Overcoming the Challenges of High Quality RNA Extraction from Core Needle Biopsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tumor Samples
2.2. RNA Extraction from Core Needle Biopsies
2.2.1. Preparation of Core Needle Biopsies for Snap Freezing
2.2.2. Homogenization and Lysis of Snap-Frozen Samples
2.2.3. Total RNA Extraction from Lysed Samples
2.3. Preparation of FFPE Tumor Specimens
2.3.1. Protocol for FFPE Preparation
2.3.2. Ribonuclease-Free Macrodissection of FFPE Tumor Specimens
2.3.3. RNA Extraction from Collected FFPE Curls
2.4. Quality Control of the Extracted Total RNA
2.5. Functionality Control of Extracted Total RNA
2.6. Statistical Analysis
3. Results and Discussion
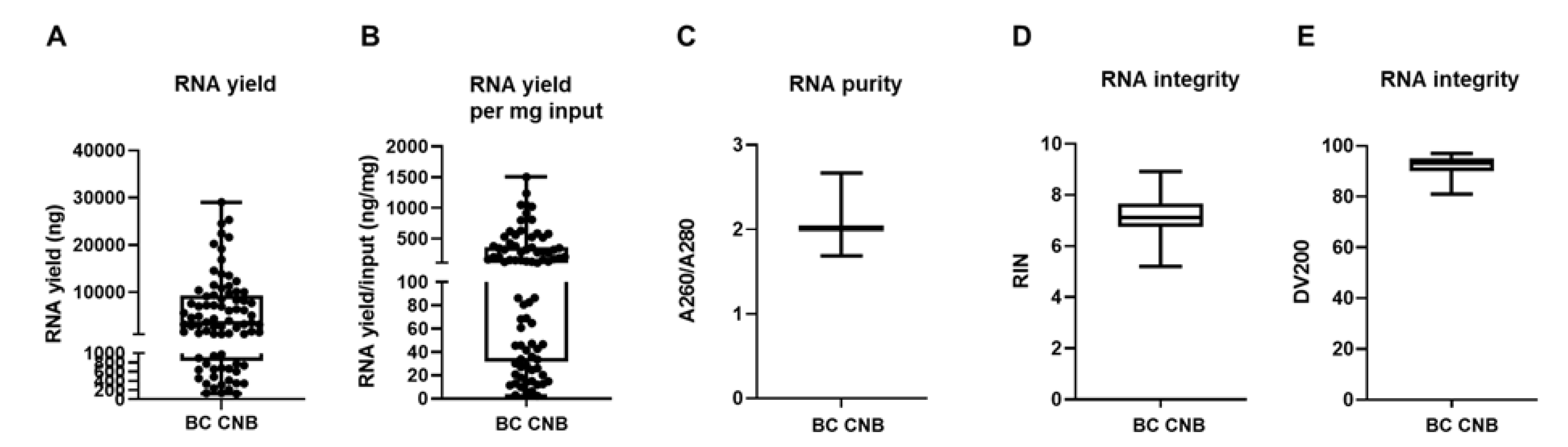
3.1. RNA Extraction from Fresh-Frozen Core Needle Biopsies Requires Mechanical Disruption
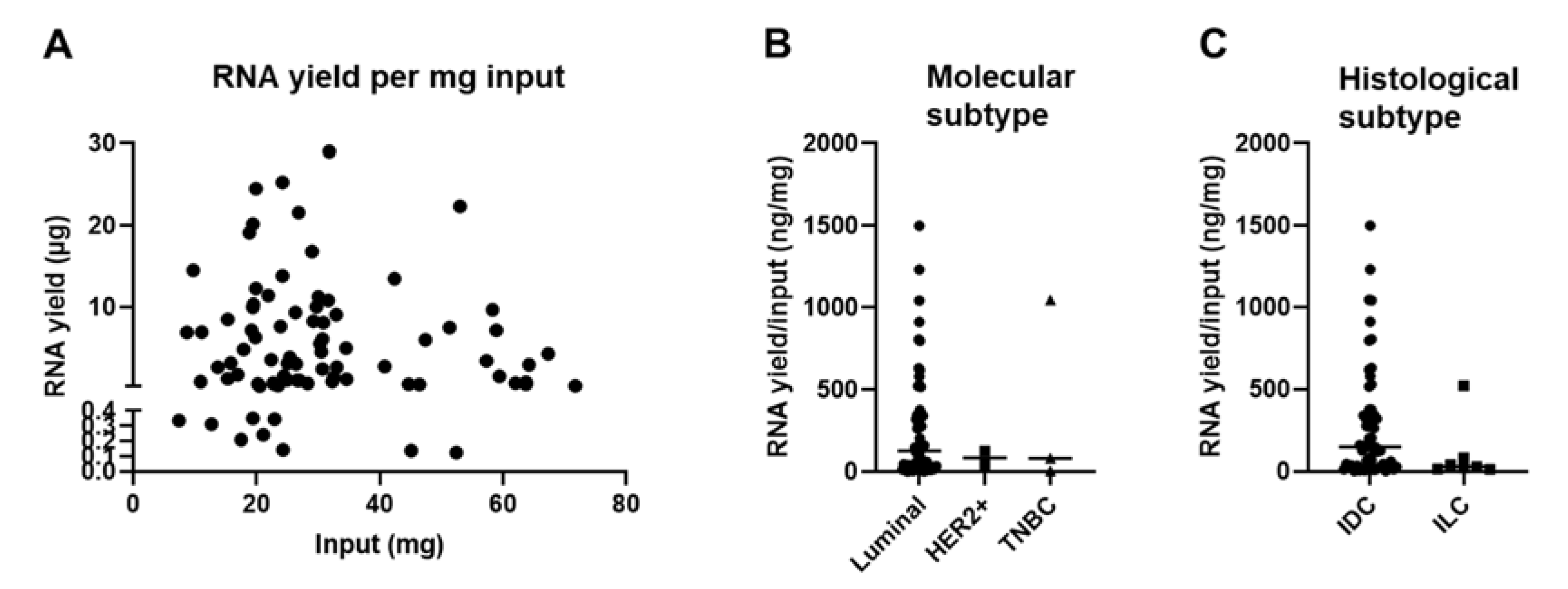
3.2. The Tissue Composition of Snap Frozen Core Needle Biopsies Affects RNA Yield
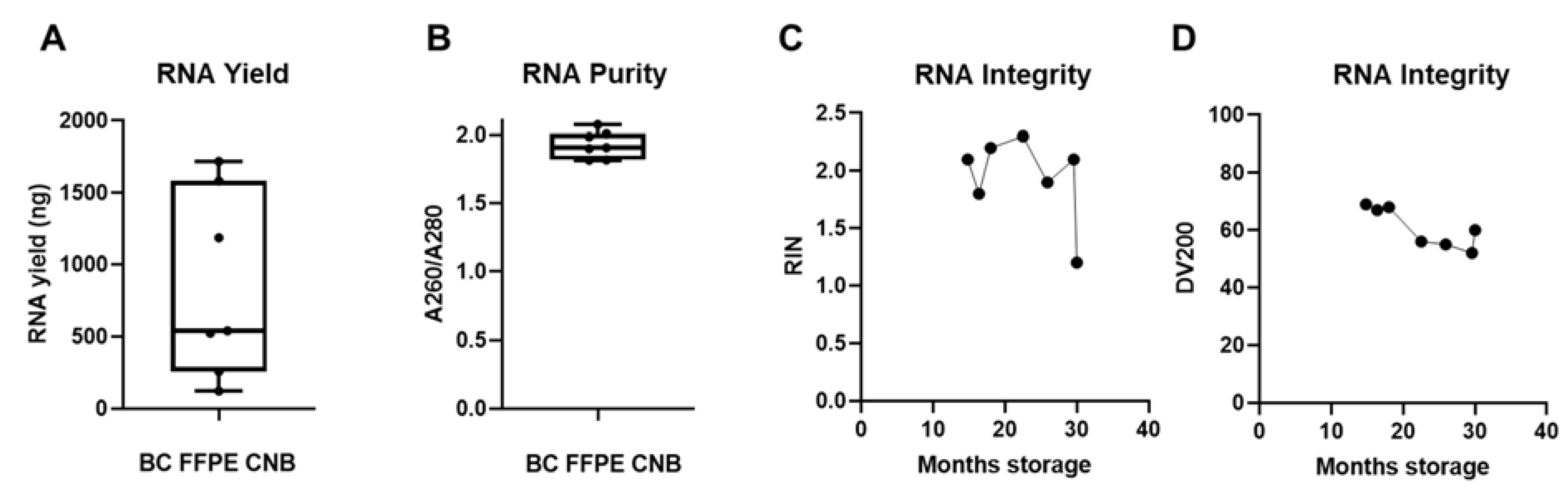
3.3. FFPE Samples Can Serve as a Back-Up Source for RNA Extraction
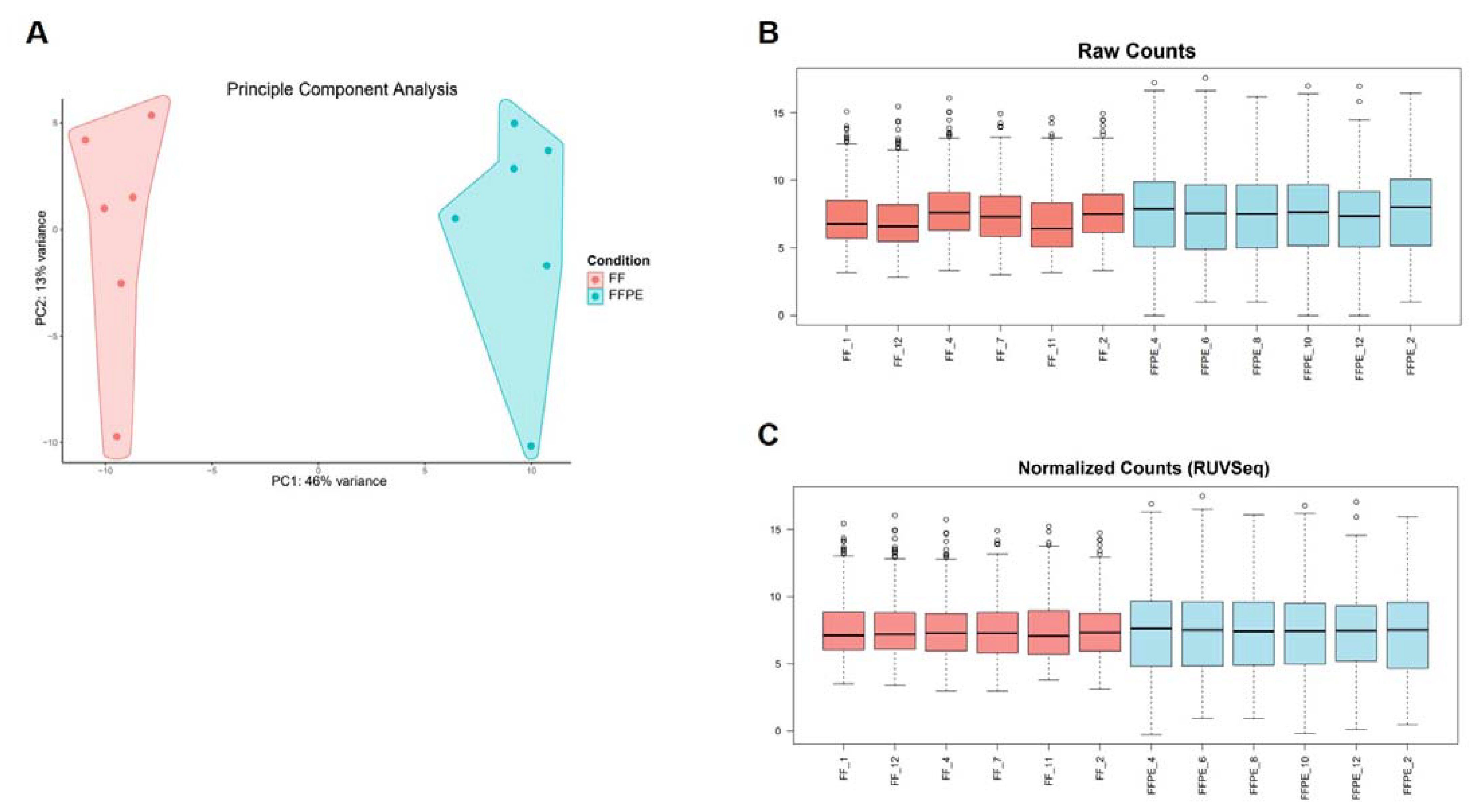
3.4. RNA Extracted from FF or FFPE BC Core Needle Biopsies Can Be Used in nanoString nCounter® Technology
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | breast cancer |
| βME | β-mercaptoethanol |
| CNB | core needle biopsy |
| ER | estrogen receptor |
| EtOH | ethanol |
| FFPE | formalin-fixed paraffin-embedded |
| FF | fresh-frozen |
| GEP | gene expression profiling |
| HER2 | human epidermal growth factor receptor 2 |
| IDC | invasive ductal carcinoma |
| ILC | invasive lobular carcinoma |
| IRG | immune-related gene |
| PCA | principal component analysis |
| rcf | relative centrifugal force |
| RIN | RNA integrity number |
| RNA | ribonucleic acid |
| RNase | ribonuclease |
| RUV | remove unwanted variation |
| SOP | standard operating procedure |
| UZ Brussel | Universitair Ziekenhuis Brussel |
| VUB | Vrije Universiteit Brussel |
Appendix A
| Hazardous Compound | Kit/Buffer | Storage | Danger | Precaution | Action |
|---|---|---|---|---|---|
| 2-mercaptoethanol | Added to RLT buffer (Qiagen RNeasy kit) | 2–8 °C Store in a well-ventilated place. Keep container tightly closed. | Toxic if swallowed or if inhaled. Fatal in contact with skin, causes skin reaction, may cause an allergic skin reaction. Causes serious eye damage. May cause damage to organs through prolonged or repeated exposure if swallowed. Very toxic to aquatic life, with long-lasting effects. | Avoid breathing dust/fume/gas/mist/vapors/spray. Wear protective gloves/protective clothing/eye protection/face protection. | If swallowed: Immediately call a poison center/doctor, rinse mouth. If on skin: Wash with plenty of water, immediately call a poison center/doctor. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do. Continue rinsing. Immediately call a poison center/doctor. |
