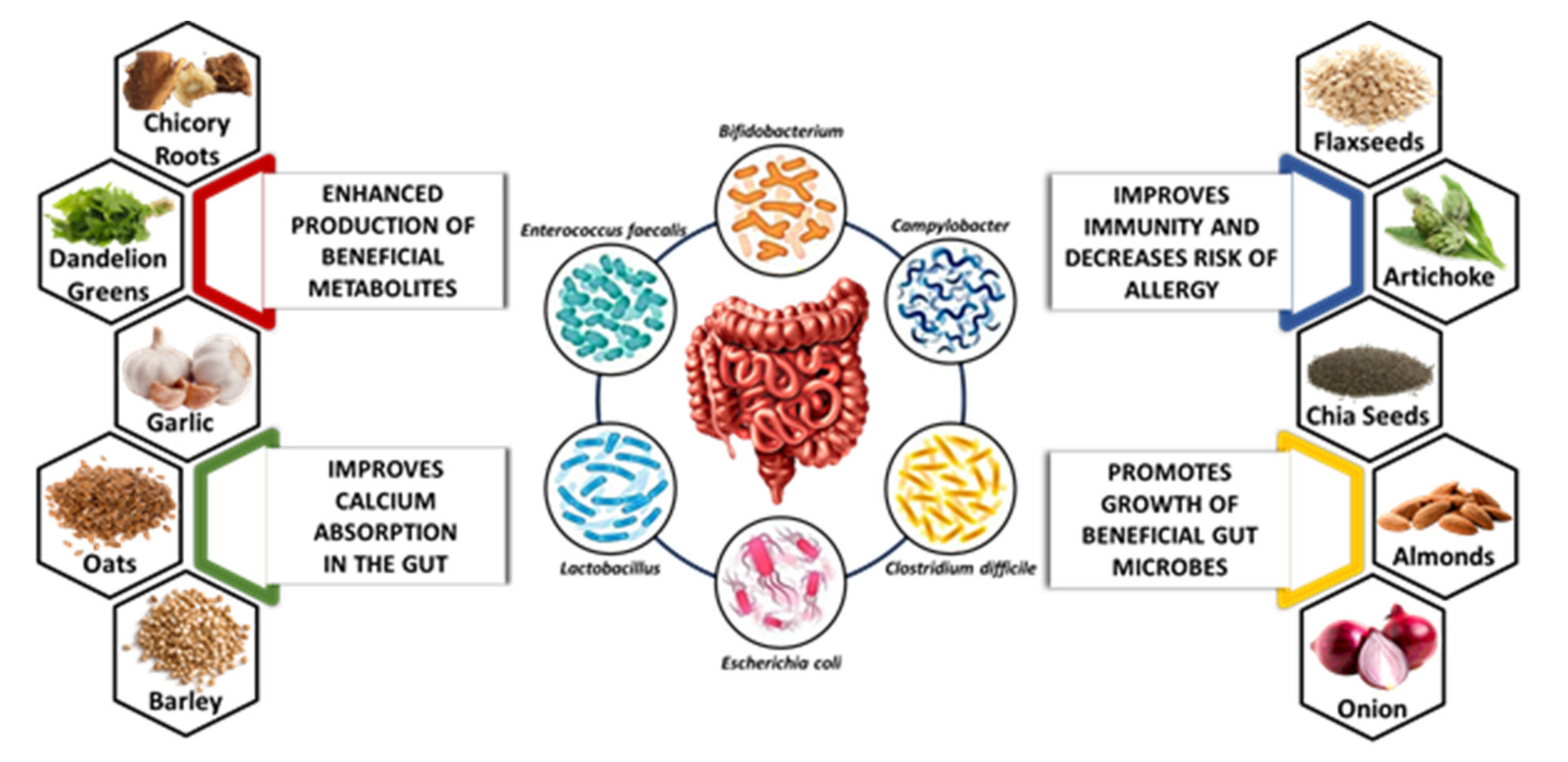
4.2. Sources of Natural Prebiotics
4.2.1. Dandelion Greens
4.2.2. Chicory Roots
4.2.3. Chia Seeds
4.2.4. Artichoke
4.2.5. Garlic
4.2.6. Almonds
4.2.7. Flaxseeds
4.2.8. Onion
4.2.9. Oats
4.2.10. Barley
5. Mode of Action of Prebiotics
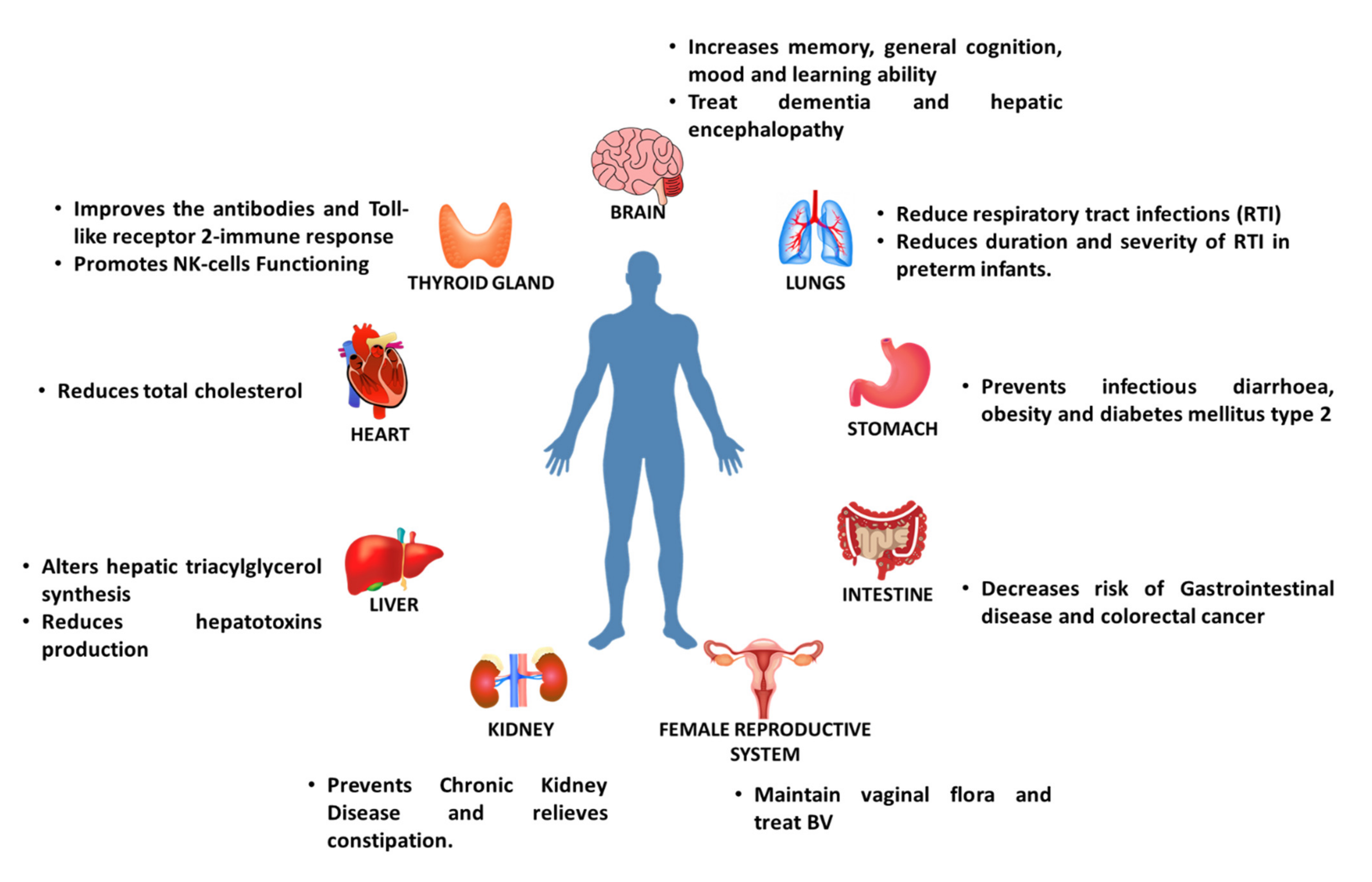
6. Health Benefits of Prebiotics
6.1. Effect of Prebiotics on Gut Microbes
6.2. Effect of Prebiotics on Metabolite Production
6.3. Effect of Prebiotics on Mineral Absorption
6.4. Effect of Prebiotics on Allergies
6.5. Effect of Prebiotics on Diabetes
6.6. Effect of Prebiotics on Necrotizing Enterocolitis
6.7. Effect of Prebiotics on Metabolic Disorders
6.8. Effect on Hepatic Encephalopathy
6.9. Effect on Female Reproductive Health
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lockyer, S.; Stanner, S. Prebiotics—An added benefit of some fiber types. Nutr. Bull. 2019, 44, 74–91. [Google Scholar] [CrossRef] [Green Version]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khangwal, I.; Shukla, P. Prospecting prebiotics, innovative evaluation methods, and their health applications: A review. 3 Biotech 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Rolim, P.M. Development of prebiotic food products and health benefits. Food Sci. Technol. 2015, 35, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Wei, J.-D.; Lu, Y.-F.; Sun, Y.-L.; Wang, Q.; Zhang, R.-L. Galacto-oligosaccharides modulate gut microbiota dysbiosis and intestinal permeability in rats with alcohol withdrawal syndrome. J. Funct. Foods 2019, 60, 103423. [Google Scholar] [CrossRef]
