Fractionation of Heavy Metals in Multi-Contaminated Soil Treated with Biochar Using the Sequential Extraction Procedure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples and Biochar
2.2. USEPA Extraction
2.3. Sequential Extraction Procedure
3. Results
3.1. Biochars Description
3.2. Effects of Biochar on Soil Properties
3.3. Heavy Metals Mobility
3.4. Heavy Metals Redistribution
3.4.1. Acid-Soluble Fraction
3.4.2. Bound to Fe and Mn Oxides
3.4.3. Bound to Organic Matter
3.4.4. Residual Fraction
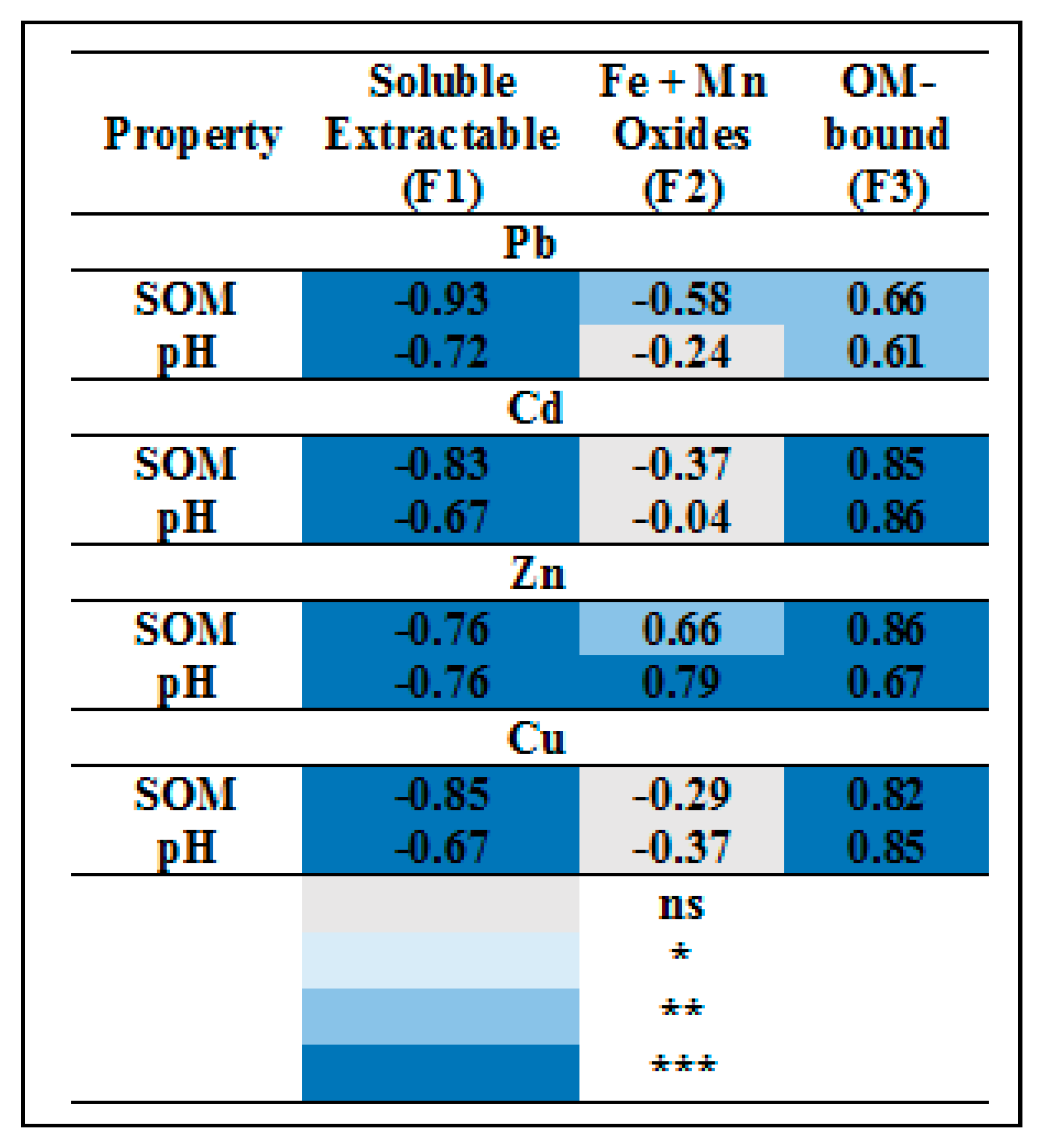
3.4.5. Correlation Analysis
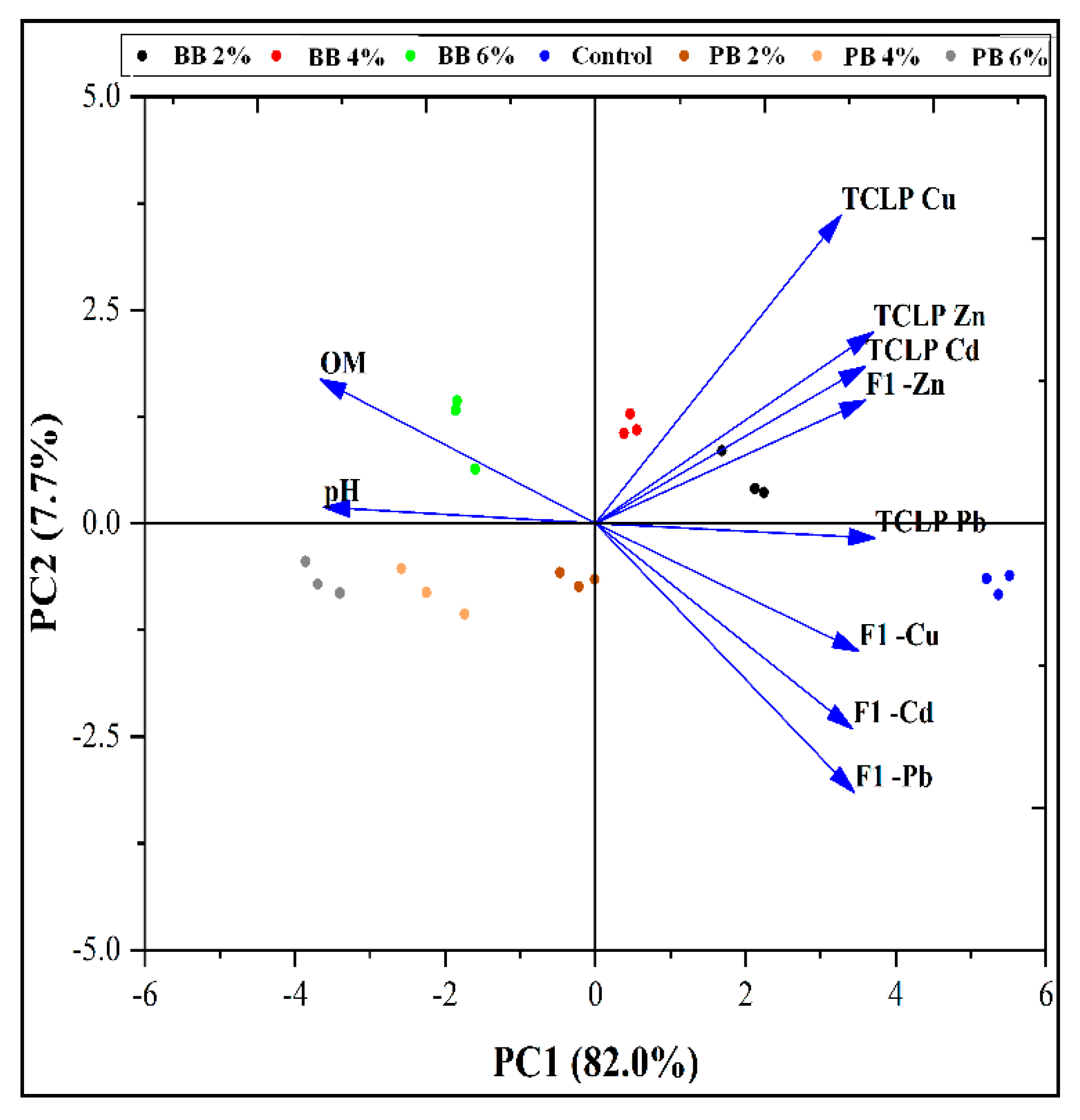
3.4.6. Principal Component Analysis (PCA)
4. Discussion
4.1. Biochar Type Effect
4.2. Biochar Rate Effect
4.3. Heavy Metals Mobility and Redistribution
4.4. Mechanisms of Heavy Metals Immobilization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A review of biochar-based sorbents for separation of heavy metals from water. Int. J. Phytoremed. 2020, 22, 111–126. [Google Scholar] [CrossRef]
- Awad, M.; Moustafa-Farag, M.; Wei, L.; Huang, Q.; Liu, Z. Effect of garden waste biochar on the bioavailability of heavy metals and growth of Brassica juncea (L.) in a multi-contaminated soil. Arab. J. Geosci. 2020, 13, 439. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection PRC and Ministry of Land and Resources PRC, Report on Soil Pollution in China. 2014. Available online: http://www.sdpc.gov.cn/fzgggz/ncjj/zhdt/201404/t20140418-607888.html (accessed on 6 March 2021).
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Li, J.; Pandey, A.K.; Khan, J. An Overview of the Potential of Bioremediation for Contaminated Soil from Municipal Solid Waste Site. In Emerging and Eco-Friendly Approaches for Waste Management; Springer Singapore: Singapore, 2019; pp. 59–68. ISBN 9789811086694. [Google Scholar]
- Botté, S.E.; Freije, R.H.; Marcovecchio, J.E. Distribution of Several Heavy Metals in Tidal Flats Sediments within Bahía Blanca Estuary (Argentina). Water Air Soil Pollut. 2010, 210, 371–388. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; de Matias, M.I.A.S.; Andrade, K.R.; de Jesus, A.N.; da Cunha, G.C.; de Andrade, R.S.; de Santos, J.C.J. Aged biochar changed copper availability and distribution among soil fractions and influenced corn seed germination in a copper-contaminated soil. Chemosphere 2020, 240, 124828. [Google Scholar] [CrossRef]
- LI, J.; XU, Y. Immobilization of Cd in paddy soil using moisture management and amendment. Environ. Sci. Pollut. Res. 2015, 22, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, S.; Islam, E.; Chen, J.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Lu, K. Lead accumulation and tolerance of Moso bamboo (Phyllostachys pubescens) seedlings: Applications of phytoremediation. J. Zhejiang Univ. B 2015, 16, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a Soil Amendment to Remediate Heavy Metal-Contaminated Agricultural Soil: Mechanisms, Efficacy, Problems, and Strategies. Water Air Soil Pollut. 2016, 227, 359. [Google Scholar] [CrossRef]
- Kabiri, P.; Motaghian, H.; Hosseinpur, A. Effects of Walnut Leaves Biochars on Lead and Zinc Fractionation and Phytotoxicity in a Naturally Calcareous Highly Contaminated Soil. Water Air Soil Pollut. 2019, 230, 263. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.-C.; Li, Z.; Chen, Z.; Wang, Y. Co-pyrolysis of sewage sludge and organic fractions of municipal solid waste: Synergistic effects on biochar properties and the environmental risk of heavy metals. J. Hazard. Mater. 2021, 412, 125200. [Google Scholar] [CrossRef]
- Awad, M.Y.M.; El-Desoky, M.A.; Ghallab, A.; Abdel-Mawly, S.E. Changes in Soil Zn and Mn Forms of Some Contaminated Egyptian Soils Treated with Organic Materials. Assiut J. Agric. Sci. 2017, 48, 269–285. [Google Scholar] [CrossRef]
- Zang, F.; Wang, S.; Nan, Z.; Ma, J.; Wang, Y.; Chen, Y.; Zhang, Q.; Li, Y. Influence of pH on the release and chemical fractionation of heavy metals in sediment from a suburban drainage stream in an arid mine-based oasis. J. Soils Sediments 2017, 17, 2524–2536. [Google Scholar] [CrossRef]
- Awad, M.Y.M.; El-Desoky, M.; Abdel-Mawly, S.E.; Ghallab, A. Mobility of Heavy Metals in Some Contaminated Egyptian Soils Treated with Certain Organic Materials. Ph.D. Thesis, Assiut University, Assiut, Egypt, 2007. [Google Scholar]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Y.; Qi, H.; Li, H.; Zhang, W. Biochar amendment changed soil-bound fractions of silver nanoparticles and ions but not their uptake by radish at an environmentally-relevant concentration. Biochar 2020, 2, 307–317. [Google Scholar] [CrossRef]
- Patel, A.K.; Das, N.; Goswami, R.; Kumar, M. Arsenic mobility and potential co-leaching of fluoride from the sediments of three tributaries of the Upper Brahmaputra floodplain, Lakhimpur, Assam, India. J. Geochem. Explor. 2019, 203, 45–58. [Google Scholar] [CrossRef]
- Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Tarnawski, M.; Szara, M.; Gorczyca, O.; Koniarz, T. The influence of the quantity and quality of sediment organic matter on the potential mobility and toxicity of trace elements in bottom sediment. Environ. Geochem. Health 2019, 41, 2893–2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.; Ok, Y.S.; Rajapaksha, A.U.; Lim, J.E.; Kim, B.-Y.; Ahn, J.-H.; Lee, Y.H.; Al-Wabel, M.I.; Lee, S.-E.; Lee, S.S. Lead and copper immobilization in a shooting range soil using soybean stover- and pine needle-derived biochars: Chemical, microbial and spectroscopic assessments. J. Hazard. Mater. 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; John Wiley & Sons: New York, NY, USA, 1982; pp. 539–579. [Google Scholar]
- Burt, R. Soil Survey Laboratory Methods Manual; Soil survey investigations report no. 42, version 4.0; Natural Resources Conservation Service, US Department of Agriculture: Washington, DC, USA, 2004.