| Sodium dodecyl sulfate | RNase ZAP | 15–25 °C | Causes mild skin irritation. | Wear protective gloves/protective clothing/eye protection/face protection. | If skin irritation occurs get medical advice/attention. |
| Ethanol (100%) | Added to buffer RPE (Qiagen RNeasy kit). Added (70% EtOH) to lysate (Qiagen RNeasy kit) 100% EtOH as such (RNase free macrodissection) Added to buffer RPE (Qiagen RNeasy FFPE kit) 100% EtOH to sample (Qiagen RNeasy FFPE kit) | 15–25 °C Store in a well-ventilated place. Keep container tightly closed. | Highly flammable liquid and vapor. Causes serious eye irritation. | Keep away from heat/sparks/open flames/hot surfaces. No smoking. Ground/bond container and receiving equipment. | If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do. Continue rinsing. |
| Guanidine thiocyanate Guanidine thiocyanate + Ethanol | Buffer RLT (Qiagen RNeasy kit) Buffer RW (Qiagen RNeasy kit) | 15–25 °C | Flammable liquid and vapor. Harmful if swallowed. May be harmful in contact with skin or if inhaled. Causes serious eye damage. Harmful to aquatic life, with long-lasting effects. Contact with acids liberates very toxic gas. | Keep away from heat/sparks/open flames/hot surfaces. No smoking. Wear protective gloves/protective clothing/eye protection/face protection. | If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a poison center or doctor/physician. |
| Guanidium chloride | Buffer RBC (Qiagen RNeasy FFPE kit) | Store in a well-ventilated place | Causes skin irritation. Causes serious eye irritation. Harmful if swallowed or inhaled. | Avoid breathing dust/fume/gas/mist/vapors/spray. Wear protective gloves/protective clothing/eye protection/face protection. Wear respiratory protection. | If inhaled: If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing. If eye irritation persists: Get medical advice/attention. If respiratory symptoms: Call a poison center or doctor/physician. Take off contaminated clothing and wash it before reuse. Dispose of contents/container to an approved waste disposal plant. |