- Zeng, J.; Song, M.; Jia, T.; Gao, H.; Zhang, R.; Jiang, J. Immunomodulatory influences of Sialylated lactuloses in mice. Biochem. Biophys. Res. Commun. 2019, 514, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Santos, D.; Bedani, R.; Perego, P.; Converti, A.; Saad, S.L. acidophilus La-5, fructo-oligosaccharides and inulin may improve sensory acceptance and texture profile of a synbiotic diet mousse. LWT 2019, 105, 329–335. [Google Scholar] [CrossRef]
- Dos Santos, D.X.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Bedani, R.; Lima, E.D.; Saad, S.M.I. Impact of probiotics and prebiotics targeting metabolic syndrome. J. Funct. Foods 2020, 64, 103666. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Bedani, R.; Saad, S.M.I. Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: An update for current perspectives and future challenges. Br. J. Nutr. 2015, 114, 1993–2015. [Google Scholar] [CrossRef]
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Fernández, E.N.; Putz, P.; Rowland, I.; Swann, J.; Türk, J.; Vermeiren, J.; et al. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur. J. Nutr. 2017, 57, 25–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [Green Version]
- Ashley, J.M.; Jarvis, W.T. Position of the American Dietetic Association: Health implications of dietary fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Food Labelling: Revision of the Nutrition and Supplement Facts Label 21 CFR 101; Food and Drug Administration: Silver Spring, MD, USA, 2016.
