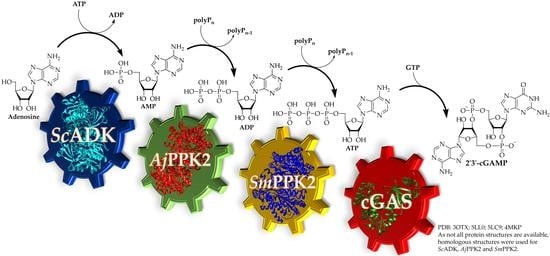
A Multi-Enzyme Cascade Reaction for the Production of 2′3′-cGAMP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids and Strains
2.2. Recombinant Enzyme Expression
2.3. Enzyme Purification
2.4. Enzyme Cascade Reactions
2.5. In Vitro Enzyme Assays
2.6. Quantification of 2′3′-cGAMP, Adenosine, AMP, ADP, ATP, and GTP
3. Results and Discussion
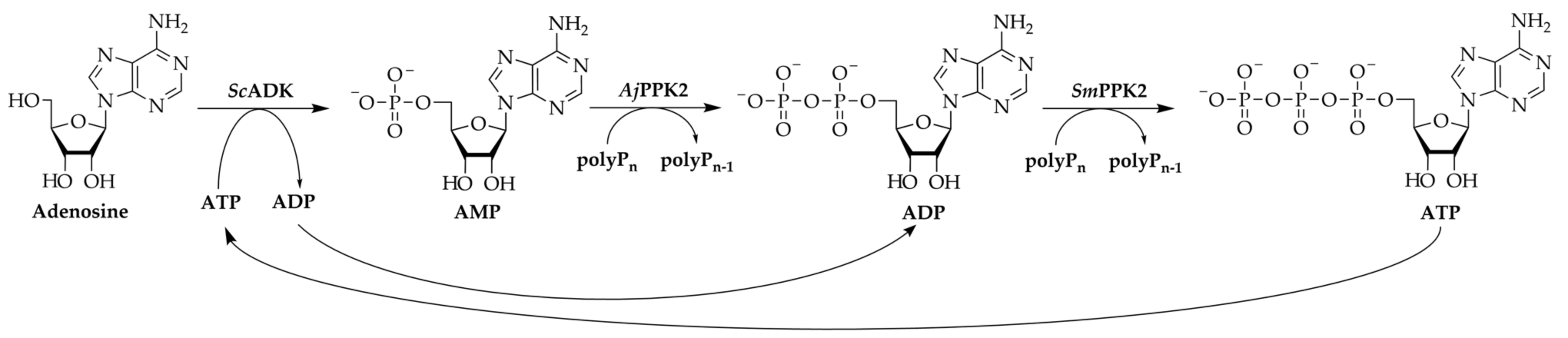
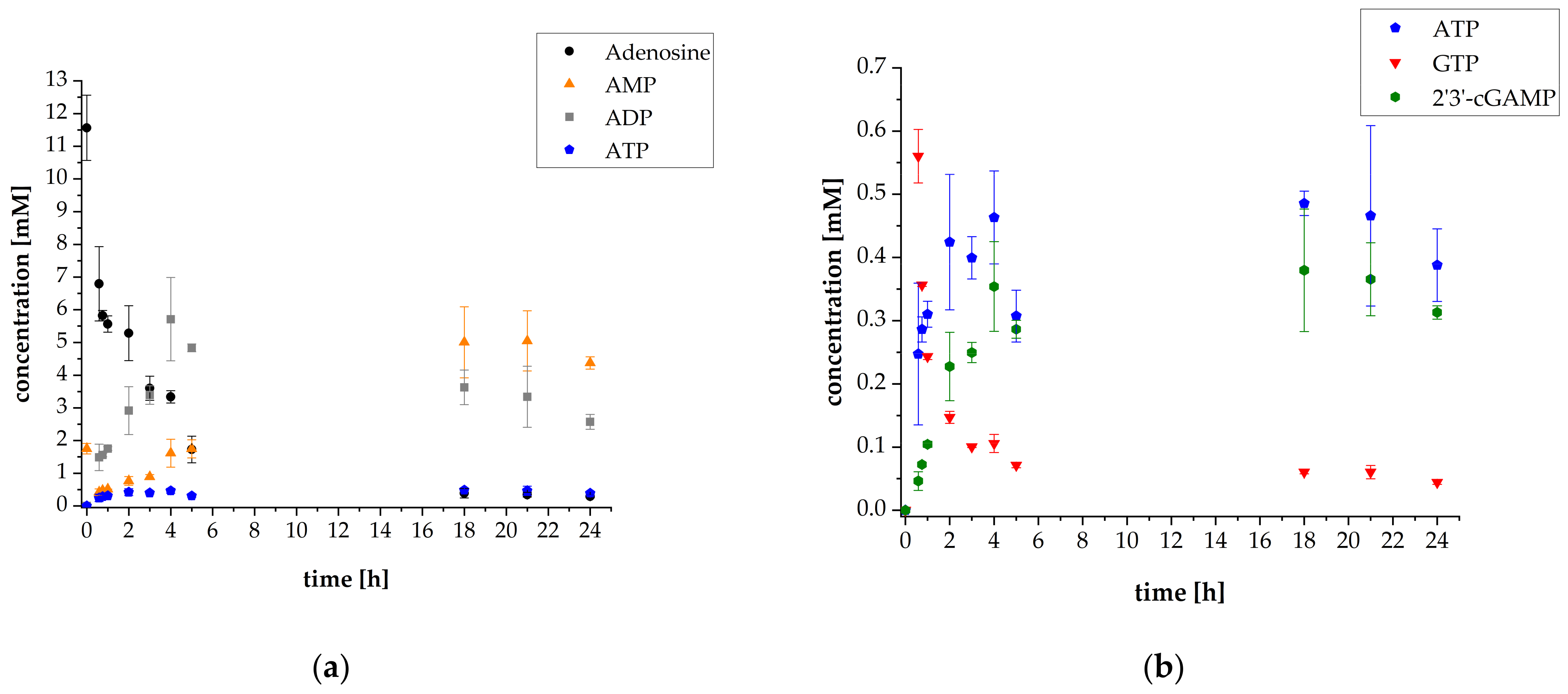
3.1. 2′3′-cGAMP Synthesis by a Multi-Enzyme Cascade Reaction
3.2. Specific Activities of the Kinases
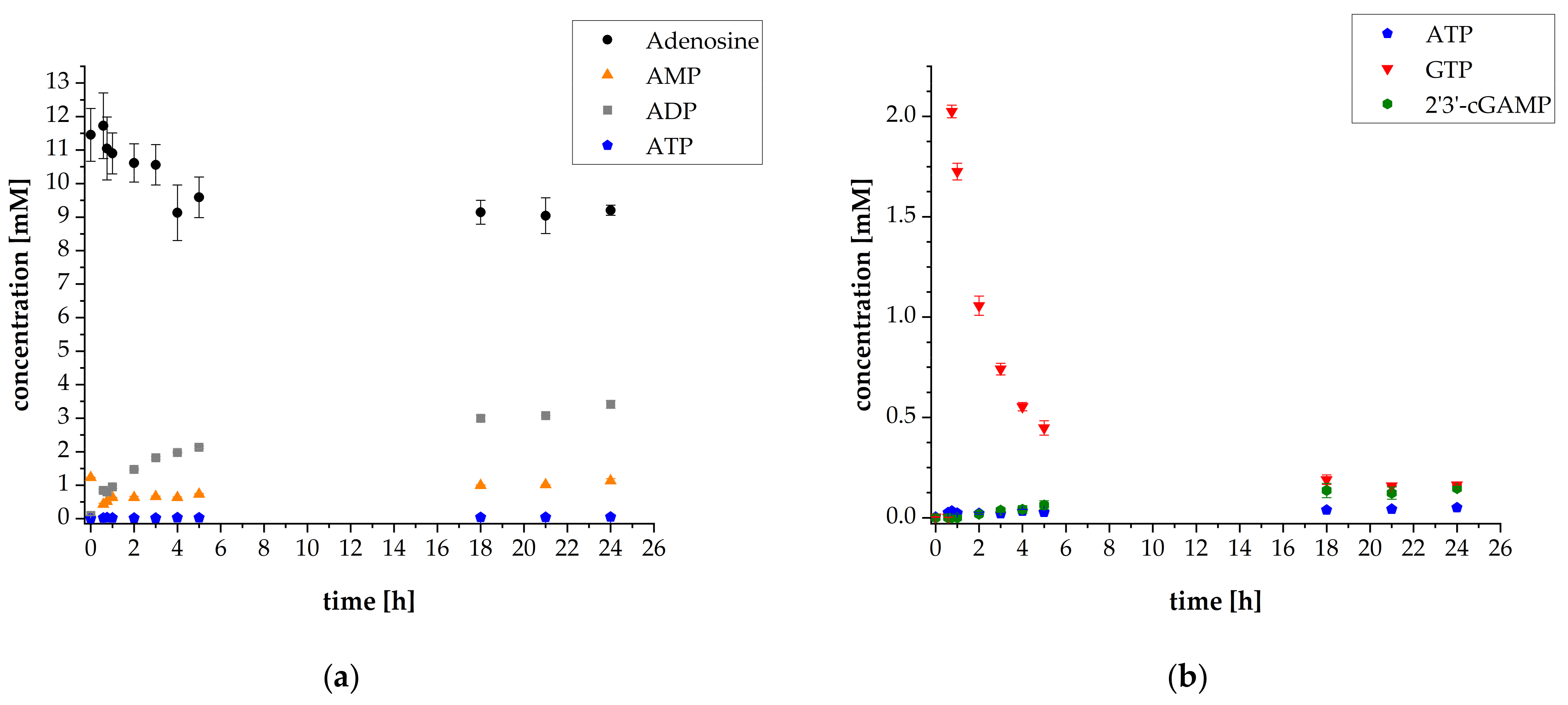
3.3. Adaptation of the Assay Composition in the Multi-Enzyme Cascade Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sperl, J.M.; Sieber, V. Multienzyme Cascade Reactions—Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Abu, R.; Woodley, J.M. Application of Enzyme Coupling Reactions to Shift Thermodynamically Limited Biocatalytic Reactions. ChemCatChem 2015, 7, 3094–3105. [Google Scholar] [CrossRef] [Green Version]
- Muschiol, J.; Peters, C.; Oberleitner, N.; Mihovilovic, M.D.; Bornscheuer, U.T.; Rudroff, F. Cascade Catalysis-Strategies and Challenges En Route to Preparative Synthetic Biology. Chem. Commun. 2015, 51, 5798–5811. [Google Scholar] [CrossRef]
- Dvorak, P.; Kurumbang, N.P.; Bendl, J.; Brezovsky, J.; Prokop, Z.; Damborsky, J. Maximizing the Efficiency of Multienzyme Process by Stoichiometry Optimization. ChemBioChem 2014, 15, 1891–1895. [Google Scholar] [CrossRef]
- Zhang, Y.; Hess, H. Toward Rational Design of High-Efficiency Enzyme Cascades. ACS Catal. 2017, 7, 6018–6027. [Google Scholar] [CrossRef] [Green Version]
- Sehl, T.; Hailes, H.C.; Ward, J.M.; Wardenga, R.; Von Lieres, E.; Offermann, H.; Westphal, R.; Pohl, M.; Rother, D. Two Steps in One Pot: Enzyme Cascade for the Synthesis of nor(Pseudo)Ephedrine from Inexpensive Starting Materials. Angew. Chem. Int. Ed. 2013, 52, 6772–6775. [Google Scholar] [CrossRef]