| DNase I | DNase I (Qiagen RNeasy (FFPE) kit) | Store in a well-ventilated place | May cause an allergic skin reaction. May cause allergy or asthma symptoms or breathing difficulties if inhaled. | Avoid breathing dust/fume/gas/mist/vapors/spray. Wear protective gloves/protective clothing/eye protection/face protection. Wear respiratory protection. | If inhaled: If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing. If eye irritation persists: Get medical advice/attention. If respiratory symptoms: Call a poison center or doctor/physician. Take off contaminated clothing and wash it before reuse. Dispose of contents/container to an approved waste disposal plant. |
| Proteinase K | Proteinase K (Qiagen RNeasy FFPE kit) | Store in a well-ventilated place | May cause allergy or asthma symptoms or breathing difficulties if inhaled. Causes mild skin irritation. | Avoid breathing dust/fume/gas/mist/vapors/spray. Wear protective gloves/protective clothing/eye protection/face protection. Wear respiratory protection. | If inhaled: If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing. If eye irritation persists: Get medical advice/attention. If respiratory symptoms: Call a poison center or doctor/physician. Take off contaminated clothing and wash it before reuse. Dispose of contents/container to an approved waste disposal plant. |
| Dimethyl sulfoxide (DMSO) | Qubit RNA HS assay kit (RNA reagent, 20× concentrated) Agilent RNA 6000 nano/pico kit (dye/gel) | Store in a well-ventilated place. | Combustible liquid | Keep away from heat/hot surfaces/sparks/open flames and other ignition sources. No smoking. Wear protective gloves/protective clothing/eye protection/face protection. | In case of fire: Use dry sand, dry chemical, or alcohol-resistant foam for extinction. Dispose of contents/container to an approved waste disposal plant. |
| Sodium hydroxide | NaOH as such (RNase free macrodissection) | Store in a cool, dry, well-ventilated place. Separate from incompatible materials. Keep container tightly closed. | Highly reactive. Incompatible with many common chemicals. Reacts violently with water. Contact with metals liberates flammable hydrogen gas. Extremely corrosive. Causes severe skin burns and eye damage (resulting in blindness). If airborne, dust or mist can cause severe irritation of the nose and throat. If ingested, can burn lips, tongue, throat, and stomach and be fatal. | Avoid direct skin contact and wear protective clothing. Avoid direct contact. Wear chemical protective gloves/eye protection. | If on skin: Take off contaminated clothing, shoes quickly and gently blot or brush away excess chemical. Immediately flush with lukewarm, gently flowing water for at least 60 min. Do not interrupt flushing. If it can be done safely, continue flushing during transport to hospital. Immediately call a poison center or doctor. If in eyes: Quickly and gently blot or brush chemical off the face. Immediately flush the contaminated eye(s) with lukewarm, gently flowing water for at least 60 min while holding the eyelid(s) open. If a contact lens is present do not delay flushing or attempt to remove the lens. Take care not to rinse contaminated water into the unaffected eye or onto the face. Immediately call a poison center or doctor. If in eyes: Quickly and gently blot or brush chemical off the face. Immediately flush the contaminated eye(s) with lukewarm, gently flowing water for at least 60 min while holding the eyelid(s) open. If a contact lens is present do not delay flushing or attempt to remove the lens. Take care not to rinse contaminated water into the unaffected eye or onto the face. Immediately call a poison center or doctor. If ingested: Rinse mouth with water. If vomiting occurs naturally, have victim lean forward to reduce risk of aspiration. Have victim rinse mouth with water again. Immediately call a poison center or doctor. |
| Hexadecane | Deparaffinization solution | Store in a dry and well-ventilated place. Keep container tightly closed. | May be harmful if inhaled. Repeatedly exposure can cause dry or burst skin Can be fatal when compound ends up in airways when swallowing. | Avoid breathing dust/fume/gas/mist/vapors/spray. Wear protective gloves/protective clothing/eye protection/face protection. | If swallowed: Contact a poison center or doctor/physician immediately, rinse mouth with water. If on skin: Wash with soap and an excess of water, remove all contaminated clothing and shoes. If symptoms are persistent, contact a doctor/physician. If in eyes: Remove contact lenses if present and easy to do. Rinse thoroughly with an excess of water for at least 15 min and contact a doctor/physician. |
References
- Haferlach, T.; Schmidts, I. The power and potential of integrated diagnostics in acute myeloid leukaemia. Br. J. Haematol. 2019, 188, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, D.G.; Bachas, C.; Heuvel-Eibrink, M.M.V.D.; Arentsen-Peters, S.T.; Kwidama, Z.J.; Schuurhuis, G.J.; Assaraf, Y.G.; De Haas, V.; Kaspers, G.J.; Cloos, J. Harnessing Gene Expression Profiles for the Identification of Ex Vivo Drug Response Genes in Pediatric Acute Myeloid Leukemia. Cancers 2020, 12, 1247. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Gao, Z.; Huo, X.; Zhang, Y.; Wang, W.; Qi, J.; Zheng, S. Analysis of gene expression profiles of non-small cell lung cancer at different stages reveals significantly altered biological functions and candidate genes. Oncol. Rep. 2017, 37, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Xu, Y.; Cao, J.; Chen, L. Genomic analysis of drug resistant small cell lung cancer cell lines by combining mRNA and miRNA expression profiling. Oncol. Lett. 2017, 13, 4077–4084. [Google Scholar] [CrossRef] [Green Version]