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of action of prebiotics and their effects on gastro-intestinal disorders in adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [Green Version]
- Howlett, J.F.; Betteridge, V.A.; Champ, M.; Craig, S.A.; Méheust, A.; Jones, J.M. The definition of dietary fiber—Discussions at the Ninth Vahouny Fiber Symposium: Building scientific agreement. Food Nutr. Res. 2010, 54. [Google Scholar] [CrossRef] [PubMed]
- Alimentarius, C. Guidelines on Nutrition Labelling CAC/GL 2-1985 as Last Amended 2010; FAO: Rome, Italy, 2010. [Google Scholar]
- Dayib, M.; Larson, J.; Slavin, J. Dietary fibers reduce obesity-related disorders. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; de Vos, W.M.; Martens, E.C.; Gilbert, J.A.; Menon, R.S.; Soto-Vaca, A.; Hautvast, J.; Meyer, P.D.; Borewicz, K.; Vaughan, E.E.; et al. Effect of fructans, prebiotics and fibers on the human gut microbiome assessed by 16S rRNA-based ap-proaches: A review. Benef. Microbes 2020, 11, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Dashtdar, M.; Kardi, K. Benefits of gum arabic, for a solitary kidney under adverse conditions: A case study. Chin. Med. Cult. 2018, 1, 88. [Google Scholar] [CrossRef]
- Presti, A.L.; Zorzi, F.; Del Chierico, F.; Altomare, A.; Cocca, S.; Avola, A.; De Biasio, F.; Russo, A.; Cella, E.; Reddel, S.; et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease. Front. Microbiol. 2019, 10, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Besten, G.D.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. New view on dietary fiber selection for predictable shifts in gut microbiota. mBio 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; Hayek, S.; Song, D. Recent application of probiotics in food and agricultural science. In Probiotics; Rigobelo, E.C., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Bali, V.; Panesar, P.S.; Bera, M.B.; Panesar, R. Fructo-oligosaccharides: Production, purification and potential applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1475–1490. [Google Scholar] [CrossRef]
- Khanvilkar, S.S.; Arya, S.S. Fructooligosaccharides: Applications and health benefits. Agro FOOD Ind. Hi Tech. 2015, 26, 6. [Google Scholar]
- Aqil, F.; Munagala, R.; Agrawal, A.K.; Gupta, R. Anticancer Phytocompounds: Experimental and Clinical Updates; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128146200. [Google Scholar]
- Alander, M.; Mättö, J.; Kneifel, W.; Johansson, M.; Kögler, B.; Crittenden, R.; Mattila-Sandholm, T.; Saarela, M. Effect of galacto-oligosaccharide supplementation on human faecal microflora and on survival and persistence of Bifidobacterium lactis Bb-12 in the gastrointestinal tract. Int. Dairy J. 2001, 11, 817–825. [Google Scholar] [CrossRef]
- Mohanty, D.; Misra, S.; Mohapatra, S.; Sahu, P.S. Prebiotics and synbiotics: Recent concepts in nutrition. Food Biosci. 2018, 26, 152–160. [Google Scholar] [CrossRef]
- Lecerf, J.-M.; Dépeint, F.; Clerc, E.; Dugenet, Y.; Niamba, C.N.; Rhazi, L.; Cayzeele, A.; Abdelnour, G.; Jaruga, A.; Younes, H.; et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 2012, 108, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Goyal, A. Functional oligosaccharides: Production, properties and applications. World J. Microbiol. Biotechnol. 2011, 27, 1119–1128. [Google Scholar] [CrossRef]
- Sorndech, W.; Na Nakorn, K.; Tongta, S.; Blennow, A. Isomalto-oligosaccharides: Recent insights in production technology and their use for food and medical applications. LWT 2018, 95, 135–142. [Google Scholar] [CrossRef]
- Franco-Robles, E.; López, M.G. Implication of Fructans in health: Immunomodulatory and antioxidant mechanisms. Sci. World J. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mudgil, D.; Barak, S.; Patel, A.; Shah, N. Partially hydrolyzed guar gum as a potential prebiotic source. Int. J. Biol. Macromol. 2018, 112, 207–210. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, 127. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J.; Michel, C. How to manipulate the microbiota: Prebiotics. In Advances in Experimental Medicine and Biology; Crusio, W.E., Dong, H., Lambris, J.D., Radeke, H.H., Rezaei, N., Eds.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; Volume 902, pp. 119–142. [Google Scholar]
- Kumar, C.G.; Sripada, S.; Poornachandra, Y. Status and future prospects of fructooligosaccharides as nutraceuticals. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 451–503. [Google Scholar]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Sánchez-Martínez, M.J.; Soto-Jover, S.; Antolinos, V.; Martínez-Hernández, G.B.; López-Gómez, A. Manufacturing of short-chain fructooligosaccharides: From laboratory to industrial scale. Food Eng. Rev. 2020, 12, 149–172. [Google Scholar] [CrossRef]
- De La Rosa, O.; Flores-Gallegos, A.C.; Muñíz-Marquez, D.; Nobre, C.; Contreras-Esquivel, J.C.; Aguilar, C.N. Fructooligosaccharides production from agro-wastes as alternative low-cost source. Trends Food Sci. Technol. 2019, 91, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.R.; Braun, M.M.; Wigertz, K.; Bryant, R.; Zhao, Y.; Lee, W.; Kempa-Steczko, A.; Weaver, C.M. Fructo-oligosaccharides and calcium absorption and retention in adolescent girls. J. Am. Coll. Nutr. 2010, 29, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Flores-Maltos, D.A.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Teixeira, J.A.; Aguilar, C.N. Biotechnological production and application of fructooligosaccharides. Crit. Rev. Biotechnol. 2016, 36, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkerman, R.; Faas, M.M.; De Vos, P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, T.; Mokoena, M.P.; Olaniran, A.O.; Wilhelmi, B.S.; Whiteley, C.G. Microbial enzymatic production and applications of short-chain fructooligosaccharides and inulooligosaccharides: Recent advances and current perspectives. J. Ind. Microbiol. Biotechnol. 2014, 41, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T. Novel physiological function of fructooligosaccharides. BioFactors 2004, 21, 89–94. [Google Scholar] [CrossRef]
- Singh, J.J.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic oligosaccharides: Special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef]