- Bornadel, A.; Hatti-Kaul, R.; Hollmann, F.; Kara, S. A Bi-Enzymatic Convergent Cascade for ε-Caprolactone Synthesis Employing 1,6-Hexanediol as a “Double-Smart Cosubstrate”. ChemCatChem 2015, 7, 2442–2445. [Google Scholar] [CrossRef]
- Citoler, J.; Derrington, S.R.; Galman, J.L.; Bevinakatti, H.; Turner, N.J. A Biocatalytic Cascade for the Conversion of Fatty Acids to Fatty Amines. Green Chem. 2019, 21, 4932–4935. [Google Scholar] [CrossRef]
- Mordhorst, S.; Andexer, J.N. Round, Round We Go-Strategies for Enzymatic Cofactor Regeneration. Nat. Prod. Rep. 2020, 37, 1316–1333. [Google Scholar] [CrossRef]
- Baughn, R.L.; Adalsteinsson, O.; Whitesides, G.M. Large-Scale Enzyme-Catalyzed Synthesis of ATP from Adenosine and Acetyl Phosphate: Regeneration of ATP from AMP. J. Am. Chem. Soc. 1978, 100, 304–306. [Google Scholar] [CrossRef]
- Bolte, J.; Whitesides, G.M. Enzymatic Synthesis of Arginine Phosphate with Coupled ATP Cofactor Regeneration. Bioorg. Chem. 1984, 12, 170–175. [Google Scholar] [CrossRef]
- Andexer, J.N.; Richter, M. Emerging Enzymes for ATP Regeneration in Biocatalytic Processes. ChemBioChem 2015, 16, 380–386. [Google Scholar] [CrossRef]
- Mordhorst, S.; Siegrist, J.; Müller, M.; Richter, M.; Andexer, J.N. Catalytic Alkylation Using a Cyclic S-Adenosylmethionine Regeneration System. Angew. Chem. Int. Ed. 2017, 56, 4037–4041. [Google Scholar] [CrossRef]
- Popadić, D.; Mhaindarkar, D.; Dang Thai, M.H.N.; Hailes, H.C.; Mordhorst, S.; Andexer, J.N. A Bicyclic S-Adenosylmethionine Regeneration System Applicable with Different Nucleosides or Nucleotides as Cofactor Building Blocks. RSC Chem. Biol. 2021. [Google Scholar] [CrossRef]
- Petchey, M.R.; Rowlinson, B.; Lloyd, R.C.; Fairlamb, I.J.S.; Grogan, G. Biocatalytic Synthesis of Moclobemide Using the Amide Bond Synthetase McbA Coupled with an ATP Recycling System. ACS Catal. 2020, 10, 4659–4663. [Google Scholar] [CrossRef]
- Fehlau, M.; Kaspar, F.; Hellendahl, K.F.; Schollmeyer, J.; Neubauer, P.; Wagner, A. Modular Enzymatic Cascade Synthesis of Nucleotides Using a (d)ATP Regeneration System. Front. Bioeng. Biotechnol. 2020, 8, 854. [Google Scholar] [CrossRef]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. cGAS Produces a 2′-5′-Linked Cyclic Dinucleotide Second Messenger That Activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [Green Version]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubensky, T.W.; Kanne, D.B.; Leong, M.L. Rationale, Progress and Development of Vaccines Utilizing STING-Activating Cyclic Dinucleotide Adjuvants. Ther. Adv. Vaccines 2013, 1, 131–143. [Google Scholar] [CrossRef]
- Novotná, B.; Vaneková, L.; Zavřel, M.; Buděšínský, M.; Dejmek, M.; Smola, M.; Gutten, O.; Tehrani, Z.A.; Pimková Polidarová, M.; Brázdová, A.; et al. Enzymatic Preparation of 2′-5′,3′-5′-Cyclic Dinucleotides, Their Binding Properties to Stimulator of Interferon Genes Adaptor Protein, and Structure/Activity Correlations. J. Med. Chem. 2019, 62, 10676–10690. [Google Scholar] [CrossRef]
- Rosenthal, K.; Becker, M.; Rolf, J.; Siedentop, R.; Hillen, M.; Nett, M.; Lütz, S. Catalytic Promiscuity of cGAS: A Facile Enzymatic Synthesis of 2′-3′-Linked Cyclic Dinucleotides. ChemBioChem 2020, 21, 3225–3228. [Google Scholar] [CrossRef]
- Rolf, J.; Siedentop, R.; Lütz, S.; Rosenthal, K. Screening and Identification of Novel cGAS Homologues Using a Combination of In Vitro and In Vivo Protein Synthesis. Int. J. Mol. Sci. 2020, 21, 105. [Google Scholar] [CrossRef] [Green Version]
- Vodnala, M.; Fijolek, A.; Rofougaran, R.; Mosimann, M.; Mäser, P.; Hofer, A. Adenosine Kinase Mediates High Affinity Adenosine Salvage in Trypanosoma brucei. J. Biol. Chem. 2008, 283, 5380–5388. [Google Scholar] [CrossRef] [Green Version]
- Kameda, A.; Shiba, T.; Kawazoe, Y.; Satoh, Y.; Ihara, Y.; Munekata, M.; Ishige, K.; Noguchi, T. A Novel ATP Regeneration System Using Polyphosphate-AMP Phosohotransferase and Polyphosphate Kinase. J. Biosci. Bioeng. 2001, 91, 557–563. [Google Scholar] [CrossRef]
- Lu, X.-B.; Wu, H.-Z.; Ye, J.; Fan, Y.; Zhang, H.-Z. Expression, Purification, and Characterization of Recombinant Saccharomyces cerevisiae Adenosine Kinase. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao 2003, 35, 666–670. [Google Scholar]
- Meng, L.; Liu, Y.; Yin, X.; Zhou, H.; Wu, J.; Wu, M.; Yang, L. Effects of His-Tag on Catalytic Activity and Enantioselectivity of Recombinant Transaminases. Appl. Biochem. Biotechnol. 2020, 190, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.M.; Wu, J.P.; Yang, L.R.; Xu, G. Soluble and Functional Expression of a Recombinant Enantioselective Amidase from Klebsiella Oxytoca KCTC 1686 in Escherichia coli and Its Biochemical Characterization. Process Biochem. 2015, 50, 1264–1271. [Google Scholar] [CrossRef]
- Resnick, S.M.; Zehnder, A.J.B. In Vitro ATP Regeneration from Polyphosphate and AMP by Polyphosphate:AMP Phosphotransferase and Adenylate Kinase from Acinetobacter johnsonii 210A. Appl. Environ. Microbiol. 2000, 66, 2045–2051. [Google Scholar] [CrossRef] [Green Version]
- Batten, L.E.; Parnell, A.E.; Wells, N.J.; Murch, A.L.; Oyston, P.C.F.; Roach, P.L. Biochemical and Structural Characterization of Polyphosphate Kinase 2 from the Intracellular Pathogen Francisella tularensis. Biosci. Rep. 2016, 36, e00294. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cui, X.; Li, Z. Characterization of Two Polyphosphate Kinase 2 Enzymes Used for ATP Synthesis. Appl. Biochem. Biotechnol. 2020, 191, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Ralph, E.C.; Shanker, S.; Wang, H.; Byrnes, L.J.; Horst, R.; Wong, J.; Brault, A.; Dumlao, D.; Smith, J.F.; et al. The Catalytic Mechanism of Cyclic GMP-AMP Synthase (cGAS) and Implications for Innate Immunity and Inhibition. Protein Sci. 2017, 26, 2367–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffney, B.L.; Veliath, E.; Zhao, J.; Jones, R.A. One-Flask Syntheses of c-di-GMP and the Rp,Rp and Rp,Sp Thiophosphate Analogues. Org. Lett. 2010, 12, 3269–3271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shi, H.; Wu, J.; Zhang, X.; Sun, L.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP Containing Mixed Phosphodiester Linkages Is An Endogenous High-Affinity Ligand for STING. Mol. Cell 2013, 51, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishige, K.; Zhang, H.; Kornberg, A. Polyphosphate Kinase (PPK2), a Potent, Polyphosphate-Driven Generator of GTP. Proc. Natl. Acad. Sci. USA 2002, 99, 16684–16688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motomura, K.; Hirota, R.; Okada, M.; Ikeda, T.; Ishida, T.; Kuroda, A. A New Subfamily of Polyphosphate Kinase 2 (Class III PPK2) Catalyzes Both Nucleoside Monophosphate Phosphorylation and Nucleoside Diphosphate Phosphorylation. Appl. Environ. Microbiol. 2014, 80, 2602–2608. [Google Scholar] [CrossRef] [Green Version]
- Nocek, B.; Kochinyan, S.; Proudfoot, M.; Brown, G.; Evdokimova, E.; Osipiuk, J.; Edwards, A.M.; Savchenko, A.; Joachimiaka, A.; Yakunin, A.F. Polyphosphate-Dependent Synthesis of ATP and ADP by the Family-2 Polyphosphate Kinases in Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 17730–17735. [Google Scholar] [CrossRef] [Green Version]
- Tavanti, M.; Hosford, J.; Lloyd, R.C.; Brown, M.J.B. ATP Regeneration by a Single Polyphosphate Kinase Powers Multigram-Scale Aldehyde Synthesisin Vitro. Green Chem. 2021, 23, 828–837. [Google Scholar] [CrossRef]
- Ma, S.K.; Gruber, J.; Davis, C.; Newman, L.; Gray, D.; Wang, A.; Grate, J.; Huisman, G.W.; Sheldon, R.A. A Green-by-Design Biocatalytic Process for Atorvastatin Intermediate. Green Chem. 2010, 12, 81–86. [Google Scholar] [CrossRef]
- Honda, K. Industrial Applications of Multistep Enzyme Reactions. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 433–450. [Google Scholar]


| Enzyme | Enzyme Concentration | Reaction Rate (μmol L−1 min−1) | Specific Activity (U mg−1) | |
|---|---|---|---|---|
| (mg L−1) | (nM) | |||
| ScADK | 5 | 130 | 21.7 ± 4.4 | 4.3 ± 0.9 |
| AjPPK2 | 0.5 | 9 | 34.6 ± 5.4 | 69.2 ± 10.8 |
| SmPPK2 | 5 | 140 | 13.8 ± 3.1 | 2.8 ± 0.6 |
| ScADK:AjPPK2: SmPPK2:thscGAS Ratio | 2′3′-cGAMP Reaction Rates (μmol L−1 min−1) | thscGAS Specific Activity (U mg−1) | (mol2′3′-cGAMP molAdo−1) | Enzyme Usage (mg2′3′-cGAMP mgenzyme−1) | |
|---|---|---|---|---|---|
| (mg:mg:mg:mg) | (µmol:µmol:µmol:µmol) | ||||
| 50:50:50:40 | 1.3:0.9:1.4:0.7 | 2.10 ± 0.60 | 0.052 ± 0.014 | 0.030 ± 0.001 | 1.12 ± 0.04 |
| 50:5:50:120 | 1.3:0.09:1.4:2.1 | 1.42 ± 0.50 | 0.012 ± 0.004 | 0.070 ± 0.004 | 1.87 ± 0.19 |
| 5:0.5:5:120 | 0.13:0.009:0.14:2.1 | 0.25 ± 0.14 | 0.002 ± 0.001 | 0.077 ± 0.030 | 0.75 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, M.; Nikel, P.; Andexer, J.N.; Lütz, S.; Rosenthal, K. A Multi-Enzyme Cascade Reaction for the Production of 2′3′-cGAMP. Biomolecules 2021, 11, 590. https://doi.org/10.3390/biom11040590
Becker M, Nikel P, Andexer JN, Lütz S, Rosenthal K. A Multi-Enzyme Cascade Reaction for the Production of 2′3′-cGAMP. Biomolecules. 2021; 11(4):590. https://doi.org/10.3390/biom11040590
Chicago/Turabian StyleBecker, Martin, Patrick Nikel, Jennifer N. Andexer, Stephan Lütz, and Katrin Rosenthal. 2021. "A Multi-Enzyme Cascade Reaction for the Production of 2′3′-cGAMP" Biomolecules 11, no. 4: 590. https://doi.org/10.3390/biom11040590
APA StyleBecker, M., Nikel, P., Andexer, J. N., Lütz, S., & Rosenthal, K. (2021). A Multi-Enzyme Cascade Reaction for the Production of 2′3′-cGAMP. Biomolecules, 11(4), 590. https://doi.org/10.3390/biom11040590