- Apostolou, P.; Iliopoulos, A.C.; Parsonidis, P.; Papasotiriou, I. Gene expression profiling as a potential predictor between normal and cancer samples in gastrointestinal carcinoma. Oncotarget 2019, 10, 3328–3338. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31164955 (accessed on 30 November 2020). [CrossRef] [PubMed] [Green Version]
- Maeda, O.; Matsuoka, A.; Furukawa, K.; Miyahara, R.; Hirooka, Y.; Ando, Y. Alterations in gene expression and DNA methylation profiles in gastric cancer cells obtained from ascitic fluids collected before and after chemotherapy. Mol. Clin. Oncol. 2019, 11, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Bing, Z.; Wu, J.; Zhang, J.; Zhou, W.; Ni, M.; Meng, Z.; Liu, S.; Tian, J.; Zhang, X.; et al. Integrative Gene Expression Profiling Analysis to Investigate Potential Prognostic Biomarkers for Colorectal Cancer. Med. Sci. Monit. 2020, 26, e918906. [Google Scholar] [CrossRef]
- Pan, F.; Chen, T.; Sun, X.; Li, K.; Jiang, X.; Försti, A.; Zhu, Y.; Lai, M. Prognosis Prediction of Colorectal Cancer Using Gene Expression Profiles. Front. Oncol. 2019, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.C.; Qian, H.X. Screening for implicated genes in colorectal cancer using whole‑genome gene expression profiling. Mol. Med. Rep. 2018, 17, 8260–8268. [Google Scholar] [CrossRef]
- Bonavida, B. Drug Resistance in Colorectal Cancer: Molecular Mechanisms and Therapeutic Strategies; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Lu, W.; Li, N.; Liao, F. Identification of Key Genes and Pathways in Pancreatic Cancer Gene Expression Profile by Integrative Analysis. Genes 2019, 10, 612. [Google Scholar] [CrossRef] [Green Version]
- Quiñonero, F.; Mesas, C.; Doello, K.; Cabeza, L.; Perazzoli, G.; Jimenez-Luna, C.; Rama, A.R.; Melguizo, C.; Prados, J. The challenge of drug resistance in pancreatic ductal adenocarcinoma: A current overview. Cancer Biol. Med. 2019, 16, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Zhao, Y.; Liang, K.; Huang, Y. Identification of Potential Key Genes for Pathogenesis and Prognosis in Prostate Cancer by Integrated Analysis of Gene Expression Profiles and the Cancer Genome Atlas. Front. Oncol. 2020, 10, 809. [Google Scholar] [CrossRef]