- Michel, M.R.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Gonzalez-Herrera, S.M.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Fructosyltransferase sources, production, and applications for prebiotics production. In Probiotics and Prebiotics in Human Nutrition and Health; Rao, V., Rao, L., Eds.; InTech: London, UK, 2016. [Google Scholar]
- Vallejo-García, L.C.; Rodríguez-Alegría, M.E.; Munguía, A.L. Enzymatic process yielding a diversity of inulin-type microbial fructooligosaccharides. J. Agric. Food Chem. 2019, 67, 10392–10400. [Google Scholar] [CrossRef]
- Monsan, P.F.; Ouarné, F. Oligosaccharides derived from sucrose. In Prebiotics and Probiotics Science and Technology; Rastall, R.A., Charalampopoulos, D., Eds.; Springer International Publishing: Geneva, Switzerland, 2009; pp. 293–336. [Google Scholar]
- Lateef, A.; Oloke, J.; Gueguim-Kana, E.; Raimi, O. Production of fructosyltransferase by a local isolate of Aspergillus nigerin both submerged and solid substrate media. Acta Aliment. 2012, 41, 100–117. [Google Scholar] [CrossRef]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.C.; Gomez-Zavaglia, A. Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Frau, F.; Ruiz-Matute, A.I.; Montilla, A.; Belloch, C.; Manzanares, P.; Corzo, N. Production of lactulose oligosaccharides by isomerisation of transgalactosylated cheese whey permeate obtained by β-galactosidases from dairy Kluyveromyces. J. Dairy Res. 2015, 82, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, D.P.; Gonçalves, M.D.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmacci, E.R.; Plante, O.J.; Hewitt, M.C.; Seeberger, P.H. Automated synthesis of oligosaccharides. Helv. Chim. Acta 2003, 86, 3975–3990. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2007, 104, 305–344. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A Changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Marín-Manzano, M.C.; Abecia, L.; Hernández-Hernández, O.; Sanz, M.L.; Montilla, A.; Olano, A.; Rubio, L.A.; Moreno, F.J.; Clemente, A. Galacto-oligosaccharides derived from lactulose exert a selective stimulation on the growth of bifidobacterium animalis in the large intestine of growing rats. J. Agric. Food Chem. 2013, 61, 7560–7567. [Google Scholar] [CrossRef] [Green Version]
- Palcic, M.M. Biocatalytic synthesis of oligosaccharides. Curr. Opin. Biotechnol. 1999, 10, 616–624. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic synthesis of galacto-oligosaccharides and other lactose derivatives (hetero-oligosaccharides) from lactose. Int. Dairy J. 2012, 22, 116–122. [Google Scholar] [CrossRef]
- Weijers, C.A.; Franssen, M.C.; Visser, G.M. Glycosyltransferase-catalyzed synthesis of bioactive oligosaccharides. Biotechnol. Adv. 2008, 26, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.L.; Wilbey, R.A.; Grandison, A.S.; Roseiro, L.B. Milk oligosaccharides: A review. Int. J. Dairy Technol. 2015, 68, 305–321. [Google Scholar] [CrossRef] [Green Version]
- Zeuner, B.; Teze, D.; Muschiol, J.; Meyer, A.S. Synthesis of human milk oligosaccharides: Protein engineering strategies for improved enzymatic transglycosylation. Molecules 2019, 24, 2033. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Lu, J.; Lin, W.; Wei, X.; Li, H.; Zhao, X.; Jiang, A.; Yuan, J. Effects of a galacto-oligosaccharide-rich diet on fecal microbiota and metabolite profiles in mice. Food Funct. 2018, 9, 1612–1620. [Google Scholar] [CrossRef]
- Karlsson, E.N.; Schmitz, E.; Linares-Pastén, J.A.; Adlercreutz, P. Endo-xylanases as tools for production of substituted xylooligosaccharides with prebiotic properties. Appl. Microbiol. Biotechnol. 2018, 102, 9081–9088. [Google Scholar] [CrossRef] [Green Version]
- Jain, I.; Kumar, V.; Satyanarayana, T. Xylooligosaccharides: An economical prebiotic from agroresidues and their health benefits. Indian J. Exp. Boil. 2015, 53, 131–142. [Google Scholar]
- Zúñiga, M.; Monedero, V.; Yebra, M.J. Utilization of host-derived glycans by intestinal lactobacillus and bifidobacterium species. Front. Microbiol. 2018, 9, 1917. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Ślizewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Childs, C.E.; Röytiö, H.; Alhoniemi, E.; Fekete, A.A.; Forssten, S.D.; Hudjec, N.; Ni Lim, Y.; Steger, C.J.; Yaqoob, P.; Tuohy, K.M.; et al. Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: A double-blind, placebo-controlled, randomised, factorial cross-over study. Br. J. Nutr. 2014, 111, 1945–1956. [Google Scholar] [CrossRef] [Green Version]
- Qiang, X.; YongLie, C.; QianBing, W. Health benefit application of functional oligosaccharides. Carbohydr. Polym. 2009, 77, 435–441. [Google Scholar] [CrossRef]
- Lin, S.-H.; Chou, L.-M.; Chien, Y.-W.; Chang, J.-S.; Lin, C.-I. Prebiotic Effects of xylooligosaccharides on the improvement of microbiota balance in human subjects. Gastroenterol. Res. Pract. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bosscher, D. Fructan Prebiotics Derived from Inulin. In Prebiotics and Probiotics Science and Technology; Rastall, R.A., Charalampopoulos, D., Eds.; Springer International Publishing: Geneva, Switzerland, 2009; pp. 163–205. [Google Scholar]
- Ende, W.V.D. Novel fructan exohydrolase: Unique properties and applications for human health. J. Exp. Bot. 2018, 69, 4227–4231. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Kok, N. Effects of fructans-type prebiotics on lipid metabolism. Am. J. Clin. Nutr. 2001, 73, 456s–458s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, T.S.M.; Miguel, A.S.M.; Fernández, D.E.R.; Ortiz, G.M.D. Biotechnological production of oligosaccharides–Applications in the food industry. In Food Production and Industry; Eissa, A.A., Ed.; InTech: London, UK, 2015. [Google Scholar]
- Gänzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.P.; Singh, J.; Boparai, R.K.; Zhu, J.; Mantri, S.; Khare, P.; Khardori, R.; Kondepudi, K.K.; Chopra, K.; Bishnoi, M. Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharm. Res. 2017, 123, 103–113. [Google Scholar] [CrossRef]
- Švejstil, R.; Musilová, S.; Rada, V. Raffinose-series oligosaccharides in soybean products. Sci. Agric. Bohem. 2015, 46, 73–77. [Google Scholar] [CrossRef] [Green Version]
