Podophyllotoxin: History, Recent Advances and Future Prospects
Abstract
:1. Introduction
2. Structural Characteristics of Podophyllotoxin
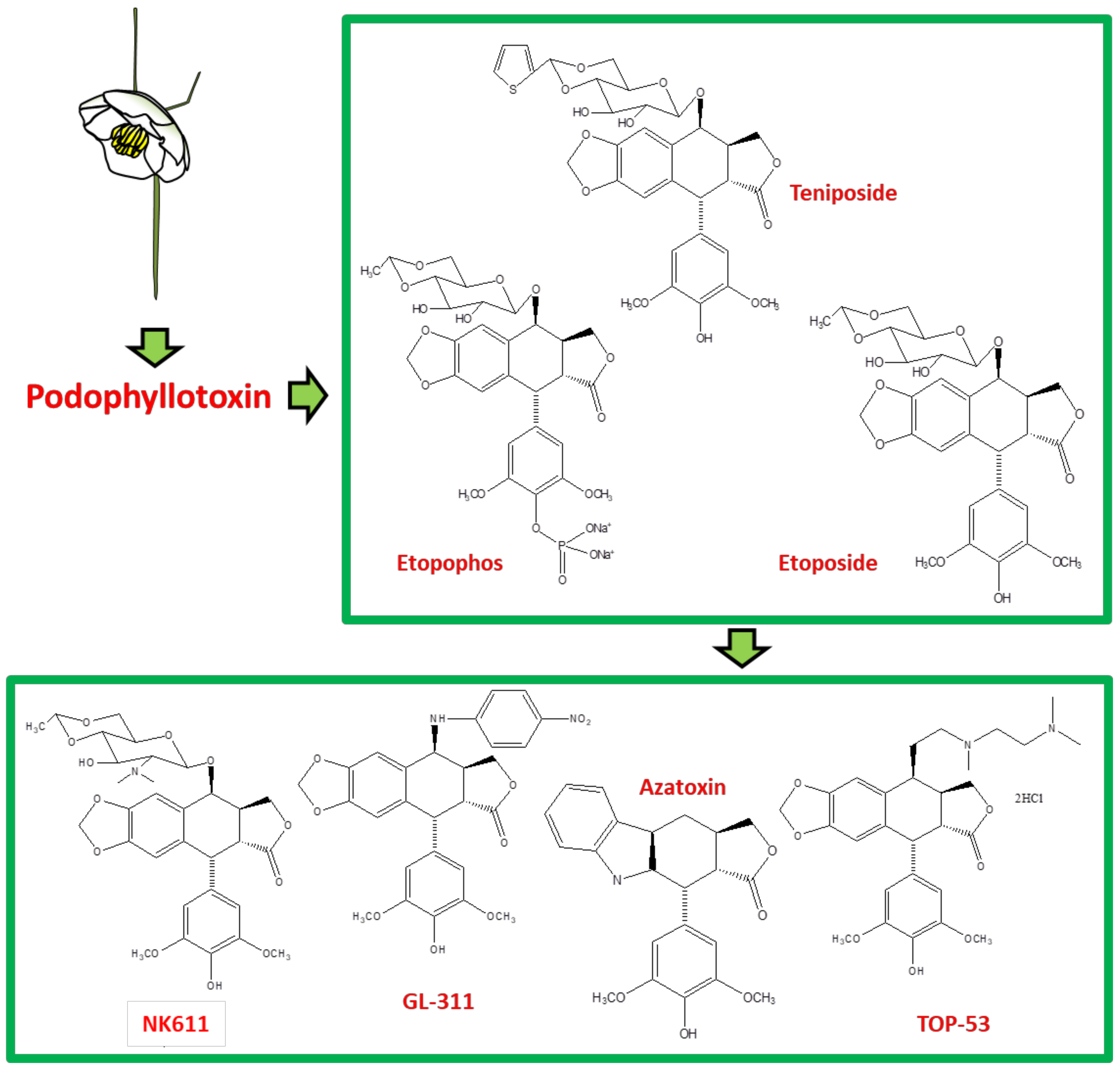
3. Derivatives, Analogues and Hybrids of Podophyllotoxin
4. Plant Sources of Podophyllotoxin
5. Biosynthesis of Plant Derived Podophyllotoxin
6. Microbial Sources of Podophyllotoxin
7. Parametric Analysis of Podophyllotoxin Biosynthesis
8. Pharmacological Significance of Podophyllotoxin and Its Derivatives
| Activity | Podophyllotoxin Derivative | Mechanism | Conclusion | Ref. |
|---|---|---|---|---|
| Cytotoxic activity | Cleistantoxin | Activity was checked against MCF-7, MCF-7R, KBand HT29 cancer cell lines | Cleistantoxin showed strong cytotoxic activity | [146] |
| Antibacterial activity | New precursors of podophyllotoxin were synthesized, and screened to check their antibacterial activity | Activity was checked against Klebsiella pneumoniae, Streptococcus faecalis, Citrobacter sp., Pseudomonas aeruginosa, Escherchia coli, Salmonella typhi and Shigella sonnei | Ethyl-2-(3′-methyl-4′-methoxybenzoyl)-3-(4″ methoxyphenol)-cyclopropane-1-carboxylic acid and Ethyl-2-(3′-methyl-4′-methoxybenzyol-3-1 3″, 4″-dimethoxyphenyl)-cyclopropane-carboxylic acid, both of them showed significant antibacterial activity | [147] |
| Antitumour activity | VP 16-213 (NSC-141540) | Activity was checked against L1210 ascites tumour in N/D mice | In 24 hours, divided treatment after every 3 hours, resulted in significant cure, hence, VP 16-213 is a cell cycle specific drug | [148] |
| Insecticidal activity | 20 podophyllotoxin analogues were tested | Activity was checked against fifth-instar larvae of Brontispa longissima in vivo | Among 20 analogues Deoxypodophyllotoxin showed more protential for insecticidal activity than a commercial insecticide (toosendanin) | [149] |
| Antineoplastic activity | New hybrids of podophyllotoxin and indirubin | Activity was checked against human leukemia cancer cells as a multifunctional anti-MDR agent | Podophyllotoxin-indirubin hybrid (Da-1) showed potential to overcome drug resistance. It is a novel hybrid havingpotent antiproliferative activity | [150] |
| Cyclolignans, derived from podophyllotoxin | Activity was checked againstA-549 human lung carcinoma, P-388 murine leukemia and HT-29 colon carcinoma | A number of substances were active in assay at concentrations below 1 pM; deoxypodophyllotoxin being the most potent compound in all cases | [151] |
9. Patents
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cane, C.; Moracs, R.M. Podophyllotoxin. Phytochemistry 2000, 54, 115–120. [Google Scholar] [CrossRef]
- Gordaliza, M.; Garcia, P.A.; Miguel del Corral, J.M. Podophyllotoxin: Distribution, sources, applications, and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, J.L.; Schrecker, A.W. Components of Podophyllin. V. The Constitution of Podophyllotoxin. J. Am. Chem. Soc. 1951, 73, 2909–2916. [Google Scholar] [CrossRef]
- Ayres, D.C.; Loike, J.D. Lignans, Chemical, Biological and Clinical Properties; Cambridge University Press: Cambridge, UK, 1990; Chapters 3 and 4. [Google Scholar]
- King, J. Discovery of Podophyllin. Coll. J. M. Sci. 1875, 2, 557–559. [Google Scholar]
- Liu, C.J.; Hou, S.S. Current Research Status of Podophyllotoxin Lignans. Nat. Prod. Res. Dev. 1997, 9, 81–89. [Google Scholar]
- Chen, Y.H. A Study of the Resources of Chinese Podophyllin Plants. Acta Pharm. Sin. 1979, 14, 101–107. [Google Scholar]
- Yang, X.Z.; Shao, H.; Zhang, L.Q.; Zhou, C.; Xuan, Q.; Yang, C.Y. Present Situation of Studies on Resources of Podophyllotoxin. Chin. Tradit. Herb. Drugs 2001, 32, 1042–1044. [Google Scholar]
- Liu, H.J.; Xu, Y.; Su, G.Q.; Li, C.Y.; Wang, L.; Liu, Y.J. Research Progress in Sinopodophyllum emodi. Chin. Tradit. Herb. Drugs 2004, 35, 98–100. [Google Scholar]
- Macrae, D.W.; Towers, N.G.H. Biological Activity of Lignans. Phytochemistry 1984, 23, 1207–1220. [Google Scholar] [CrossRef]
- Bohlin, L.; Rosen, B. Podophyllotoxin derivatives: Drug discovery and development. Drug Discov. Today 1996, 1, 343–351. [Google Scholar] [CrossRef]
- Cragg, G.M.; Kingston, D.G. Anticancer Agents from Natural Products, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 97. [Google Scholar]
- Podwyssotzki, V. The active constituents of podophyllotoxin. Pharm. J. Trans. 1881, 12, 217–218. [Google Scholar]
- Podwyssotzki, V. On the active constituents of podophyllin. Am. J. Pharm. 1882, 12, 102–115. [Google Scholar]
- Podwyssotzki, V. Pharmakologische Studien uber podophyllum peltatum. NaunynSchmied Arch. Exp. Path. Phar. 1884, 13, 29–52. [Google Scholar] [CrossRef] [Green Version]
- Species Plantrum; Salvius: Stockholm, Sweden, 1753; p. 505.
- Yu, X.; Che, Z.P.; Xu, H. Recent Advances in the Chemistry and Biology of Podophyllotoxins. Chem. Eur. J. 2016, 10, 1002–1006. [Google Scholar] [CrossRef]
- Stoll, A.; Renz, J.; Wartburg, A.V. The isolation of podophyllotoxin glucoside. J. Am. Chem. Soc. 1954, 76, 3103–3104. [Google Scholar] [CrossRef]
- Stoll, A.; von Wartburg, A.; Angliker, E.; Renz, J. The isolation of 4′- Demethylpodophyllotoxin glucoside from rhizomes podophyllum emodi wall. J. Am. Chem. Soc. 1954, 76, 5004–5005. [Google Scholar] [CrossRef]
- Emmenggger, H.; Stahelin, H.; Rutschmann, J.; von Wartburg, A. Chemistry and Pharmacology of podophyllum glucosides and derivatives. I. Arzneim. 1961, 11, 327–333. [Google Scholar]