- Deng, L.; Gu, X.; Zeng, T.; Xu, F.; Dong, Z.; Liu, C.; Chao, H. Identification and characterization of biomarkers and their functions for docetaxel‑resistant prostate cancer cells. Oncol. Lett. 2019, 18, 3236–3248. [Google Scholar] [CrossRef]
- Grossman, D.; Okwundu, N.; Bartlett, E.K.; Marchetti, M.A.; Othus, M.; Coit, D.G.; Hartman, R.I.; Leachman, S.A.; Berry, E.G.; Korde, L.; et al. Prognostic Gene Expression Profiling in Cutaneous Melanoma. JAMA Dermatol. 2020, 156, 1004–1011. [Google Scholar] [CrossRef]
- Györffy, B.; Serra, V.; Materna, V.; Schäfer, R.; Dietel, M.; Schadendorf, D.; Lage, H. Analysis of gene expression profiles in melanoma cells with acquired resistance against antineoplastic drugs. Melanoma Res. 2006, 16, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Koroknai, V.; Patel, V.; Szász, I.; Ádány, R.; Balazs, M. Gene Expression Signature of BRAF Inhibitor Resistant Melanoma Spheroids. Pathol. Oncol. Res. 2020, 26, 2557–2566. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Roberto, M.; Panebianco, M.; Botticelli, A.; Mazzuca, F.; Marchetti, P. Drug resistance of BRAF-mutant melanoma: Review of up-to-date mechanisms of action and promising targeted agents. Eur. J. Pharmacol. 2019, 862, 172621. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Pareja, F.G. New Advances in Molecular Breast Cancer Pathology. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Shaheen, S.; Fawaz, F.; Shah, S.; Büsselberg, D. Differential Expression and Pathway Analysis in Drug-Resistant Triple-Negative Breast Cancer Cell Lines Using RNASeq Analysis. Int. J. Mol. Sci. 2018, 19, 1810. [Google Scholar] [CrossRef] [Green Version]
- Finak, G.; Bertos, N.R.; Pepin, F.; Sadekova, S.; Souleimanova, M.; Zhao, H.; Chen, H.; Omeroglu, G.; Meterissian, S.; Omeroglu, A.; et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008, 14, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Latha, N.R.; Rajan, A.; Nadhan, R.; Achyutuni, S.; Sengodan, S.K.; Hemalatha, S.K.; Varghese, G.R.; Thankappan, R.; Krishnan, N.; Patra, D.; et al. Gene expression signatures: A tool for analysis of breast cancer prognosis and therapy. Crit. Rev. Oncol. 2020, 151, 102964. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Zheng, J.; Hu, S.; Zhang, W.; Zhou, H.; Li, X.; Liu, Z.-Q. Construction and validation of an immunity-related prognostic signature for breast cancer. Aging 2020, 12, 21597–21612. [Google Scholar] [CrossRef]
- Samadani, A.A.; Nikbakhsh, N.; Fattahi, S.; Pourbagher, R.; Mir, S.M.A.; Kani, N.M.; Abedian, Z.; Akhavan-Niaki, H. RNA Extraction from Animal and Human’s Cancerous Tissues: Does Tissue Matter? Int. J. Mol. Cell. Med. 2015, 4, 54–59. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25815283 (accessed on 30 November 2020).