- González-Rodríguez, I.; Ruiz, L.; Gueimonde, M.; Margolles, A.; Sánchez, B. Factors involved in the colonization and survival of bifidobacteria in the gastrointestinal tract. Fems Microbiol. Lett. 2012, 340, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Niittynen, L.; Kajander, K.; Korpela, R. Galacto-oligosaccharides and bowel function. Scand. J. Food Nutr. 2007, 51, 62–66. [Google Scholar] [CrossRef]
- Battistini, C.; Gullón, B.; Ichimura, E.S.; Gomes, A.M.P.; Ribeiro, E.P.; Kunigk, L.; Moreira, J.U.V.; Jurkiewicz, C. Development and characterization of an innovative synbiotic fermented beverage based on vegetable soybean. Braz. J. Microbiol. 2018, 49, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibson, G.R.; Rastall, R.A. In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl. Environ. Microbiol. 2005, 71, 8383–8389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-López, E.; Cela, D.; Costabile, A.; Mateos-Aparicio, I.; Rupérez, P. In vitro fermentability and prebiotic potential of soyabean Okara by human faecal microbiota. Br. J. Nutr. 2016, 116, 1116–1124. [Google Scholar] [CrossRef] [Green Version]
- Carlson, J.; Esparza, J.; Swan, J.; Taussig, D.; Combs, J.; Slavin, J. In vitro analysis of partially hydrolyzed guar gum fermentation differences between six individuals. Food Funct. 2016, 7, 1833–1838. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health effects and sources of prebiotic dietary fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Mola, G.T.; Stadler, F.J. Guar gum and its composites as potential materials for diverse applications: A review. Carbohydr. Polym. 2018, 199, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Reider, S.J.; Moosmang, S.; Tragust, J.; Trgovec-Greif, L.; Tragust, S.; Perschy, L.; Przysiecki, N.; Sturm, S.; Tilg, H.; Stuppner, H.; et al. Prebiotic effects of partially hydrolyzed guar gum on the composition and function of the human microbiota—Results from the PAGODA Trial. Nutrients 2020, 12, 1257. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Harholt, J.; Suttangkakul, A.; Scheller, H.V. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babbar, N.; Dejonghe, W.; Sforza, S.; Elst, K. Enzymatic pectic oligosaccharides (POS) production from sugar beet pulp using response surface methodology. J. Food Sci. Technol. 2017, 54, 3707–3715. [Google Scholar] [CrossRef]
- Cano, M.E.; García-Martin, A.; Morales, P.C.; Wojtusik, M.; Santos, V.E.; Kovensky, J.; Ladero, M. Production of oligosaccharides from agrofood wastes. Fermentation 2020, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Larsen, N.; De Souza, C.B.; Krych, L.; Cahú, T.B.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2015, 36, 1–7. [Google Scholar] [CrossRef]
- Maier, T.V.; Lucio, M.; Lee, L.H.; Verberkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. mBio 2017, 8, e01343-17. [Google Scholar] [CrossRef] [Green Version]
- Kalmokoff, M.; Zwicker, B.; O’Hara, M.; Matias, F.; Green, J.; Shastri, P.; Green-Johnson, J.; Brooks, S. Temporal change in the gut community of rats fed high amylose cornstarch is driven by endogenous urea rather than strictly on carbohydrate availability. J. Appl. Microbiol. 2013, 114, 1516–1528. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.-L.; Zhao, X.-H. In vitro fermentation of a retrograded maize starch by healthy adult fecal extract and impacts of exogenous microorganisms on three acids production. Starch Stärke 2012, 65, 330–337. [Google Scholar] [CrossRef]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2012, 83, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghazzewi, F.H.; Khanna, S.; Tester, R.F.; Piggott, J. The potential use of hydrolysed konjac glucomannan as a prebiotic. J. Sci. Food Agric. 2007, 87, 1758–1766. [Google Scholar] [CrossRef]
- Connolly, M.L.; Lovegrove, J.A.; Tuohy, K.M. Konjac glucomannan hydrolysate beneficially modulates bacterial composition and activity within the faecal microbiota. J. Funct. Foods 2010, 2, 219–224. [Google Scholar] [CrossRef]
- Chen, H.-L.; Cheng, H.-C.; Wu, W.-T.; Liu, Y.-J.; Liu, S.-Y. Supplementation of Konjac glucomannan into a low-fiber chinese diet promoted bowel movement and improved colonic ecology in constipated adults: A placebo-controlled, diet-controlled trial. J. Am. Coll. Nutr. 2008, 27, 102–108. [Google Scholar] [CrossRef]
- Chen, H.-L.; Cheng, H.-C.; Liu, Y.-J.; Liu, S.-Y.; Wu, W.-T. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrients 2006, 22, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Harmayani, E.; Aprilia, V.; Marsono, Y. Characterization of glucomannan from Amorphophallus oncophyllus and its prebiotic activity in vivo. Carbohydr. Polym. 2014, 112, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Prado, S.B.R.D.; Castro-Alves, V.C.; Ferreira, G.F.; Fabi, J.P. Ingestion of non-digestible carbohydrates from plant-source foods and decreased risk of colorectal cancer: A review on the biological effects and the mechanisms of action. Front. Nutr. 2019, 6, 72. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sa`id, A.M.; Mustapha, H.; Mashi, J.; Muhammad, Y.; Abubakar, S.; Gadanya, A. Nutritional and pharmacological potential of ethanol leaves extract of taraxacum officinale. Asian J. Biol. Sci. 2019, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, S.; Adil, S.; El-Hack, M.A.; Alagawany, M.; Farag, M. Beneficial uses of dandelion herb (Taraxacum officinale) in poultry nutrition. World’s Poult. Sci. J. 2017, 73, 591–602. [Google Scholar] [CrossRef]
- Mahboubi, M.; Mahboubi, M. Hepatoprotection by dandelion (Taraxacum officinale) and mechanisms. Asian Pac. J. Trop. Biomed. 2020, 10, 1. [Google Scholar] [CrossRef]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Fatima, T.; Bashir, O.; Naseer, B.; Zameer Hussain, S.; Tabasum Fatima, C.; Fatima, T.; Zameer Hussain, S. Dandelion: Phytochemistry and clinical potential. J. Med. Plants Stud. 2018, 6, 198–202. [Google Scholar]
- Ivanov, I.G. Polyphenols Content and Antioxidant Activities of Taraxacum officinale F.H. Wigg (Dandelion) Leaves. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 889–893. Available online: www.ijppr.com (accessed on 10 October 2020).