- Kuhn, M.; von Wartburg, A. Mitosis-inhibiting substances. XXI. Synthesis of epipodophyllotoxin b-D-glucopyranoside. Helv. Chim. Acta 1968, 51, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; von Wartburg, A. Mitosis-inhibiting substances. glycosidation process.II. Glycosides of 4″-demethylepipodo-phyllotoxin. Helv. Chim. Acta 1969, 52, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Keller-Juslen, C.; Kuhn, M.; Von Wartburg, A.; Staehelin, H. Synthesis and antimitotic activity of glycosidic lignan derivatives related to podophyllotoxin. J. Med. Chem. 1971, 14, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Joel, S. The clinical pharmacology of etoposide. An update. Cancer Treat. Rev. 1996, 22, 179–221. [Google Scholar] [CrossRef]
- Stahelin, H.F.; von Wartburg, A. The Chemical and Biological Route from Podophyllotoxin Glucoside to Etoposide: Ninth Cain Memorial Award Lecture. Cancer Res. 1991, 51, 5–15. [Google Scholar]
- Ardalani, H.; Avan, A.; Ghayour-Mobarhan, M. Podophyllotoxin: A novel potential natural anticancer agent. Avicenna J. Phytomed. 2017, 7, 285–294. [Google Scholar]
- Mross, K.; Huttmann, A.; Herbst, K.; Hanauske, A.R.; Schilling, T.; Manegold, C.; Burk, K.; Hossfeld, D.K. Pharmacokinetics and pharmacodynamics of the new podophyllotoxin derivative NK 611 A study by the AIO groups PHASE-I and APOH. Cancer Chemother. Pharmacol. 1996, 38, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Lee, C.C.; Chao, Y.; Shu, C.H.; Chen, L.T.; Chen, L.L.; Chen, M.H.; Yuan, C.C.; Whang-Peng, J. A novel podophyllotoxin-derived compound GL331 is more potent than its congener VP-16 in killing refractory cancer cells. Pharm. Res. 1999, 16, 997–1002. [Google Scholar] [CrossRef]
- Lee, C.C.; Huang, T.S. A novel topoisomerase GL331 preferentially induces DNA cleavage at (C/G)T sites and can cause telomere DNA damage. Pharm. Res. 2001, 18, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Solary, E.; Leteurtre, F.; Paull, K.D.; Scudiero, D.; Hamel, E.; Pommier, Y. Dual inhibition of topoisomerase II and tubulin polymerization by azatoxin, a novel cytotoxic agent. Biochem. Pharm. 1993, 45, 2449–2456. [Google Scholar] [CrossRef]
- Arora, R. Medicinal Plant Biotechnology; CAB International: Wallingford, UK, 2010. [Google Scholar]
- You, Y. Podophyllotoxin derivatives: Current synthetic approaches for new anticancer agents. J. Curr. Pharm. Des. 2005, 11, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, L. Polyether-modified poly (acrylic acid): Synthesis and applications. Ind. Eng. Chem. Res. 1998, 37, 4267–4274. [Google Scholar] [CrossRef]
- Von-Schreier, E. Partial synthese der 6, 7-Dimethoxy-Analogen von Podophyllotoxin, Epi-, Neo-, und Desoxy-podophyllotoxin. Helv. Chim. Acta 1964, 47, 1529–1554. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Hu, H.; Chen, H.X.; Cheng, Y.C.; Lee, K.H. New 4 beta-substituted aniline derivatives of 6,7-O,O-demethylene-4’-O-demethylpodophyllotoxin and related compounds as potent inhibitors of human DNA topoisomerase II. J. Med. Chem. 1992, 35, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Kashiwada, Y.; Bastow, K.F.; Cheng, Y.C.; Lee, K.H. Podophenazine, 2″,3″-Dichloropo-dophenazine, Benzopodophenazine, and Their 4b-p-Nitroaniline Derivatives as Novel DNA Topoisomerase II Inhibitors. J. Med. Chem. 1996, 39, 1396–1402. [Google Scholar] [CrossRef]
- Cho, S.J.; Tropsha, A.; Suffness, M.; Cheng, Y.C.; Lee, K.H. Three-Dimensional Quantitative Structure-Activity Relationship Study of 4’-O-Demethyl-epipodophyllotoxin Analogs Using the Modified CoMFA/q2 -GRS Approach. J. Med. Chem. 1996, 39, 1383–1395. [Google Scholar] [CrossRef]
- Hartwell, J.L. α-Peltatin, a new compound isolated from podophyllum peltatum. J. Am. Chem. Soc. 1947, 69, 2918. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; von Wartburg, A.; Renz, J. The isolation of a-peltatin glucoside from the rhizomes of Podophyllum peltatum L. J. Am. Chem. Soc. 1955, 77, 1710–1711. [Google Scholar] [CrossRef]
- Nagar, N.; Jat, R.K.; Saharan, R.; Verma, S.; Sharma, D.; Bansal, K. Podophyllotoxin and their Glycosidic derivatives. Pharmacophore 2011, 2, 87–97. [Google Scholar]
- Bedows, E.; Hatfield, G.M. An Investigation of the Antiviral Activity of Podophyllum peltatum. J. Nat. Prod. 1982, 45, 725–729. [Google Scholar] [CrossRef]
- Thurston, L.S.; Irie, H.; Tani, S.; Han, F.S.; Liu, Z.C.; Cheng, Y.C.; Lee, K.H. Inhibition of human DNA topoisomerase II by podophyllotoxin and alpha-peltatin analogs. J. Med. Chem. 1986, 29, 1547–1550. [Google Scholar] [CrossRef]
- Thurston, L.S.; Imakura, Y.; Haruna, M.; Li, D.H.; Liu, Z.C.; Liu, S.Y.; Cheng, Y.C.; Lee, K.H. Inhibition of human DNA topoisomerase II by cytotoxic ether and ester derivatives of podophyllotoxin and alpha-peltatin. J. Med. Chem. 1989, 32, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Beers, S.A.; Imakura, Y.; Dai, H.J.; Li, D.H.; Cheng, Y.C.; Lee, K.H. Synthetic Ring C Aromatized Podophyllotoxin Analogues as Potential Inhibitors of Human DNA Topoisomerase II. J. Nat. Prod. 1988, 51, 901–905. [Google Scholar] [CrossRef]
- Canjanamurthy, K.; Lokanatharai, M. Synthesis of Podophyllotoxin & related analogues: Part II-Synthesis of ß-apopicrophyllin analogues with modified Hydroaromatic ring-B. Indian J. Chem. 1985, 24, 505–508. [Google Scholar]