- Choi, Y.; Kim, A.; Kim, J.; Lee, J.; Lee, S.Y.; Kim, C. Optimization of RNA Extraction from Formalin-Fixed Paraffin-Embedded Blocks for Targeted Next-Generation Sequencing. J. Breast Cancer 2017, 20, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Landolt, L.; Marti, H.-P.; Beisland, C.; Flatberg, A.; Eikrem, O.S. RNA extraction for RNA sequencing of archival renal tissues. Scand. J. Clin. Lab. Investig. 2016, 76, 426–434. [Google Scholar] [CrossRef] [Green Version]
- McDonough, S.J.; Bhagwate, A.; Sun, Z.; Wang, C.; Zschunke, M.; Gorman, J.A.; Kopp, K.J.; Cunningham, J.M. Use of FFPE-derived DNA in next generation sequencing: DNA extraction methods. PLoS ONE 2019, 14, e0211400. [Google Scholar] [CrossRef] [Green Version]
- Wimmer, I.; Tröscher, A.R.; Brunner, F.; Rubino, S.J.; Bien, C.G.; Weiner, H.L.; Lassmann, H.; Bauer, J. Systematic evaluation of RNA quality, microarray data reliability and pathway analysis in fresh, fresh frozen and formalin-fixed paraffin-embedded tissue samples. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Lee, J.; Lee, E.H.; Park, H.Y.; Kim, W.W.; Lee, R.K.; Chae, Y.S.; Lee, S.J.; Kim, J.-E.; Kang, B.-I.; Park, J.Y.; et al. Efficacy of an RNA-based multigene assay with core needle biopsy samples for risk evaluation in hormone-positive early breast cancer. BMC Cancer 2019, 19, 388. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.; Greytak, S.; Odeh, H.; Guan, P.; Powers, J.; Bavarva, J.; Moore, H.M. Deleterious effects of formalin-fixation and delays to fixation on RNA and miRNA-Seq profiles. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gaffney, E.F.; Riegman, P.H.; Grizzle, W.E.; Watson, P.H. Factors that drive the increasing use of FFPE tissue in basic and translational cancer research. Biotech. Histochem. 2018, 93, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Micke, P.; Ohshima, M.; Tahmasebpoor, S.; Ren, Z.-P.; Östman, A.; Pontén, F.; Botling, J. Biobanking of fresh frozen tissue: RNA is stable in nonfixed surgical specimens. Lab. Investig. 2006, 86, 202–211. [Google Scholar] [CrossRef]
- Sun, H.; Sun, R.; Hao, M.; Wang, Y.; Zhang, X.; Liu, Y.; Cong, X. Effect of Duration of Ex Vivo Ischemia Time and Storage Period on RNA Quality in Biobanked Human Renal Cell Carcinoma Tissue. Ann. Surg. Oncol. 2015, 23, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Hamilton, A.M.; Furberg, H.; Pietzak, E.; Purdue, M.P.; Troester, M.A.; Hoadley, K.A.; Love, M.I. An approach for normalization and quality control for NanoString RNA expression data. Brief. Bioinform. 