- USEPA. Method 1311 TCLP-Toxicity Characteristic Leaching Procedure. In Test Methods for Evaluating Solid Waste, 3rd ed.; Environmental Protection Agency: Washington, DC, USA, 1992. [Google Scholar]
- Žemberyová, M.; Barteková, J.; Hagarová, I. The utilization of modified BCR three-step sequential extraction procedure for the fractionation of Cd, Cr, Cu, Ni, Pb and Zn in soil reference materials of different origins. Talanta 2006, 70, 973–978. [Google Scholar] [CrossRef]
- Awad, M.Y.M. Effect of Some Organic Compounds on Soil Properties and Plant Growth. Master’s Thesis, Menoufia University, Al Minufya, Egypt, 2001. [Google Scholar]
- Rekaby, S.A.; Awad, M.Y.M.; Hegab, S.A.; Eissa, M.A. Effect of some organic amendments on barley plants under saline condition. J. Plant Nutr. 2020, 43, 1840–1851. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Prasad, D.M.R. Sorption of Heavy Metals onto Biochar. In Applications of Biochar for Environmental Safety; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Lin, H.; Li, G.; Dong, Y.; Li, J. Effect of pH on the release of heavy metals from stone coal waste rocks. Int. J. Miner. Process. 2017, 165, 1–7. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Guo, D.; Zhang, Y.; Sun, X.; Jiang, S.; Guo, Z.; Huang, H.; Liang, W.; Li, R.; Zhang, Z. Using bamboo biochar with compost for the stabilization and phytotoxicity reduction of heavy metals in mine-contaminated soils of China. Sci. Rep. 2017, 7, 2690. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Solaiman, Z.M.; Meney, K.; Murphy, D.V.; Rengel, Z. Biochars immobilize soil cadmium, but do not improve growth of emergent wetland species Juncus subsecundus in cadmium-contaminated soil. J. Soils Sediments 2013, 13, 140–151. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, R.; Jiang, T.; Li, Z. Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard. Mater. 2012, 229–230, 145–150. [Google Scholar] [CrossRef]
- Ozcan, N.; Altundag, H. Speciation of heavy metals in street dust samples from sakarya I. Organized industrial district using the bcr sequential extraction procedure by ICP-OES. Bull. Chem. Soc. Ethiop. 2013, 27, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Golui, D.; Datta, S.P.; Dwivedi, B.S.; Meena, M.C.; Trivedi, V.K.; Jaggi, S.; Bandyopadhyay, K.K. Assessing Geoavailability of Zinc, Copper, Nickel, Lead and Cadmium in Polluted Soils Using Short Sequential Extraction Scheme. Soil Sediment Contam. Int. J. 2021, 30, 74–91. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhai, L.M.; Tan, W.F.; Liu, F.; He, J.Z. Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ. Pollut. 2007, 147, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Lu, H.; Lonappan, L.; Brar, S.K.; He, L.; Chen, J.; Yang, S. Biochar application as a soil amendment for decreasing cadmium availability in soil and accumulation in Brassica chinensis. J. Soils Sediments 2018, 18, 2511–2519. [Google Scholar] [CrossRef]
- Lucchini, P.; Quilliam, R.S.; DeLuca, T.H.; Vamerali, T.; Jones, D.L. Increased bioavailability of metals in two contrasting agricultural soils treated with waste wood-derived biochar and ash. Environ. Sci. Pollut. Res. 2014, 21, 3230–3240. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Shi, Y.; Qiu, Y.; Che, L.; Xue, C. Performance and mechanisms of emerging animal-derived biochars for immobilization of heavy metals. Sci. Total Environ. 2019, 646, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.; Lee, S.J.; Bolan, N.; Chung, J.W.; Edraki, M. Comparative Sorption of Pb and Cd by Biochars and Its Implication for Metal Immobilization in Soils. Water Air Soil Pollut. 2013, 224, 1711. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef] [PubMed]


| Property | Unit | Soil | Biochar | |
|---|---|---|---|---|
| Paulownia | Bamboo | |||
| Clay | (g kg−1) | 260 | - | - |
| Silt | (g kg−1) | 320 | - | - |
| Sand | (g kg−1) | 420 | - | - |
| Texture | loam | - | - | |
| Organic matter | (g kg−1) | 24.40 | 840 | 732 |
| EC (1:5) | (dS m‒1) | 0.90 | 1.80 | 0.75 |
| pH (1:2.5) | 5.50 | 10.50 | 10.00 | |
| Total Cd | (mg kg−1) | 2.50 | ND * | ND * |
| Total Pb | (mg kg−1) | 970 | 4.00 | ND * |
| Total Zn | (mg kg−1) | 1010 | 40.00 | 30.50 |
| Total Cu | (mg kg−1) | 36 | 15.50 | 3.88 |
| Biochar Type | Biochar Rate | OM | pH | EC |
|---|---|---|---|---|
| CK | 0 | 2.40 ± 0.05 d | 5.54 ± 0.10 d | 0.64 ± 0.10 b |
| PB | 2 | 3.58 ± 0.04 c | 5.75 ± 0.07 c | 0.34 ± 0.09 c |
| 4 | 4.31 ± 0.30 b | 6.21 ± 0.11 a | 0.38 ± 0.11 c | |
| 6 | 5.47 ± 0.04 a | 6.048 ± 0.7 ab | 0.39 ± 0.08 c | |
| BB | 2 | 3.51 ± 0.16 c | 5.58 ± 0.10 d | 0.65 ± 0.10 b |
| 4 | 4.28 ± 0.23 b | 5.92 ± 0.08 b | 0.68 ± 0.10 b | |
| 6 | 5.21 ± 0.16 a | 6.05 ± 0.10 b | 0.76 ± 0.08 a |
| Biochar Type | Biochar Rate | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 49.95 ± 0.63 a | 1.45 ± 0.20 a | 132.03 ± 1.77 a | 0.850 ± 0.08 a |
| PB | 2 | 42.06 ± 0.58 c | 1.26 ± 0.08 c | 113.51 ± 1.88 d | 0.698 ± 0.05 c |
| 4 | 40.01 ± 0.76 d | 1.23 ± 0.09 d | 107.63 ± 1.67 f | 0.546 ± 0.08 d | |
| 6 | 38.90 ± 0.35 d | 1.21 ± 0.10 e | 99.94 ± 1.26 g | 0.470 ± 0.07 e | |
| BB | 2 | 48.34 ± 0.44 b | 1.40 ± 0.08 b | 128.10 ± 1.23 b | 0.774 ± 0.08 b |
| 4 | 42.51 ± 0.44 c | 1.39 ± 0.11 b | 123.98 ± 1.78 c | 0.774 ± 0.03 b | |
| 6 | 39.73 ± 0.51 d | 1.24 ± 0.09 d | 112.00 ± 1.43 e | 0.749 ± 0.07 b |
| Biochar Type | Biochar Rate | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 137.46 ± 0.05 a | 1.85 ± 0.04 a | 168.01 ± 1.00 a | 2.05 ± 0.08 a |
| PB | 2 | 126.45 ± 0.034 b | 1.71 ± 0.04 b | 145.15 ± 3.00 c | 1.65 ± 0.7 b |
| 4 | 125.46 ± 0.05 c | 1.64 ± 0.03 bc | 144.92 ± 4.00 c | 1.53 ± 0.5 b | |
| 6 | 123.48 ± 1.0 d | 1.60 ± 0.02 c | 141.75 ± 1.00 c | 1.27 ± 0.05 c | |
| BB | 2 | 129.13 ± 0.58 e | 1.68 ± 0.05 b | 155.84 ± 1.00 b | 1.66 ± 0.08 b |
| 4 | 124.50 ± 0.07 f | 1.66 ± 0.06 bc | 153.59 ± 1.00 b | 1.66 ± 0.07 b | |
| 6 | 120.14 ± 0.55 g | 1.64 ± 0.04 bc | 151.79 ± 2.00 b | 1.41 ± 0.06 bc |
| Biochar Type | Biochar Rate | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 609.33 ± 16 a | 0.491 ± 0.05 ab | 85.16 ± 2.00 c | 6.71 ± 0.13 bc |
| PB | 2 | 549.45 ± 6 d | 0.487 ± 0.06 ab | 89.04 ± 1.00 b | 6.45 ± 0.35 cd |
| 4 | 592.63 ± 5 ab | 0.498 ± 0.03 a | 97.89 ± 2.00 a | 6.25 ± 0.05 d | |
| 6 | 557.78 ± 9 cd | 0.477 ± 0.02 ab | 97.76 ± 3.00 a | 5.85 ± 0.07 e | |
| BB | 2 | 576.00 ± 3 bc | 0.481 ± 0.04 ab | 90.77 ± 2.00 b | 7.14 ± 0.22 a |
| 4 | 572.67 ± 1 bc | 0.488 ± 0.05 ab | 94.60 ± 1.00 a | 7.04 ± 0.05 ab | |
| 6 | 526.18 ± 15 e | 0.463 ± 0.05 b | 90.25 ± 1.00 b | 7.05 ± 0.08 ab |
| Biochar Type | Biochar Rate | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 61.70 ± 2 c | 0.104 ± 0.02 d | 140.82 ± 1.00 f | 7.34 ± 0.08 d |
| PB | 2 | 91.33 ± 2 c | 0.147 ± 0.05 bc | 165.67 ± 2.00 e | 9.05 ± 0.22 c |
| 4 | 98.62 ± 2 a | 0.164 ± 0.06 a | 185.11 ± 3.00 c | 10.45 ± 0.38 b | |
| 6 | 99.96 ± 4 a | 0.168 ± 0.07 a | 189.35 ± 2.00 b | 11.80 ± 0.58 a | |
| BB | 2 | 98.96 ± 2 a | 0.144 ± 0.08 c | 172.41 ± 1.00 d | 8.49 ± 0.31 c |
| 4 | 99.49 ± 3 a | 0.147 ± 0.08 bc | 189.90 ± 1.00 b | 8.79 ± 0.22 c | |
| 6 | 99.63 ± 3 a | 0.155 ± 0.05 b | 200.01 ± 1.00 a | 8.93 ± 0.08 c |
| Biochar Type | Biochar Rate | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 195.95 ± 2.00 a | 0.127 ± 0.05 b | 658.00 ± 15 a | 16.89 ± 0.10 a |
| PB | 2 | 186.60 ± 2.00 bcd | 0.146 ± 0.06 a | 645.60 ± 7 a | 15.86 ± 0.11 a |
| 4 | 193.53 ± 3.00 ab | 0.146 ± 0.08 a | 667.15 ± 2 a | 15.79 ± 0.11 a | |
| 6 | 180.81 ± 5.00 d | 0.146 ± 0.07 a | 649.75 ± 2 a | 15.25 ± 1.57 a | |
| BB | 2 | 192.06 ± 4.00 abc | 0.145 ± 0.04 a | 629.78 ± 3 a | 15.92 ± 0.33 a |
| 4 | 185.10 ± 4.00 cd | 0.141 ± 0.06 a | 667.77 ± 10 a | 15.31 ± 0.81 a | |
| 6 | 173.99 ± 2.00 e | 0.140 ± 0.03 a | 664.73 ± 11 a | 15.63 ± 0.88 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.; Liu, Z.; Skalicky, M.; Dessoky, E.S.; Brestic, M.; Mbarki, S.; Rastogi, A.; EL Sabagh, A. Fractionation of Heavy Metals in Multi-Contaminated Soil Treated with Biochar Using the Sequential Extraction Procedure. Biomolecules 2021, 11, 448. https://doi.org/10.3390/biom11030448
Awad M, Liu Z, Skalicky M, Dessoky ES, Brestic M, Mbarki S, Rastogi A, EL Sabagh A. Fractionation of Heavy Metals in Multi-Contaminated Soil Treated with Biochar Using the Sequential Extraction Procedure. Biomolecules. 2021; 11(3):448. https://doi.org/10.3390/biom11030448
Chicago/Turabian StyleAwad, Mahrous, Zhongzhen Liu, Milan Skalicky, Eldessoky S. Dessoky, Marian Brestic, Sonia Mbarki, Anshu Rastogi, and Ayman EL Sabagh. 2021. "Fractionation of Heavy Metals in Multi-Contaminated Soil Treated with Biochar Using the Sequential Extraction Procedure" Biomolecules 11, no. 3: 448. https://doi.org/10.3390/biom11030448