- Canjanamurthy, K.; Lokanatharai, M. Synthesis of Podophyllotoxin & related analogues: Part III-Synthesis of ß-apopicrophyllin analogues with modified lactone ring. Indian J. Chem. 1987, 26, 131–135. [Google Scholar]
- He, Y.; Ma, W.Y.; Zhang, C.N. The effects of unsaturated factors on ring C to chemical Properties of podophyllotoxin analogues. J. Chin. Pharm. Sci. 2001, 10, 81–84. [Google Scholar]
- Kamal, A.; Reddy, S.T.; Polepalli, S.; Shalini, N.; Reddy, V.G.; Subba Rao, A.V.; Shankaraiah, N. Synthesis and biological evaluation of podophyllotoxin congeners as tubulin polymerization inhibitors. Bioorg. Med. Chem. 2014, 22, 5466–5475. [Google Scholar] [CrossRef]
- Guo, H.Z.; Guo, D.A.; Fei, X.Y.; Cui, Y.J.; Zheng, J.H. Biotransformation of podophyllotoxin to picropodophyllin by microbes. J. Asian Nat. Prod. Res. 1998, 1, 99–102. [Google Scholar] [CrossRef]
- Larsson, M.A. Cyclolignans as inhibitors of the insulin-like growth factor-I receptor. Cancer Res. 2007, 67, 2899. [Google Scholar] [CrossRef] [Green Version]
- Vasilcanu, R.; Vasilcanu, D.; Rosengren, L.; Natalishvili, N.; Sehat, B.; Yin, S.; Girnita, A.; Axelson, M.; Girnita, L.; Larsson, O. Picropodophyllin induces down regulation of the insulin-like growth factor 1 receptor: Potential mechanistic involvement of Mdm2 and beta-arrestin. Oncogene 2008, 27, 1629–1638. [Google Scholar] [CrossRef]
- Walter, J.; Gensler, C.D.; Murthy, M.H.T. Non-enolizable podophyllotoxin derivatives. J. Med. Chem. 1977, 20, 635–644. [Google Scholar]
- Glinski, M.B.; Freed, J.C.; Durst, T. Preparation of 2-substituted podophyllotoxin derivatives. J. Org. Chem. 1987, 52, 2749–2753. [Google Scholar] [CrossRef]
- Van-Vliet, D.S.; Lee, K.H. A high yield preparation of 2-fluoropodophyllotoxin. Tetrahedron. Lett. 1999, 40, 2259–2262. [Google Scholar] [CrossRef]
- Van-Vliet, D.S.; Tachibana, Y.; Bastow, K.F.; Huang, E.S.; Lee, K.H. Design, Synthesis, and Biological Testing of 4b-Anilino-2-fluoro-4’-demethylpodophyllo-toxin Analogues as Cytotoxic and Antiviral Agents. J. Med. Chem. 2001, 44, 1422–1428. [Google Scholar] [CrossRef]
- Laatsch, H.; Ernst, B.P.; Noltemeyer, M. Synthesis of Sterically Fixed Podophyllotoxin. Liebigs Ann. 1996, 1996, 731–737. [Google Scholar] [CrossRef]
- Zhou, X.M.; Lee, K.J.H.; Cheng, J.; Wu, S.S.; Chen, H.X.; Guo, X.; Cheng, Y.C.; Lee, K.H. New gamma-lactone ring-modified arylamino etoposide analogs as inhibitors of human DNA topoisomerase II. J. Med. Chem. 1994, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M.; Faircloth, G.T.; Castro, M.A.; Miguel del Corral, J.M.; Lopez-Vazquez, M.L.; Feliciano, S.A. Immunosuppressive cyclolignans. J. Med. Chem. 1996, 39, 2865–2868. [Google Scholar] [CrossRef]
- Subrahmanyam, D.; Renuka, B.; Laxmana-Rao, C.V.; Sagar, S.P.; Deevi, D.S.; Babu, J.M.; Vyas, K. Novel D-ring analogues of podophyllotoxin as potent anti-cancer agents. Bioorg. Med. Chem. Lett. 1998, 8, 1391–1396. [Google Scholar] [CrossRef]
- Subrahmanyam, D.; Renuka, B.; Sunil, K.G.; Vandana, V.; Deevi, D.S. 9-Deoxopodophyllotoxin derivatives as anti-cancer agents. Bioorg. Med. Chem. Lett. 1999, 9, 2131–2134. [Google Scholar] [CrossRef]
- Gaspar, N.; Occean, B.V.; Pacquement, H.; Bompas, E.; Bouvier, C.; Brisse, H.J.; Castex, M.P.; Cheurfa, N.; Corradini, N.; Delaye, J.; et al. Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. Eur. J. Cancer 2018, 88, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef] [Green Version]
- Jia, K.Z.; Zhan, X.; Li, H.M.; Shen, Y.; Qi, Q.; Zhang, Y.; Li, Y.Z.; Tang, Y.J. A novel podophyllotoxin derivative with higher anti-tumor activity produced via 4′-demethylepipodophyllotoxin biotransformation by Penicillium purpurogenum. Process Biochem. 2020, 96, 220–227. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Yang, L.; Tian, X. Podophyllotoxin: Current Perspectives. Curr. Bioact. Compd. 2007, 3, 37–66. [Google Scholar]
- Hernandez, A.P.; Diez, P.; Garcia, P.A.; Perez-Andres, M.; Ortega, P.; Jambriana, P.G.; Diez, D.; Castro, M.A.; Fuentes, M. A Novel Cytotoxic Conjugate Derived from the Natural Product Podophyllotoxin as a Direct-Target Protein Dual Inhibitor. Molecules 2020, 25, 4258. [Google Scholar] [CrossRef]
- Kumar, P.; Pal, T.; Sharma, N.; Kumar, V.; Sood, H.; Chauhan, R.S. Expression analysis of biosynthetic pathway genes vis-à-vis podophyllotoxin content in Podophyllum hexandrum Royle. Protoplasma 2015, 252, 1253–1262. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Zidorn, C.; Dobner, M.J.; Ellmerer, E.P.; Stuppner, H. Lignans, phenylpropanoids and polyacetylenes from Chaerophyllum aureum L. (Apiaceae). Z. Nat. C 2003, 58, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Al-Juaid, S.S.; Abdel-Mogib, M. A novel podophyllotoxin lignan from Justicia heterocarpa. Chem. Pharm. Bull. 2004, 52, 507–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.Z.; Wang, L.P.; Chen, Z.N. A new podophyllotoxin-type lignan from Dysosma versipellis var. tomentosa. J. Nat. Prod. 1991, 54, 1422–1424. [Google Scholar] [CrossRef]
- Bedir, E.; Khan, I.; Moraes, R.M. Bioprospecting for Podophyllotoxin. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, Egypt, 2002; pp. 545–549. [Google Scholar]
- Gohar, U.F.; Iqbal, I.; Shah, Z.; Mukhtar, H.; Zia-Ul-Haq, M. COVID-19: Recent Developments in Therapeutic Approaches. In Alternative Medicine Interventions for COVID-19; Zia-Ul-Haq, M., Bin-Jumah, M.N., Alothamn, S.I., Henidi, H.A., Eds.; Springer: Cham, Switzerland, 2021; pp. 249–274. [Google Scholar]
- Liang, Z.; Zhang, J.; Xuan, Z.; Li, J.; Xiaoqian, Z.; Zhao, C. Endophytic fungus from Sinopodophyllum emodi (Wall.) ying that produces Podophyllotoxin. J. Chromatogr. Sci. 2016, 54, 175–178. [Google Scholar] [PubMed] [Green Version]
- Karuppaiya, P.; Tsay, H.S. Enhanced production of podophyllotoxin, kaempferol, and quercetin from callus culture of Dysosma pleiantha (Hance) woodson: An endangered medicinal plant. Biotech. Appl. Biochem. 2019, 67, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Caldwell, M.E.; Cole, J.R. Antitumor agents from Bursera microphylla (Burseraceae) I. Isolation and characterization of deoxypodophyllotoxin. J. Pharm. Sci. 1968, 57, 696–697. [Google Scholar] [CrossRef] [PubMed]
- Provan, G.J.; Waterman, P.G. Picropolygamain: A New Lignan from Commiphora incisa Resin1. Planta Med. 1985, 51, 271–272. [Google Scholar] [CrossRef]
- Wickramaratne, D.B.M.; Mar, W.; Chai, H.; Castillo, J.J.; Farnsworth, N.R.; Soejarto, D.D.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic constituents of Bursera permollis. Planta Med. 1995, 61, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Jutiviboonsuk, A.; Zhang, H.; Tan, G.T.; Ma, C.; Van-Hung, N.; Cuong, N.M.; Bunyapraphatsara, N.; Soejarto, D.D.; Fong, H.H. Bioactive constituents from roots of Bursera tonkinensis. Phytochemistry 2005, 66, 2745–2751. [Google Scholar] [CrossRef]
- Velazquez-Jimenez, R.; Torres-Valencia, J.M.; Cerda-García-Rojas, C.M.; Hernandez-Hernández, J.D.; Roman-Marín, L.U.; Manríquez-Torres, J.J.; Gomez-Hurtado, M.A.; Valdez-Calderon, A.; Motilva, V.; García-Maurino, S.; et al. Absolute configuration of podophyllotoxin related lignans from Bursera fagaroides using vibrational circular dichroism. Phytochemistry 2011, 72, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, A.S.; Medarde, M.; Lopez, J.L.; Puebla, P.; Miguel del Corral, J.M.; Barrer, A.F. Lignans from Juniperus thurifera. Phytochemistry 1989, 28, 2863–2866. [Google Scholar] [CrossRef]
- Aynehchi, Y. Desoxypodophyllotoxin, the cytotoxic principle of Callitris columellaris F. Muell. J. Pharm. Sci. 1971, 60, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Van, U.W.; Pras, N.; Vossebeld, E.M.; Mol, J.N.; Malingre, T.M. Production of 5-methoxypodophyllotoxin in cell suspension cultures of Linum flavum L. Tissue Organ Cult. 1990, 20, 81–87. [Google Scholar]
- Kier, L.B.; Fitzgerald, D.B.; Burgett, S. Isolation of podophyllotoxin from Callitrus drummondii. J. Pharm. Sci. 1963, 52, 502–503. [Google Scholar] [PubMed]
- Hartwell, J.L.; Johnson, J.M.; Fitzgerald, D.B.; Belkin, M. Podophyllotoxin from Juniperus species; savinin. JACS 1953, 75, 235–236. [Google Scholar] [CrossRef]
- Muranaka, T.; Miyata, M.; Ito, K.; Tachibana, S. Production of podophyllotoxin in Juniperus chinensis callus cultures treated with oligosaccharides and a biogenetic precursor in honour of Professor GH Neil towers 75th birthday. Phytochemistry 1998, 49, 491–496. [Google Scholar] [CrossRef]
- Chang, L.C.; Song, L.L.; Park, E.J.; Luyengi, L.; Lee, K.J.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Bioactive constituents of Thuja occidentalis. J. Nat. Prod. 2000, 63, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Mikac, S.; Markulin, L.; Drouet, S.; Corbin, C.; Tungmunnithum, D.; Kiani, R.; Kabra, A.; Abbasi, B.H.; Renouard, S.; Bhambra, A.; et al. Bioproduction of Podophyllotoxin and Related Aryltretralin-Lignans in Hairy Root Cultures of Linum Flavum L. Plant Cell Tissue Differ. Second. Metab. Fundam. Appl. 2021, 503–540. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Zheljazkov, V.D.; Osbrink, W.L.; Castro, A.; Maddox, V.; Craker, L.E.; Astatkie, T. Podophyllotoxin and essential oil profile of Juniperus and related species. Ind. Crop. Prod. 2013, 43, 668–676. [Google Scholar] [CrossRef]
- Och, M.; Och, A.; Ciesla, L.; Kubrak, T.; Pecio, L.; Stochmal, A.; Kocki, J.; Bogucka-Kocka, A. Study of cytotoxic activity, podophyllotoxin, and deoxypodophyllotoxin content in selected Juniperus species cultivated in Poland. Pharm. Biol. 2015, 53, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Renouard, S.; Corbin, C.; Colas, C.; Fidel, T.; Lopez, T.; Leclerc, E.A.; Hendrawati, O.; Falguieres, A.; Doussot, J.; Ferroud, C.; et al. Aerial parts of Callitris species as a rich source of deoxypodophyllotoxin. Ind. Crop. Prod. 2015, 63, 53–57. [Google Scholar] [CrossRef]
- Wanner, J.; Jirovetz, L.; Schmidt, E. Callitris intratropica RT Baker & HG Smith as a novel rich source of deoxypodophyllotoxin. Curr. Bioact. Compd. 2015, 11, 73–77. [Google Scholar]
- Doussot, J.; Mathieu, V.; Colas, C.; Molinie, R.; Corbin, C.; Montguillon, J.; Banuls, L.M.Y.; Renouard, S.; Lamblin, F.; Dupre, P.; et al. Investigation of the lignan content in extracts from Linum, Callitris and Juniperus species in relation to their in vitro antiproliferative activities. Planta Med. 2017, 234, 574–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasparova, M.; Martin, J.; Tu, A.L.; Spilkov, A.J. Production of podophyllotoxin by plant tissue cultures of Juniperus virginiana. Nat. Prod. Commun. 2017, 12, 101–103. [Google Scholar] [PubMed]
- Broomhead, A.J.; Dewick, P.M. Aryltetralin lignans from Linum flavum and Linum capitatum. Phytochemistry 1990, 29, 3839–3844. [Google Scholar] [CrossRef]
- Smollny, T.; Wichers, H.; De Rijk, T.; Van Zwam, A.; Shasavari, A.; Alfermann, A.W. Formation of lignans in suspension cultures of Linum album. Planta Med. 1992, 58, 622–624. [Google Scholar] [CrossRef]
- Kranz, K.; Petersen, M. β-Peltatin 6-O-methyltransferase from suspension cultures of Linum nodiflorum. Phytochemistry 2003, 64, 453–458. [Google Scholar] [CrossRef]
- Kartal, M.; Konuklugil, B.; Indrayanto, G.; Alfermann, A.W. Comparison of different extraction methods for the determination of podophyllotoxin and 6-methoxypodophyllotoxin in Linum species. J. Pharm. Biomed. Anal. 2004, 35, 441–447. [Google Scholar] [CrossRef]
- Vasilev, N.; Ionkova, I. Lignan accumulation in cell cultures of Linum strictum ssp. strictum L. Acta Pharm. 2004, 54, 347–351. [Google Scholar]
- Mohagheghzadeh, A.; Gholami, A.; Soltani, M.; Hemmati, S.; Alfermann, W. Linum mucronatum: Organ to organ lignan variations. Z. Nat. C 2005, 60, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Van Furden, B.; Humburg, A.; Fuss, E. Influence of methyl jasmonate on podophyllotoxin and 6-methoxypodophyllotoxin accumulation in Linum album cell suspension cultures. Plant Cell Rep. 2005, 24, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Federolf, K.; Alfermann, A.W.; Fuss, E. Aryltetralin-lignan formation in two different cell suspension cultures of Linum album: Deoxypodophyllotoxin 6-hydroxylase, a key enzyme for the formation of 6-methoxypodophyllotoxin. Phytochemistry 2007, 68, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Jain, A.; Gupta, N.; Srivastava, A.K.; Bisaria, V.S. Co-culture of arbuscular mycorrhiza-like fungi (Piriformospora indica and Sebacina vermifera) with plant cells of Linum album for enhanced production of podophyllotoxins: A first report. Biotechnol. Lett. 2008, 30, 1671–1677. [Google Scholar] [CrossRef]
- Lautie, E.; Quintero, R.; Fliniaux, M.A.; Villarreal, M.L. Selection methodology with scoring system: Application to Mexican plants producing podophyllotoxin related lignans. J. Ethnopharmacol. 2008, 120, 402–412. [Google Scholar] [CrossRef]
- Vasilev, N.; Ebel, R.; Edrada, R.A.; Fuss, E.; Alfermann, A.W.; Ionkova, I.; Petrova, A.; Repplinger, M.; Schmidt, T.J. Metabolic profiling of lignan variability in Linum species of section Syllinum native to Bulgaria. Planta Med. 2008, 74, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Samadi, A.; Jafari, M.; Nejhad, N.M.; Hossenian, F. Podophyllotoxin and 6-methoxy podophyllotoxin production in hairy root cultures of Liunm mucronatum ssp. mucronatum. Pharmacogn. Mag. 2014, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Sasheva, P.; Ionkova, I. Podophyllotoxin production by cell cultures of Linum thracicum ssp. thracicum Degen elicited with methyl jasmonate and salicylic acid. Comptes Rendus Acad. Bulg. Sci. 2015, 68, 883–889. [Google Scholar]
- Esfandiari, M.; Sharifi, M.; Mohamadyar-Toupkanlou, F.; Hanaee-Ahwaz, H.; Yousefzadi, M.; Jafari, A.; Hosseinzadeh, S.; Soleimani, M. Optimization of cell/tissue culture of Linum persicum for production of lignans derivatives including Podophyllotoxin. Tissue Organ Cult. 2018, 133, 51–61. [Google Scholar] [CrossRef]
- Lalaleo, L.; Testillano, P.; Risueno, M.C.; Cusido, R.M.; Palazon, J.; Alcazar, R.; Bonfill, M. Effect of in vitro morphogenesis on the production of podophyllotoxin derivatives in callus cultures of Linum album. J. Plant Physiol. 2018, 228, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Ali-Hussaini, S.M.; Rahim, A.; Riyaz, S. Podophyllotoxinderivatives: A patent review (2012–2014). Expert Opin. Ther. Pat. 2015, 25, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M. Historical and Introductory Aspects of Carotenoids. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–42. [Google Scholar]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryltetralin ligans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef]
- Kour, A.; Shawl, A.S.; Rehman, S.; Sultan, P.; Qazi, P.H.; Suden, P.; Khajuria, R.K.; Verma, V. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J. Microbiol. Biotechnol. 2008, 24, 1115–1121. [Google Scholar] [CrossRef]
- Cao, L.; Huang, J.; Li, J. Fermentation conditions of Sinopodophyllum hexandrum endophytic fungus on production of podophyllotoxin. Food Ferment. Ind. 2007, 33, 28–32. [Google Scholar]
- Huang, J.X.; Zhang, J.; Zhang, X.R.; Zhang, K.; Zhang, X.; He, X.R. Mucor fragilis as a novel source of the key pharmaceutical agents podophyllotoxin and kaempferol. Pharm. Biol. 2014, 52, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M. Fusarium solani, P1, a new endophytic podophyllotoxin-producing fungus from roots of Podophyllum hexandrum. Afr. J. Microbiol. Res. 2012, 6, 2493–2499. [Google Scholar]
- Porter, J.; Eyberger, A.L.; Dondapati, R. Endophyte Fungal Isolates from Podophllum peltatum Produce Podophyllotoxin. J. Nat. Prod. 2006, 69, 1121–1124. [Google Scholar]
- Kusari, S.; Zuhlke, S.; Spiteller, M. Chemometric Evaluation of the Anti-cancer Pro-drug Podophyllotoxin and Potential Therapeutic Analogues in Juniperus and Podophyllum Species. Phytochem. Anal. 2011, 22, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Lamshoft, M.; Spiteller, M. Aspergillus fumigatus, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009, 107, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, Y.X.; Ye, Y.H.; Zheng, H.M.; Zhang, B.W.; Zhang, E.H. Screening and identification of endophytic fungi producing podophyllotoxin compounds in Sinopodophyllum hexandrum stems. Chin. J. Exp. Trad. Med. 2017, 18, 493–532. [Google Scholar]
- Tan, X.; Zhou, Y.; Zhou, X.; Xia, X.; Wei, Y.; He, L.; Tang, H.; Yu, L. Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Jiang, B.; Su, Y.; Liu, S.; Zhang, L. Podophyllotoxin and its analogues from the endophytic fungi derived from Dysosma veitchii. Biotechnology 2004, 14, 55–57. [Google Scholar]
- Zeng, S.; Shao, H.; Zhang, L. An endophytic fungus producing a substance analogous to podophyllotoxin isolated from Diphylleia sinensis. J. Microbiol. 2004, 24, 1–2. [Google Scholar]
- Yang, X.; Guo, S.; Zhang, L.; Shao, H. Select of producing podophyllotoxin endophytic fungi from podophyllin plant. Nat. Prod. Res. Dev. 2003, 15, 419–422. [Google Scholar]
- Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Ghasempour, A.; Moyano, E.; Palazon, J. The effect of light on gene expression and podophyllotoxin biosynthesis in Linum album cell culture. Plant Physiol. Biochem. 2012, 56, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Sun, P.; Li, M.F. Chilling temperature stimulates growth, gene over-expression and podophyllotoxin biosynthesis in Podophyllum hexandrum Royle. Plant Physiol. Biochem. 2016, 9428, 30231–30235. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Mehra, R.S.; Srivastava, A.K.; Bhojwani, S.S.; Bisaria, V.S. Effect of major nutrients on podophyllotoxin production in Podophyllum hexandrum suspension cultures. Appl. Microbiol. Biotechnol. 2003, 60, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Li, W.; Yang, D.L.; Zhou, L.L.; Li, T.T.; Su, X.M. Relationship between podophyllotoxin accumulation and soil nutrients and the influence of Fe2+ and Mn2+ on podophyllotoxin biosynthesis in Podophyllum hexandrum tissue culture. Plant Physiol. Biochem. 2013, 71, 96–102. [Google Scholar] [CrossRef]
- Alam, M.A.; Naik, P.K. Impact of Soil Nutrients and Environmental Factors on Podophyllotoxin Content among 28 Populations of Podophyllum Hexandrum. Commun. Soil Sci. Plant Anal. 2009, 40, 2485–2504. [Google Scholar] [CrossRef]
- Xu, H.; Lv, M.; Tian, X. A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003–2007. Curr. Med. Chem. 2009, 16, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Farkya, S.; Bisaria, V.S.; Srivastava, A.K. Biotechnological aspects of the production of the anticancer drug podophyllotoxin. Appl. Microbiol. Biotech. 2004, 65, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.F.; Loike, J.D.; Horwitz, S.B.; Sternlicht, H.; Gensler, W.J. Conformational analysis of podophyllotoxin and its congeners. Structure-activity relationship in microtubule assembly. J. Med. Chem. 1979, 22, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M. Past, Present and Future of Carotenoids Research. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 827–854. [Google Scholar]
- Desbene, S.; Giorgi-Renault, S. Drugs that inhibit tubulin polymerization: The particular case of podophyllotoxin and analogues. Curr. Med. Chem. Anti Cancer Agents 2002, 2, 71–90. [Google Scholar] [CrossRef]
- Hu, C.; Xu, D.; Du, W.; Qian, S.; Wang, L.; Lou, J.; He, Q.; Yang, B.; Hu, Y. Novel 4 beta-anilino-podophyllotoxin derivatives: Design synthesis and biological evaluation as potent DNA-topoisomerase II poisons and anti-MDR agents. Mol. Biosyst. 2010, 6, 410–420. [Google Scholar] [CrossRef]
- Ming-Jen, C.; Ming, D.; Hing, Y.; Sung, Y.; Jih, J.; Hsun, S.; Yueh, H.; Gwo, F.; Ih, S. A New Benzenoid Derivative from an Endophytic Fungus in Peperomia sui. Chem. Nat. Compd. 2018, 54, 625–627. [Google Scholar]
- Markkanen, T.; Makinen, M.L.; Nikoskelainen, J.; Ruohonen, J.; Nieminen, K.; Jokinen, P.O.; Rannio, R.; Hirvonen, T. Biological activities of lignans. Drugs Exp. Clin. Res. 1981, 7, 691. [Google Scholar]
- Fife, K.H. New treatments for genital warts less than ideal: Abstract and commentary. JAMA 1998, 279, 2003–2004. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Konno, K.; Shigeta, S.; Yokota, T. Inhibitory Effects of Podophyllotoxin Derivatives on Herpes Simplex Virus Replication. Antivir. Chem. Chemother. 1998, 9, 263–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacRae, W.D.; Hudson, J.B.; Towers, G.H. The antiviral action of lignans. Planta Med. 1989, 55, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Syed, T.A.; Cheema, K.M.; Khayyami, M.; Ahmad, S.A.; Ahmad, S.H.; Ahmad, S. Human leukocyte interferon-alpha versus podophyllotoxin in cream for the treatment of genital warts in males. A placebo-controlled, double-blind, comparative study. Dermatology 1995, 191, 129. [Google Scholar] [CrossRef]
- Beautner, K.R. Podophyllotoxin in the treatment of genital warts. In EP Eischman (edn) Sexually Transmitted Diseases. Adv. Treat. 1996, 24, 122–232. [Google Scholar]
- Zhao, W.; Cong, Y.; Li, H.M.; Li, S.; Shen, Y.; Qi, Q.; Zhang, Y.; Li, Y.Z.; Tang, Y.J. Challenges and potential for improving the druggability of podophyllotoxin-derived drugs in cancer chemotherapy. Nat. Prod. Rep. 2021, 38, 470–488. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.Q.; Yang, L.; Feng, G. Podophyllotoxin Derivatives Show Activity against Brontispa Longissima Larvae. Nat. Prod. Commun. 2010, 5, 1247–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leander, K.; Rosen, B. Medicinal Uses for Podophyllotoxin. U.S. Patent 4,788,216, 29 November 1988. Available online: https://patents.google.com/patent/US4788216A/en (accessed on 15 February 2021).