2020. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, S.; Renmans, D.; Broos, K.; Goethals, L.; Maenhout, S.K.; Benteyn, D.; Goyvaerts, C.; Du Four, S.; Van Der Jeught, K.; Bialkowski, L.; et al. Intratumoral Delivery of TriMix mRNA Results in T-cell Activation by Cross-Presenting Dendritic Cells. Cancer Immunol. Res. 2016, 4, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Burden, D.W. Guide to the disruption of biological samples. Random Primers 2012, 12, 1–25. [Google Scholar]
- Whiting, J.L. Application Note Magmax FFPE DNA/RNA Ultra Kit; COL31317 1016; Thermo Fisher Scientific: Erembodegem, Belgium, 2016. [Google Scholar]
- Ellis, M.; Davis, N.; Coop, A.; Liu, M.; Schumaker, L.; Lee, R.Y.; Srikanchana, R.; Russell, C.G.; Singh, B.; Miller, W.R.; et al. Development and validation of a method for using breast core needle biopsies for gene expression microarray analyses. Clin. Cancer Res. 2002, 8, 1155–1166. [Google Scholar]
- Hatzis, C.; Sun, H.; Yao, H.; Hubbard, R.E.; Meric-Bernstam, F.; Babiera, G.V.; Wu, Y.; Pusztai, L.; Symmans, W.F. Effects of Tissue Handling on RNA Integrity and Microarray Measurements From Resected Breast Cancers. J. Natl. Cancer Inst. 2011, 103, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Technical Note: RNA sequencing. In Expression Analysis of FFPE Samples; Pub. No. 470-2013-002; Illumina: San Diego, CA, USA, 2014.
- Bruning, O.; Rodenburg, W.; Radonic, T.; Zwinderman, A.H.; De Vries, A.; Breit, T.M.; De Jong, M. RNA isolation for transcriptomics of human and mouse small skin biopsies. BMC Res. Notes 2011, 4, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, D.; Tomo, S.; Modi, A.; Purohit, P.; Sharma, P. Optimising total RNA quality and quantity by phenol-chloroform extraction method from human visceral adipose tissue: A standardisation study. MethodsX 2020, 7, 101113. [Google Scholar] [CrossRef]
- Silk, M.T.; Mikkilineni, N.; Silk, T.C.; Zabor, E.C.; Ostrovnaya, I.; Hakimi, A.A.; Hsieh, J.J.; Ziv, E.; Rekhtman, N.; Solomon, S.B.; et al. Prospective Evaluation of Unprocessed Core Needle Biopsy DNA and RNA Yield from Lung, Liver, and Kidney Tumors: Implications for Cancer Genomics. Anal. Cell. Pathol. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Annaratone, L.; Cascardi, E.; Vissio, E.; Sarotto, I.; Chmielik, E.; Sapino, A.; Berrino, E.; Marchiò, C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology 2020, 87, 125–142. [Google Scholar] [CrossRef]
- Nagarajan, D.; McArdle, S.E.B. Immune Landscape of Breast Cancers. Biomedicines 2018, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Poudel, P.; