Green Extraction by Ultrasound, Microencapsulation by Spray Drying and Antioxidant Activity of the Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Encapsulating Agents
2.2. Sample Preparation
2.3. Extraction of Carotenoids from the Coproduct
2.4. Microparticle Preparation (MP)
2.5. Encapsulation Efficiency (EE)
2.6. Quantification of Total Carotenoids by Spectrophotometry
2.7. ABTS Assay
2.8. β-Carotene/Linoleic Acid System Assay
2.9. Physicochemical Characterization of the Microparticle
2.9.1. Infrared Analysis (FTIR)
2.9.2. Thermogravimetry Analysis (TG)
2.9.3. Differential Scanning Calorimetry (DSC)
2.9.4. Water Activity
2.9.5. Particle Morphology and Particle Size
2.10. Statistical Analysis
3. Results and Discussion
3.1. Quantification of Total Carotenoids, Encapsulation Efficiency and Antioxidant Activity
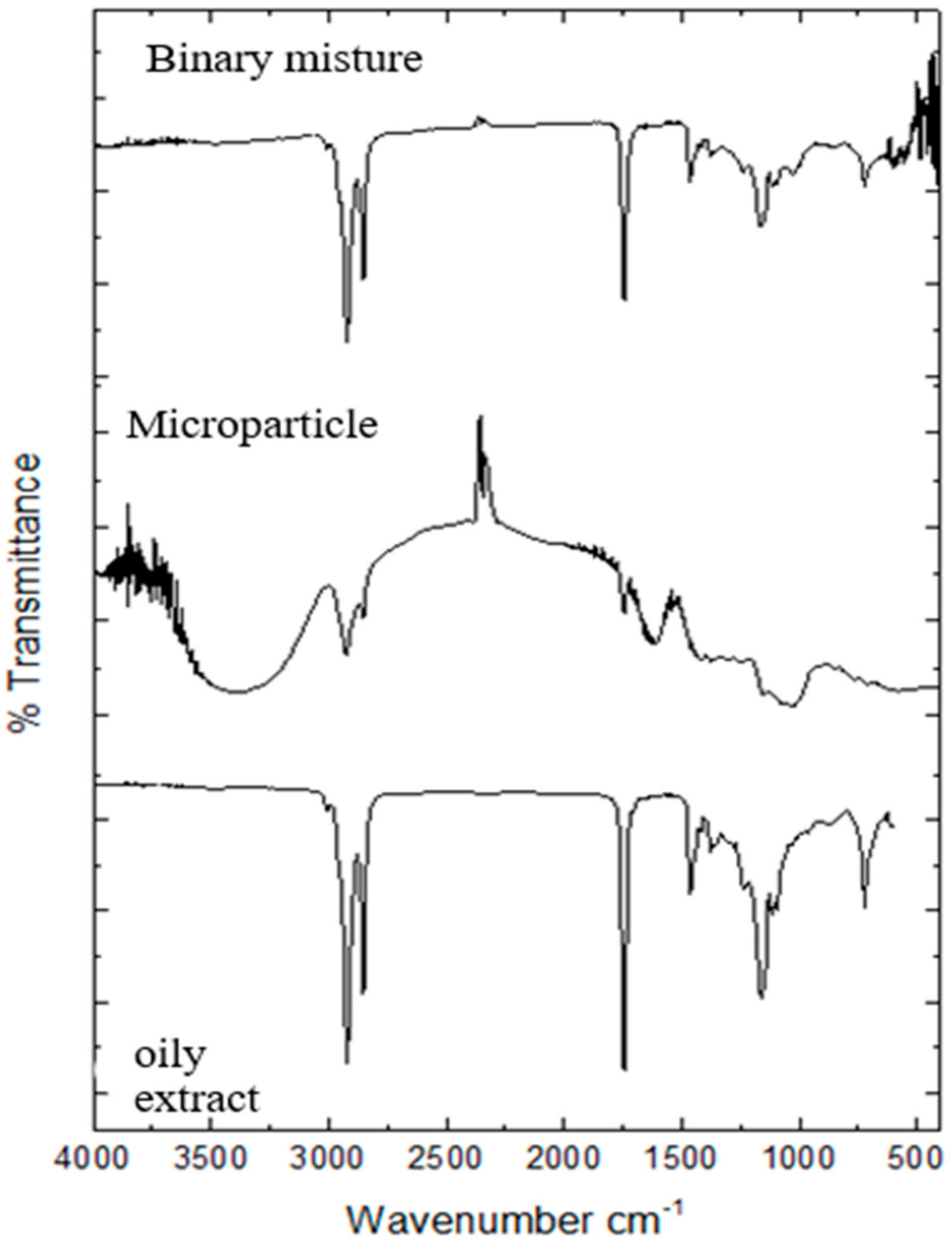
3.2. Infrared Analysis
3.3. Thermogravimetry Analysis (TG/DTG)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Moisture Content and Water Activity
3.6. Particle Morphology and Particle Size
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Maia, G.C.H.M.; Da Silva Campos, M.; Barros-Monteiro, J.; Castillo, J.E.L.; Faleiros, M.S.; De Aquino Sales, R.S.; Galeno, D.M.L.; Lira, E.; Das Chagas Do Amaral Souza, F.; Ortiz, C.; et al. Effects of Astrocaryum aculeatum Meyer (Tucumã) on Diet-Induced Dyslipidemic Rats. J. Nutr. Metab. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Pardauil, J.J.R.; de Molfetta, F.A.; Braga, M.; de Souza, L.K.C.; Filho, G.N.R.; Zamian, J.R.; da Costa, C.E.F. Characterization, thermal properties and phase transitions of amazonian vegetable oils. J. Therm. Anal. Calorim. 2017, 127, 1221–1229. [Google Scholar] [CrossRef]
- Gomes, A.T.A.; Pereira, R.R.; Duarte Junior, A.P.; da Cruz Rodrigues, A.M.; Remédios, C.M.R.; Brasil, D.d.S.B.; Morais, L.R.B.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Tucumã (Astrocaryum vulgare) fat: An Amazonian material as a pharmaceutical input for lipid nanoparticle production. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Gabbay Alves, T.V.; Silva da Costa, R.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Pinheiro Arruda, M.S.; Carréra Silva Júnior, J.O.; Converti, A.; Ribeiro Costa, R.M. Bioactive compounds and antioxidant potential for polyphenol-rich cocoa extract obtained by agroindustrial residue. Nat. Prod. Res. 2017, 6419, 1–4. [Google Scholar] [CrossRef]