- Lerndal, T.; Svensson, B. A clinical study of CPH 82 vs. methotrexate in early rheumatoid arthritis. Rheumatology 2000, 39, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordaliza, M.; Castro, M.A.; Miguel del Corra, J.M.; Feliciano, S.A. Antitumor Properties of Podophyllotoxin and Related Compounds. Curr. Pharm. Des. 2000, 6, 1811–1839. [Google Scholar] [CrossRef]
- Hensel, A.; Bauer, R.; Heinrich, M.; Spiegler, V.; Kayser, O.; Hempel, G.; Kraft, K. Challenges at the Time of COVID-19: Opportunities and innovations in the Antivirals from Nature. Planta Med. 2020, 86, 659–664. [Google Scholar] [CrossRef]
- Thanh, V.T.T.; Pham, V.C.; Mai, H.D.T.; Litaudon, M.; Gueritte, F.; Retailleau, P.; Nguyen, V.H.; Chau, V.M. Cytotoxic Lignans from Fruits of Cleistanthus indochinensis: Synthesis of Cleistantoxin Derivatives. J. Nat. Prod. 2012, 75, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Nanjundaswamy, N.; Satishi, S.; Lokanatha Rai, K.M.; Shashikanth, S.; Raveesha, K.A. Antibacterial Activity of Synthetic Precursors of Podophyllotoxin. Int. J. Biomed. Sci. 2007, 3, 112–115. [Google Scholar] [PubMed]
- Dombernowsky, P.; Nissen, N.I. Schedule dependency of the antileukemic activity of the podophyllotoxin-derivative VP 16-213 (NSC-141540) in L1210 leukemia. Acta Physiol. Microbiol. Scand. Sec. A Path. 1973, 81, 715–724. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Feng, G.; Yang, L.; Zhang, J.; Li, H.Y. Podophyllotoxin-derived insecticidal agents: Part XIII—Evaluation of insecticidal activity of podophyllotoxin derivatives against Brontispa longissima. Nat. Prod. Res. 2010, 25, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, L.; Chen, Y. Design, synthesis and antineoplastic activity of novel hybrids of podophyllotoxin and indirubin against human leukemia cancer cells as multifunctional anti-MDR agents. Bioorg. Med. Chem. Lett. 2018, 28, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X. Podophyllotoxins Derivatives. U.S. Patent US-8158809-B2, 17 April 2012. Available online: https://patents.google.com/patent/US8158809B2/en (accessed on 15 February 2021).
- Larsson, O.; Axelson, M. New Use of Specific Cyclolignans. World Intellectual Property Organization W.O. Patent WO-02/102804-A1, 19 June 2002. Available online: https://patents.google.com/patent/WO2002102804A1/en (accessed on 15 February 2021).
- Kerkar, B.; Potier, P. Novel Podophyllotoxin Derivatives, the Production. There of and the Use of the Same in Therapeutics. World Intellectual Property Organization W.O. Patent WO2003082875A3, 19 October 2003. Available online: https://patents.google.com/patent/WO2003082875A3/en (accessed on 15 February 2021).
- Zhang, Z.; Zhou, M.; Zhang, Y. Podophyllotoxin Derivative with 4-Position Nitrogen Substitution and Preparation Method and Application There of. U.S. Patent US-10639295-B2, 5 May 2020. Available online: https://patents.google.com/patent/US10639295B2/en (accessed on 15 February 2021).
- Kamal, A.; Reddy, V.G.; Subbarao, A.V.; Riyaz, S.; Nayak, V.L.; Taj, S. 4Beta-Amidotriazole Linked Podophyllotoxin Derivatives as Potential Anticancer Agents. U.S. Patent US-2020123171-A1, 28 May 2020. Available online: https://patents.google.com/patent/US20200123171A1/en (accessed on 15 February 2021).
- Quirion, J.; Deliencourt-Godefroy, G.C.; Audouard, C. Gem-Difluorinated C-Glycoide Compounds Derived from Podophyllotoxin, Their Preparation and Their Application. U.S. Patent US8236935B2, 7 August 2012. Available online: https://patents.google.com/patent/US8236935B2/en (accessed on 15 February 2021).
- Tang, Y.; Zhao, W. 4-Sulfur Substituted Podophyllotoxin Derivative and Preparation Method There of and Use There of. U.S. Patent US20200216462A1, 9 July 2020. Available online: https://patents.google.com/patent/US20200216462A1/en (accessed on 15 February 2021).
- Tang, Y.; Li, J.; Zhao, W.; Li, H. Sulfur-Substituted Podophyllotoxin Derivative, Synthesis Method There of, and Use There of. U.S. Patent US9828386B2, 28 November 2017. Available online: https://patents.google.com/patent/US9828386B2/en (accessed on 15 February 2021).
- Patents Lens Database. Available online: https://www.lens.org/lens/search/patent/list?q=Podophyllotoxin&preview=true (accessed on 15 February 2021).
- Google Patents Database. Available online: https://patents.google.com/?q=Podophyllotoxin&num=100&oq=Podophyllotoxin (accessed on 15 February 2021).














| S. No. | Family; Plant(s) | Part Used | Derivative | Ref. |
|---|---|---|---|---|
| 1 | Apiaceae; Chaerophyllum aurium | Extract of subaerial part | Deoxy podorhizone and deoxypodophyllotoxin | [67] |
| 2 | Acanthaceae; Justicia heterocarpa | Extract of aerial part | Podophyllotoxin lignan | [68] |
| 3 | Berberidaceae; Jeffersonia diphylla, Dysosma pleiantha, Dysosma versipellis var. tomentosa, Dysosma versipellis | Extract of root, Culture of Callus, | Podophyllotoxin, kaempferol and quercetin, Podophyllotoxin derivatives, 4-demethylisopodophyllotoxin | [69,70,71,72,73] |
| 4 | Burseraceae; C. incisa B. permollis B. fagaroides B. microphylla B. konkinensis | Resin, Stem bark, Exudate, Stem, Extract of root | Derivatives of podophyllotoxin, Deoxypodophyllotoxin, 4-demethyldeoxypodophyllotoxin | [74,75,76,77,78] |
| 5 | Broginaceae; L. erythrorhizon | Extract of needle leaf | Podophyllotoxin and derivatives | [79] |
| 6 | Cupressaceae; J. sabina J. virginia J. conferta C. preissii J. depressa C. rhomboidea J. virginiana J. chinensis J. horizontalis J. davurica C. endlicher J. thurifera C. drummondii C. intratropica J. squamata J. chinensis C. collumelaris J. scopulorum J. lucayana J. virginiana J. silicicola J. viriginia T. occidentalis | Extract of needle leaf, Culture of suspension, culture of callus, needle leaf aqueous suspension, extract of needle leaf, Extract of stem, Extract of wood, Aerial part, Twig and extract of needle leaf | Podophyllotoxin and derivatives, Deoxypodophyllotoxin, 5-methoxypodophyllotoxin | [79,80,81,82,83,84,85,86,87,88,89,90,91,92] |
| 7 | Linaceae; L. perenne L. scabrellum L. thracicum spp. L. capatitum L. elegans L. austriacum L. arboreum L. hirsutum L. usitatissimum L. scabrellum L. strictum spp. L. album L. flavum L. mucronatum spp. L. persicum L. nodiflorum L. mucronatum | Culture of hairy root, Culture of suspension, Seed extract, root extract, aerial part, Callus, Tissue culture, Aerial Tissue culture, cell culture | Podophyllotoxin, Podophyllotoxin derivatives, 5-methoxypodophyllotoxin, 6-methylpodophyllotoxin, 6-methoxypodophyllotoxin | [86,91,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107] |
| Class | Fungal Endophyte | Host Plant | Ref. |
|---|---|---|---|
| Sordariomycetes | Fusarium oxysporum Fusarium solani Fusarium sp. Pseudallescheria sp. | Juniperus recurva, Podophyllum hexandrum, Dysosma versipellis, Sinopodophyllum hexandrum | [111,114,118,119] |
| Agaricomycetes | Trametes hirsuta | Podophyllum peltatum | [110] |
| Leotiomycetes | Phialocephala fortinii | Podophyllum peltatum | [115] |
| Dothideomycetes | Alternaria tenuissima | Sinopodophyllum emodi | [72] |
| Eurotiomycetes | Aspergillus fumigatus, Penicillium implicatum | Juniperous communis, Dysoma veitchii, Diphylleia sinensis | [117,120,121] |
| Ascomycetes | Monilia sp. | Dysoma veitchi | [122] |
| Zygomycetes | Mucor fragilis | Sinopodophyllum hexandrum | [113] |
| Parameters | General Effect | Sub-Parameter | Podophyllotoxin Yield | Ref. |
|---|---|---|---|---|
| Light | Light can increase or decrease the biosynthesis of podophyllotoxin. | Red light | Substantial increase | [123] |
| Blue light | Slight increase | |||
| White light | Decrease | |||
| Chilling Temperature | Chilling temperature can increase or decrease the biosynthesis of podophyllotoxin | 4 °C | 5-folds increase | [124] |
| 10 °C | 3.33-folds increase | |||
| Macro Nutrients | At different concentrations major nutrients can increase or decrease the biosynthesis of podophyllotoxin | Glucose concentration | Highest levels of yield at 60 g/L | [125] |
| Phosphate concentration | Highest levels of yield at 1.25 mM | |||
| Nitrogen concentration | Highest levels of yield at 60 mM | |||
| Micro Nutrients | Different ions can influence the yield of podophyllotoxin | NO3−, PO43−, Na+, Fe2+, Mn2+ | Positive correlation | [126] |
| SO42−, K+ | Negative correlation | |||
| Mg2+, Ca2+, Cu, Zn | No correlation | |||
| Soil Nutrients | Podophyllotoxin production can be increased or decreased by acidic or basic pH and nutrient availability | pH | Podophyllotoxin content was increased significantly (more than 6.62%) when pH of soil was 4.82 | [127] |
| Nitrogen | Podophyllotoxin content was increased significantly when nitrogen content was 2.7% | |||
| Carbon | Podophyllotoxin content was increased significantly when soil organic carbon content was 3.32% |
| Sr. No | Formulae | Patent Number | Medical Application | Ref. |
|---|---|---|---|---|
| 1 |  | US-8158809-B2 | Cancer treatment | [152] |
| 2 | -NA- | WO-02/102804-A1 | Inhibit Insulin-like growth factor-1′s tyrosine-phosphorylation activity | [153] |
| 3 |  R: -CH2NHCOR2, -CH(OH)CH(Phe)(NHCOR3), & chains containing: pyrrole, thiazole, indole, naphthelene, phenyl, quinoline, pyrazine or pyridine groups. | WO-03082875-A3 | Cancer treatment | [154] |
| 4 |  And a salt thereof. | US-10639295-B2 | Elevated pharmaceutical efficacy in terms of drug concentration buildup in body | [155] |
| 5 | Assignee: Council Of Scientific and Industrial Research—status: pending | US-2020123171-A1 | NA | [156] |
| 6 |  | US-8236935-B2 | Cancer treatment | [157] |
| 7 |  R1: substitutively selected from the following  R2: -H, or –CH3 | US-2020216462-A1 | Improved antitumor activity, reduced undesired cytotoxicity | [158] |
| 8 |  R1: Any of the following  R2: -H or –CH3 | US-9828386-B2 | Elevated antitumor activity than podophyllotoxin or 4′demethylepipodophyllotoxin | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, Z.; Gohar, U.F.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-UI-Haq, M.; Toma, S.I.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules 2021, 11, 603. https://doi.org/10.3390/biom11040603
Shah Z, Gohar UF, Jamshed I, Mushtaq A, Mukhtar H, Zia-UI-Haq M, Toma SI, Manea R, Moga M, Popovici B. Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules. 2021; 11(4):603. https://doi.org/10.3390/biom11040603
Chicago/Turabian StyleShah, Zinnia, Umar Farooq Gohar, Iffat Jamshed, Aamir Mushtaq, Hamid Mukhtar, Muhammad Zia-UI-Haq, Sebastian Ionut Toma, Rosana Manea, Marius Moga, and Bianca Popovici. 2021. "Podophyllotoxin: History, Recent Advances and Future Prospects" Biomolecules 11, no. 4: 603. https://doi.org/10.3390/biom11040603
APA StyleShah, Z., Gohar, U. F., Jamshed, I., Mushtaq, A., Mukhtar, H., Zia-UI-Haq, M., Toma, S. I., Manea, R., Moga, M., & Popovici, B. (2021). Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules, 11(4), 603. https://doi.org/10.3390/biom11040603