- Trojanová, I.; Rada, V.; Kokoska, L.; Vlková, E. The bifidogenic effect of Taraxacum officinale root. Fitoterapia 2004, 75, 760–763. [Google Scholar] [CrossRef]
- Joshi, D.; Roy, S.; Banerjee, S. Prebiotics: A functional food in health and disease. In Natural Products and Drug Discovery: An Integrated Approach; MandaL, S.C., Konishi, T., Mandal, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 507–523. ISBN 9780081021040. [Google Scholar]
- Devaraj, E. Hepatoprotective properties of dandelion: Recent update. J. Appl. Pharm. Sci. 2016, 6, 202–205. [Google Scholar] [CrossRef] [Green Version]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Leroy, G.; Grongnet, J.F.; Mabeau, S.; Le Corre, D.; Baty-Julien, C. Changes in inulin and soluble sugar concentration in artichokes (Cynara scolymus L.) during storage. J. Sci. Food Agric. 2010, 90, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, N.; Das, S.; Sharma, S. Cichorium intybus: A concise report on its ethnomedicinal, botanical, and phytopharmacological aspects. Drug Dev. Ther. 2016, 7, 1. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative livestock feed supplement. Sci. World J. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Abbas, Z.K.; Saggu, S.; Sakeran, M.I.; Zidan, N.; Rehman, H.; Ansari, A.A. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J. Biol. Sci. 2015, 22, 322–326. [Google Scholar] [CrossRef] [Green Version]
- El-Kholy, W.M.; Aamer, R.A.; Ali, A.N.A. Utilization of inulin extracted from chicory (Cichorium intybus L.) roots to improve the properties of low-fat synbiotic yoghurt. Ann. Agric. Sci. 2020, 65, 59–67. [Google Scholar] [CrossRef]
- Chikkerur, J.; Samanta, A.K.; Kolte, A.P.; Dhali, A.; Roy, S. Production of short chain fructo-oligosaccharides from inulin of chicory root using fungal endoinulinase. Appl. Biochem. Biotechnol. 2019, 191, 695–715. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, J.; Salvador, A.; Hernando, I. Replacing fat and sugar with inulin in cakes: Bubble size distribution, physical and sensory properties. Food Bioprocess Technol. 2014, 7, 964–974. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [Green Version]
- Hrnčič, M.K.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia Hispanica L.): An overview-phytochemical profile, isolation methods, and application. Molecules 2020, 25, 11. [Google Scholar] [CrossRef] [Green Version]
- Santillán-Álvarez, A.; Dublán-García, O.; López-Martínez, L.X.; Quintero-Salazar, B.; Gómez-Oliván, L.M.; Díaz-Bandera, D.; Hernández-Navarro, M.D. Effect of chia seed on physicochemical and sensory characteristics of common carp restructured as functional food. J. Food Sci. Eng. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Nadeem, M.; Manzoor, M.F.; Javed, A.; Ali, Z.; Akhtar, M.N.; Ali, M.; Hussain, Y. Fatty acids characterization, oxidative perspectives and consumer acceptability of oil extracted from pre-treated chia (Salvia hispanica L.) seeds. Lipids Health Dis. 2016, 15, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamargo, A.; Cueva, C.; Laguna, L.; Moreno-Arribas, M.; Muñoz, L.A. Understanding the impact of chia seed mucilage on human gut microbiota by using the dynamic gastrointestinal model simgi. J. Funct. Foods 2018, 50, 104–111. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Kolba, N.; Martino, H.S.D.; Hart, J.; Tako, E. Soluble extracts from chia seed (Salvia hispanica L.) affect brush border membrane functionality, morphology and intestinal bacterial populations in vivo (Gallus gallus). Nutrients 2019, 11, 2457. [Google Scholar] [CrossRef] [Green Version]
- Bekheet, S.; Sota, V. Biodiversity and medicinal uses of globe artichoke (Cynara scolymus L.) plant. J. Biodivers. Conserv. Bioresour. Manag. 2019, 5, 39–54. [Google Scholar] [CrossRef]
- Praznik, W.; Cieślik, E.; Filipiak-Florkiewicz, A. Soluble dietary fibers in Jerusalem artichoke powders: Composition and ap-plication in bread. Nahrung Food 2002, 46, 151–157. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Minerals profile of two globe artichoke cultivars as affected by NPK fertilizer regimes. Food Res. Int. 2017, 100, 95–99. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D. Targeting carbohydrates and polyphenols for a healthy microbiome and healthy weight. Curr. Nutr. Rep. 2019, 8, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031–1048. [Google Scholar]
- Zhang, N.; Huang, X.; Zeng, Y.; Wu, X.; Peng, X. Study on prebiotic effectiveness of neutral garlic fructan in vitro. Food Sci. Hum. Wellness 2013, 2, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.L.; Cacciola, F.; Rich, G.; Bisignano, C.; Saija, A.; Dugo, P.; et al. Characterization of polyphenols, lipids and dietary fiber from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lapsley, K.; Blumberg, J. A nutrition and health perspective on almonds. J. Sci. Food Agric. 2006, 86, 2245–2250. [Google Scholar] [CrossRef]
- Chen, C.-Y.O.; Milbury, P.E.; Blumberg, J.B. Polyphenols in almond skins after blanching modulate plasma biomarkers of oxidative stress in healthy humans. Antioxidants 2019, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Nuenopalop, C.; Bisignano, G.; Wickham, M.S.J.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) Seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Huang, G.; Zhang, W.; Ni, L. In vitro and in vivo evaluation of the prebiotic effect of raw and roasted almonds (Prunus amygdalus). J. Sci. Food Agric. 2016, 96, 1836–1843. [Google Scholar] [CrossRef] [Green Version]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M.K. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Dzuvor, C.K.O.; Taylor, J.T.; Acquah, C.; Pan, S.; Agyei, D. Bioprocessing of functional ingredients from flaxseed. Molecules 2018, 23, 2444. [Google Scholar] [CrossRef] [Green Version]
- Power, K.A.; Lepp, D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary flaxseed modulates the colonic microenvironment in healthy C57Bl/6 male mice which may alter susceptibility to gut-associated diseases. J. Nutr. Biochem. 2016, 28, 61–69. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Yin, P.; Fan, H.; Sun, L.; Liu, Y. Flaxseed oil ameliorates alcoholic liver disease via anti-inflammation and modulating gut microbiota in mice. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Singh Bora, K.; Sharma, A. Phytoconstituents and therapeutic potential of Allium cepa Linn.—A Review. Pharmacogn. Rev. 2009, 3, 170. [Google Scholar]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, J.L. Carbohydrates, dietary fiber, and resistant starch in white vegetables: Links to health outcomes. Adv. Nutr. 2013, 4, 351S–355S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdón, B.R.; Rodríguez, C.T.; Rodríguez, E.R.; Romero, C.D. Fructans and major compounds in onion cultivars (Allium cepa). J. Food Compos. Anal. 2009, 22, 25–32. [Google Scholar] [CrossRef]
- Vinke, P.C.; El Aidy, S.; Van Dijk, G. The role of supplemental complex dietary carbohydrates and gut microbiota in promoting cardiometabolic and immunological health in obesity: Lessons from healthy non-obese individuals. Front. Nutr. 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Sargautiene, V.; Nakurte, I.; Nikolajeva, V. Broad prebiotic potential of non-starch polysaccharides from oats (Avena sativa L.): An in vitro Study. Pol. J. Microbiol. 2018, 67, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its pro-cessing as value added foods—A review. J. Food Sci. Technol. 2013, 52, 662–675. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of beta-glucan. Fibers 2019, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-glucans: The impact of processing and how it affects physiological responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [Green Version]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Blattner, F.R. Taxonomy of the genus hordeum and barley (Hordeum vulgare). Compend. Plant Genomes 2018, 11–23. [Google Scholar] [CrossRef]
- Lahouar, L.; Ghrairi, F.; El Arem, A.; Medimagh, S.; El Felah, M.; Ben Salem, H.; Achour, L. Biochemical composition and nutritional evaluation of barley rihane (Hordeum vulgare L.). Afr. J. Tradit. Complement. Altern. Med. 2016, 14, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Cereal based functional food of Indian subcontinent: A review. J. Food Sci. Technol. 2011, 49, 665–672. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One health, fermented foods, and gut microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKevith, B. Nutritional aspects of cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, I. Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards Nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Steele, K.; Dickin, E.; Keerio, M.; Samad, S.; Kambona, C.; Brook, R.; Thomas, W.; Frost, G. Breeding low-glycemic index barley for functional food. Field Crop. Res. 2013, 154, 31–39. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2012, 6, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Nath, A.; Molnár, M.A.; Csighy, A.; Kőszegi, K.; Galambos, I.; Huszár, K.P.; Koris, A.; Vatai, G. Biological activities of lac-tose-based prebiotics and symbiosis with probiotics on controlling osteoporosis, blood-lipid and glucose levels. Medicina 2018, 54, 98. [Google Scholar] [CrossRef] [Green Version]
- Khangwal, I.; Shukla, P. Potential prebiotics and their transmission mechanisms: Recent approaches. J. Food Drug Anal. 2019, 27, 649–656. [Google Scholar] [CrossRef] [Green Version]
- De Souza Aquino, J.; Batista, K.S.; Menezes, F.N.D.D.; Lins, P.P.; de Sousa Gomes, J.A.; da Silva, L.A. Models to evaluate the prebiotic potential of foods. In Functional Food—Improve Health through Adequate Food; InTech: London, UK, 2017. [Google Scholar]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.S.; Finocchiaro, T. Handbook of Prebiotics and Probiotics Ingredients: Health Benefits and Food Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Janardhana, V.; Broadway, M.M.; Bruce, M.P.; Lowenthal, J.W.; Geier, M.S.; Hughes, R.J.; Bean, A.G.D. Prebiotics modulate immune responses in the gut-associated lymphoid tissue of chickens. J. Nutr. 2009, 139, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven im-provement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Li, S.; Li, Y.; Gan, R.-Y.; Li, H.-B. Gut microbiota’s relationship with liver disease and role in hepatoprotection by dietary natural products and probiotics. Nutrients 2018, 10, 1457. [Google Scholar] [CrossRef] [Green Version]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [Green Version]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; de Vos, W.M. Next-generation beneficial microbes: The case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.L.; Lee, W.; Martin, B.R.; Story, J.A.; Campbell, J.K.; Weaver, C.M. Prebiotics Enhance magnesium absorption and inulin-based fibers exert chronic effects on calcium utilization in a postmenopausal rodent model. J. Food Sci. 2012, 77, 88–94. [Google Scholar] [CrossRef]
- Whisner, C.M.; Castillo, L.F. Prebiotics, bone and mineral metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial metabolites in nutrition, healthcare and agriculture. 3 Biotech 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nu-trients and other food components. Eur. J. Nutr. 2018, 57, 1. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain commu-nication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.; Pires, E.; et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [Green Version]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.; Giudici, F.; Fiorindi, C.; Ficari, F.; Scaringi, S.; Amedei, A. Immunomodulating activity and therapeutic effects of short chain fatty acids and tryptophan post-biotics in inflammatory bowel disease. Front. Immunol. 2019, 10, 2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kho, Z.Y.; Lal, S.K. The human gut microbiome—A potential controller of wellness and disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [Green Version]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The Potential of probiotics as a therapy for osteoporosis. In Bugs as Drugs; Cani, P.D., Britton, R.A., Eds.; American Society of Microbiology: Washington, DC, USA, 2017; Volume 5, pp. 213–233. [Google Scholar]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, M.R.; Patel, V.P. Probiotics: A promising tool for calcium absorption. Open Nutr. J. 2018, 12, 59–69. [Google Scholar] [CrossRef]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Liang, L.; Gunn, S.K.; Darlington, G.; Ellis, K.J. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am. J. Clin. Nutr. 2005, 82, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Aςil, Y.; Gluër, C.C.; Schrezenmeir, J. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J. Nutr. 2007, 137, 838S–846S. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Özdemir, Ö. Various effects of different probiotic strains in allergic disorders: An update from laboratory and clinical data. Clin. Exp. Immunol. 2010, 160, 295–304. [Google Scholar] [CrossRef]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and immune function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef] [Green Version]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and preventive effects in allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Osborn, D.A.; Sinn, J.K.H. Prebiotics in infants for prevention of allergy. Cochrane Database Syst. Rev. 2013, CD006474. [Google Scholar] [CrossRef]