- Gabbay Alves, T.V.; Silva da Costa, R.; Aguiar Gomes, A.T.; Ferreira da Costa, C.E.; Perego, P.; Carréra Silva Júnior, J.O.; Converti, A.; Ribeiro Costa, R.M. Quality control of Amazonian cocoa (Theobroma cacao L.) by-products and microencapsulated extract by thermal analysis. J. Therm. Anal. Calorim. 2018, 134, 993–1000. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef]
- da Costa, R.S.; Dos Santos, O.V.; da Lannes, S.C.S.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Ribeiro-Costa, R.M.; Converti, A.; Silva Júnior, J.O.C. Bioactive compounds and value-added applications of cupuassu (Theobroma grandiflorum schum.) agroindustrial by-product. Food Sci. Technol. 2020, 40, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Noronha Matos, K.A.; Praia Lima, D.; Pereira Barbosa, A.P.; Zerlotti Mercadante, A.; Campos Chisté, R. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- de Souza Filho, O.C.; Sagrillo, M.R.; Garcia, L.F.M.; Machado, A.K.; Cadoná, F.; Ribeiro, E.E.; Duarte, M.M.M.F.; Morel, A.F.; da Cruz, I.B.M. The In Vitro Genotoxic Effect of Tucuma (Astrocaryum aculeatum), an Amazonian Fruit Rich in Carotenoids. J. Med. Food 2013, 16, 1013–1021. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Cossetin, L.F.; Sagrillo, M.R.; Nascimento, K.; da Silva, A.S.; Machado, A.K.; da Cruz, I.B.M.; Stefani, L.M.; et al. Antihyperglycemic, antioxidant activities of tucumã oil (Astrocaryum vulgare) in alloxan-induced diabetic mice, and identification of fatty acid profile by gas chromatograph: New natural source to treat hyperglycemia. Chem. Biol. Interact. 2017, 270, 51–58. [Google Scholar] [CrossRef]
- Veronezi, C.M.; Jorge, N. Carotenoides Em Abóboras. Bol. Cent. Pesqui. Process. Aliment. 2011, 29, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Razi Parjikolaei, B.; Bahij El-Houri, R.; Fretté, X.C.; Christensen, K.V. Influence of green solvent extraction on carotenoid yield from shrimp (Pandalus borealis) processing waste. J. Food Eng. 2015, 155, 22–28. [Google Scholar] [CrossRef]
- Pu, J.; Bechtel, P.J.; Sathivel, S. Extraction of shrimp astaxanthin with flaxseed oil: Effects on lipid oxidation and astaxanthin degradation rates. Biosyst. Eng. 2010, 107, 364–371. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Mahendrakar, N.S. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresour. Technol. 2005, 96, 1195–1200. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Ginies, C.; Chemat, F. Direct green extraction of volatile aroma compounds using vegetable oils as solvents: Theoretical and experimental solubility study. LWT Food Sci. Technol. 2014, 59, 724–731. [Google Scholar] [CrossRef]
- Buriol, L.; Finger, D.; Schmidt, E.M.; Dos Santos, J.M.T.; Da Rosa, M.R.; Quináia, S.P.; Torres, Y.R.; Santa, H.S.D.; Pessoa, C.; De Moraes, M.O.; et al. Composição química e atividade biológica de extrato oleoso de própolis: Uma alternativa ao extrato etanólico. Quim. Nova 2009, 32, 296–302. [Google Scholar] [CrossRef]
- Handayani, A.D.; Indraswati, N.; Ismadji, S. Extraction of astaxanthin from giant tiger (Panaeus monodon) shrimp waste using palm oil: Studies of extraction kinetics and thermodynamic. Bioresour. Technol. 2008, 99, 4414–4419. [Google Scholar] [CrossRef]
- Achat, S.; Tomao, V.; Madani, K.; Chibane, M.; Elmaataoui, M.; Dangles, O.; Chemat, F. Direct enrichment of olive oil in oleuropein by ultrasound-assisted maceration at laboratory and pilot plant scale. Ultrason. Sonochem. 2012, 19, 777–786. [Google Scholar] [CrossRef]
- Dobiáš, P.; Pavlíková, P.; Adam, M.; Eisner, A.; Beňová, B.; Ventura, K. Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. Cent. Eur. J. Chem. 2010, 8, 87–95. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Liang, Z.; Wang, W. Investigation on ultrasound-assisted extraction of salvianolic acid B from Salvia miltiorrhiza root. Ultrason. Sonochem. 2010, 17, 61–65. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Knorr, D.; Ade-Omowaye, B.I.O.; Heinz, V. Nutritional improvement of plant foods by non-thermal processing. Proc. Nutr. Soc. 2002, 61, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Mason, T.J.; Chemat, F.; Vinatoru, M. The Extraction of Natural Products using Ultrasound or Microwaves. Curr. Org. Chem. 2010, 15, 237–247. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Condón, S.; Álvarez, I.; Raso, J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep. Purif. Technol. 2014, 136, 130–136. [Google Scholar] [CrossRef]
- Eh, A.L.S.; Teoh, S.G. Novel modified ultrasonication technique for the extraction of lycopene from tomatoes. Ultrason. Sonochem. 2012, 19, 151–159. [Google Scholar] [CrossRef]
- Almahy, H.A.; Ali, M.A.; Band-Ali, A.A. Extraction of carotenoids as natural dyes from the daucus carota linn (carrot) using ultrasound in Kingdom of Saudi Arabia. Res. J. Chem. Sci. 2013, 3, 63–66. [Google Scholar]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound assisted extraction of lycopene from tomato processing wastes. J. Food Sci. Technol. 2014, 51, 4102–4107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Çam, M.; Içyer, N.C.; Erdoǧan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Murugesan, R.; Orsat, V. Spray Drying for the Production of Nutraceutical Ingredients-A Review. Food Bioprocess Technol. 2012, 5, 3–14. [Google Scholar] [CrossRef]
- Vasconcelos, E.A.F.; Medeiros, M.G.F.; Raffin, F.N.; Moura, T.F.A.L. Influência da temperatura de secagem e da concentração de Aerosil®200 nas características dos extratos secos por aspersão da Schinus terebinthifolius Raddi (Anacardiaceae). Rev. Bras. Farmacogn. 2005, 15, 243–249. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Pinzón-Zarate, L.X.; González-Salcedo, L.O. Optimization of ultrasonic-assisted extraction of total carotenoids from peach palm fruit (Bactris gasipaes) by-products with sunflower oil using response surface methodology. Ultrason. Sonochem. 2015, 27, 560–566. [Google Scholar] [CrossRef]
- De Cássia Almeida Sampaio, R.; Da Costa, R.S.; De Souza, C.R.F.; Duarte Júnior, A.P.; Ribeiro-Costa, R.M.; Da Costa, C.E.F.; De Oliveira, W.P.; Converti, A.; Silva Júnior, J.O.C. Thermal characterization of Arrabidaea chica (Humb. & Bonpl.) B. Verl. dry extracts obtained by spray dryer. J. Therm. Anal. Calorim. 2016, 123, 2469–2475. [Google Scholar] [CrossRef]
- Norulfairuz, D.; Zaidel, A.; Aqilah, N.; Mohd, Y.M. Efficiency and Thermal Stability of Encapsulated Anthocyanins from Red Dragon Fruit (Hylocereus polyrhizus (Weber) Britton & Rose) using Microwave-assisted Technique. Chem. Eng. Trans. 2015, 43, 127–132. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Ogawa, M.; Maia, E.L.; Fernandes, A.C.; Nunes, M.L.; de Oliveira, M.E.B.; Freitas, S.T. Resíduos do beneficiamento do camarão cultivado: Obtenção de pigmentos carotenóides. Ciência e Tecnol. Aliment. 2007, 27, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggenete, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Trolox Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Dos Santos, R.J.; Genovese, M.I.; Lajolo, F.M. Avaliação da atividade antioxidante utilizando sistema β-caroteno/ácido linoléico e método de seqüestro de radicais DPPH•. Cienc. e Tecnol. Aliment. 2006, 26, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, R.S.; Negrão, C.A.B.; Camelo, S.R.P.; Ribeiro-Costa, R.M.; Barbosa, W.L.R.; Da Costa, C.E.F.; Silva Júnior, J.O.C. Investigation of thermal behavior of Heliotropium indicum L. lyophilized extract by TG and DSC. J. Therm. Anal. Calorim. 2013, 111, 1959–1964. [Google Scholar] [CrossRef]
- da Saúde, M.; Agência Nacional de Vigilância Sanitária (ANVISA). Farmacopeia Brasileira, 5th ed.; Companhia Editora Nacional: São Paulo, Brazil, 2010; Volume 1.
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Gabbay Alves, T.V.; Silva da Costa, R.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Carréra Silva Júnior, J.O.; Ribeiro Costa, R.M.; Converti, A. Microencapsulation of Theobroma cacao L. waste extract: Optimization using response surface methodology. J. Microencapsul. 2017, 34, 111–120. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; Longo, E.; Mattoso, L.H.C. Investigação do processo de absorção de água de hidrogéis de polissacarídeo: Efeito da carga iônica, presença de sais, concentrações de monômero e polissacarídeo. Polímeros 2012, 22, 311–317. [Google Scholar] [CrossRef] [Green Version]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Vieira, M.; Silva, D.A. Disponibilidade de carotenoides nos domicílios brasileiros. Availab. Carotenoids Braz. Househ. 2012, 227–244. [Google Scholar] [CrossRef]
- Rao, A.V.; Shen, H. Effect of low dose lycopene intake on lycopene bioavailability and oxidative stress. Nutr. Res. 2002, 22, 1125–1131. [Google Scholar] [CrossRef]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Lagazzo, A.; Botter, R.; Perego, P. Microencapsulation of phenolic compounds from olive pomace using spray drying: A study of operative parameters. LWT Food Sci. Technol. 2015, 62, 177–186. [Google Scholar] [CrossRef]
- Caliskan, G.; Nur Dirim, S. The effects of the different drying conditions and the amounts of maltodextrin addition during spray drying of sumac extract. Food Bioprod. Process. 2013, 91, 539–548. [Google Scholar] [CrossRef]
- Gaur, P.K.; Mishra, S.; Bajpai, M. Formulation and evaluation of controlled-release of telmisartan microspheres: In vitro/in vivo study. J. Food Drug Anal. 2014, 22, 542–548. [Google Scholar] [CrossRef] [Green Version]
- do Carmo, E.L.; Fernandes, R.V.d.B.; Borges, S.V. Microencapsulação por spray drying, novos biopolímeros e aplicações na tecnologia de alimentos. J. Eng. Exact Sci. 2018, 1, 30–44. [Google Scholar] [CrossRef]
- Álvarez-Henao, M.V.; Saavedra, N.; Medina, S.; Jiménez Cartagena, C.; Alzate, L.M.; Londoño-Londoño, J. Microencapsulation of lutein by spray-drying: Characterization and stability analyses to promote its use as a functional ingredient. Food Chem. 2018, 256, 181–187. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Lopera, S.M.C.; Guzmán, C.O.; Cataño, C.R.; Gallardo, C.C. Desarrollo y caracterización de micropartículas de ácido fólico formadas por secado por aspersión, utilizando goma arábiga y maltodextrina como materiales de pared. Vitae 2009, 16, 55–65. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Truscott, G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: Potential effects for human health. Mol. Nutr. Food Res. 2012, 56, 205–216. [Google Scholar] [CrossRef]
- Kljak, K.; Grbeša, D. Carotenoid content and antioxidant activity of hexane extracts from selected Croatian corn hybrids. Food Chem. 2015, 167, 402–408. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Pinto, A.C.; Veiga, V.F., Jr.; Grynberg, N.F.; Echevarria, A. Plantas medicinais: A necessidade de estudos multidisciplinares. Quim. Nova 2002, 25, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Silva-Júnior, A.A.; Scarpa, M.V.; Pestana, K.C.; Mercuri, L.P.; de Matos, J.R.; de Oliveira, A.G. Thermal analysis of biodegradable microparticles containing ciprofloxacin hydrochloride obtained by spray drying technique. Thermochim. Acta 2007, 467, 91–98. [Google Scholar] [CrossRef]
- Spricigo, R.; Botelho, K.C.A.; Consiglieri, V.O.; Serra, C.H.R. Obtenção avaliação de complexos de inclusão de furosemida com β-ciclodextrina e hidroxipropil-β-ciclodextrina: Efeitos sobre as propriedades de dissolução do fármaco. Lat. Am. J. Pharm. 2008, 27, 645–653. [Google Scholar]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of spray drying microencapsulation of almond oil into taro starch spherical aggregates. LWT 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Pereira, R.R.; Testi, M.; Rossi, F.; Silva Junior, J.O.C.; Ribeiro-Costa, R.M.; Bettini, R.; Santi, P.; Padula, C.; Sonvico, F. Ucuùba (Virola surinamensis) fat-based nanostructured lipid carriers for nail drug delivery of ketoconazole: Development and optimization using box-behnken design. Pharmaceutics 2019, 11, 284. [Google Scholar] [CrossRef] [Green Version]