- Jerram, S.T.; Dang, M.N.; Leslie, R.D. The role of epigenetics in Type 1 diabetes. Curr. Diabetes Rep. 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, Á.; Berná, G.; Rojas, A.; Martín, F.; Soria, B. Gene-diet interactions in type 2 diabetes: The chicken and egg debate. Int. J. Mol. Sci. 2017, 18, 1188. [Google Scholar] [CrossRef]
- Harsch, I.A.; Konturek, P.C. The role of gut microbiota in obesity and Type 2 and Type 1 diabetes mellitus: New insights into “old” diseases. Med. Sci. 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Nam, K.; Chung, S.-J. Effect of nutrient composition in a mixed meal on the postprandial glycemic response in healthy people: A preliminary study. Nutr. Res. Pract. 2019, 13, 126–133. [Google Scholar] [CrossRef]
- Russell, W.R.; Baka, A.; Björck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of diet composition on blood glucose regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef]
- Mirmiran, P. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: Role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef] [Green Version]
- Yang, J. Influence of Native and Processed Cereal Grain Fibers on Gut Health. Ph.D. Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2015. [Google Scholar]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia 2015, 58, 2206–2217. [Google Scholar] [CrossRef]
- Elgendy, M.M.; Othman, H.; Aly, H.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [CrossRef]
- Gephart, S.M.; McGrath, J.M.; Effken, J.A.; Halpern, M.D. Necrotizing enterocolitis risk. Adv. Neonatal Care 2012, 12, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.; Ross, R.P.; Ryan, C.A.; Hussey, S.; Murphy, B.; Fitzgerald, G.F.; Stanton, C. Role of gut microbiota in early infant development. Clin. Med. Pediatr. 2009, 3, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Cavallo, L.; Francavilla, R. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J. Pediatr. 2008, 152, 801–806. [Google Scholar] [CrossRef]
- Raskov, H.; Burcharth, J.; Pommergaard, H.-C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef] [Green Version]
- Spiller, R. Review article: Probiotics and prebiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 385–396. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currò, D.; Ianiro, G.; Pecere, S.; Bibbò, S.; Cammarota, G. Probiotics, fiber and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017, 174, 1426–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Gastroenterol. Res. Pract. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gulzar, N.; Saleem, I.M.; Rafiq, S.; Nadeem, M. Therapeutic Potential of probiotics and prebiotics. In Oral Health by Using Probiotic Products; Mahmoudi, R., Moosazad, S., Aghaei, K., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Chung, W.S.F.; Walker, A.W.; Louis, P.; Parkhill, J.; Vermeiren, J.; Bosscher, D.; Duncan, S.H.; Flint, H.J. Modulation of the human gut microbiota by dietary fibers occurs at the species level. BMC Biol. 2016, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Salminen, S. Handbook of Probiotics and Prebiotics; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Verdenelli, M.C.; Cecchini, C.; Coman, M.M.; Silvi, S.; Orpianesi, C.; Coata, G.; Cresci, A.; Di Renzo, G.C. Impact of probiotic synbio® administered by vaginal suppositories in promoting vaginal health of apparently healthy women. Curr. Microbiol. 2016, 73, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Recine, N.; Palma, E.; Domenici, L.; Giorgini, M.; Imperiale, L.; Sassu, C.; Musella, A.; Marchetti, C.; Muzii, L.; Panici, P.B. Restoring vaginal microbiota: Biological control of bacterial vaginosis. A prospective case–control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch. Gynecol. Obs. 2016, 293, 101–107. [Google Scholar] [CrossRef]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobaclli and probiotics in maintaining vaginal health. Arch. Gynecol. Obs. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- Li, J.; McCormick, J.; Bocking, A.; Reid, G. Importance of vaginal microbes in reproductive health. Reprod. Sci. 2012, 19, 235–242. [Google Scholar] [CrossRef]
- Yang, S.; Reid, G.; Challis, J.R.G.; Kim, S.O.; Gloor, G.B.; Bocking, A.D. Is there a role for probiotics in the prevention of preterm birth? Front. Immunol. 2015, 6, 62. [Google Scholar] [CrossRef] [Green Version]
| Types of Prebiotics | Chemical Structure | Production Methods | Potential Benefits | Reference |
|---|---|---|---|---|
| Fructooligosaccharides (FOS) | Glucose and Fructose units linked by β (2→1) glycosidic linkages | Polymerization of fructose monomers | Improve mineral absorption, decrease triglycerides, improve immunity, inhibit pathogenic microorganisms, prevent cancer, and control diabetes | [35,36,37] |
| Galactooligosaccharides (GOS) | Galactose and Glucose bound by β (1→3) and β (1→4) linkages | Transgalactosylation of lactose using β-galactosidase | Increase bifidogenic activity | [4,38] |
| Xylooligosaccharides (XOS) | xylose units linked through β (1→4) bonds | Enzymatic hydrolysis of plant xylans | Non-carcinogenic nature, exhibit a positive effect on the intestinal flora, non-digestibility | [39,40] |
| Soybean oligosaccharides (SOS) | galactose α-(1-6) linked to glucose (Raffinose)galactose α-(1-6) linked to terminal galactose (Stachyose) | NS | Increase the level of IgG, modulate body weight and the immune system | [41] |
| Isomalto-oligosaccharides (IMO) | Glucose bonds by α (1→4) type | Transglucosylation of liquefied starch | Improve gastrointestinal flora | [42] |
| Fructans | fructose with β (2→1) linkage | Enzymatic hydrolysis using Fructozyme L | Modulate gut physiology to provide protection from pathogens, improve the level of glucose | [43] |
| Guar gum | β-D-mannopyranosyl (1-4) linked with α-D-galactopyranosyl (1-6) residues | Enzymatic hydrolysis using cellulase | Improve cholesterol, glycemia | [44] |
| Pectinoligosaccharides (POS) | (1-4)-α-D-GalA (galacturonic acid) -(1,2)-α-L-Rha | Enzymatic hydrolysis by pectinase | Anti-inflammatory effect | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kuča, K.; Chopra, C.; Singh, R.; Kumar, H.; Șen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. https://doi.org/10.3390/biom11030440
Kaur AP, Bhardwaj S, Dhanjal DS, Nepovimova E, Cruz-Martins N, Kuča K, Chopra C, Singh R, Kumar H, Șen F, et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules. 2021; 11(3):440. https://doi.org/10.3390/biom11030440
Chicago/Turabian StyleKaur, Amrit Pal, Sonali Bhardwaj, Daljeet Singh Dhanjal, Eugenie Nepovimova, Natália Cruz-Martins, Kamil Kuča, Chirag Chopra, Reena Singh, Harsh Kumar, Fatih Șen, and et al. 2021. "Plant Prebiotics and Their Role in the Amelioration of Diseases" Biomolecules 11, no. 3: 440. https://doi.org/10.3390/biom11030440