- Peighambardoust, S.H.; Golshan Tafti, A.; Hesari, J. Application of spray drying for preservation of lactic acid starter cultures: A review. Trends Food Sci. Technol. 2011, 22, 215–224. [Google Scholar] [CrossRef]
- Mestry, A.P.; Mujumdar, A.S.; Thorat, B.N. Optimization of spray drying of an innovative functional food: Fermented mixed juice of carrot and watermelon. Dry Technol. 2011, 29, 1121–1131. [Google Scholar] [CrossRef]
- Sandulachi, E. Water Activity Concept and Its Role in Food Preservation. Meridian Ingineresc. 2012, 4, 40–48. [Google Scholar]
- Gamboa-Santos, J.; Soria, A.C.; Villamiel, M.; Montilla, A. Quality parameters in convective dehydrated carrots blanched by ultrasound and conventional treatment. Food Chem. 2013, 141, 616–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, O.W.; Petrovick, P.R. Secagem por aspersão (spray drying) de extratos vegetais: Bases e aplicações. Braz. J. Pharmacogn. 2010, 20, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Barros, S.L.; Santos, N.C.; Almeida, R.L.J.; Silva, S.N.; Nascimento, A.P.S.; Almeida, R.D.; Ribeiro, V.H.D.A.; Silva, W.P.; Gomes, J.P.; Silva, V.M.D.A.; et al. Influence of Pulp, Sugar and Maltodextrin Addiction in the Formulation of Kiwi Jellies With Lemon Grass Tea. J. Agric. Sci. 2019, 11, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Simon-Brown, K.; Solval, K.M.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Dzandu, B.; Kyereh, E.; Goldson Barnaby, A.; Thompson, I.; et al. Microencapsulation of ginger (Zingiber officinale) extract by spray drying technology. LWT Food Sci. Technol. 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Botrel, D.A.; de Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of wall matrix systems on the properties of spray-dried microparticles containing fish oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156. [Google Scholar] [CrossRef]



| Sample | Total Carotenoids (mg/g) | Encapsulation Efficiency (%) | ABTS+ (µM trolox) | β-Carotene/ Linoleic Acid (%) |
|---|---|---|---|---|
| OE | 3.305 ± 0.01 a | 584.75 ± 0.00 a | 49.9 ± 1.8 a | |
| MP | 2.559 ± 0.01 b | 77.42 ± 5.44 | 537.12 ± 0.01 b | 43.3 ± 2.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.M.d.M.C.; Pereira, R.R.; Carvalho, F.B.d.; Silva Santos, A.; Ribeiro-Costa, R.M.; Carréra Silva Júnior, J.O. Green Extraction by Ultrasound, Microencapsulation by Spray Drying and Antioxidant Activity of the Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Biomolecules 2021, 11, 545. https://doi.org/10.3390/biom11040545
Ferreira LMdMC, Pereira RR, Carvalho FBd, Silva Santos A, Ribeiro-Costa RM, Carréra Silva Júnior JO. Green Extraction by Ultrasound, Microencapsulation by Spray Drying and Antioxidant Activity of the Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Biomolecules. 2021; 11(4):545. https://doi.org/10.3390/biom11040545
Chicago/Turabian StyleFerreira, Lindalva Maria de Meneses Costa, Rayanne Rocha Pereira, Fernanda Brito de Carvalho, Alberdan Silva Santos, Roseane Maria Ribeiro-Costa, and José Otávio Carréra Silva Júnior. 2021. "Green Extraction by Ultrasound, Microencapsulation by Spray Drying and Antioxidant Activity of the Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds" Biomolecules 11, no. 4: 545. https://doi.org/10.3390/biom11040545
APA StyleFerreira, L. M. d. M. C., Pereira, R. R., Carvalho, F. B. d., Silva Santos, A., Ribeiro-Costa, R. M., & Carréra Silva Júnior, J. O. (2021). Green Extraction by Ultrasound, Microencapsulation by Spray Drying and Antioxidant Activity of the Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Biomolecules, 11(4), 545. https://doi.org/10.3390/biom11040545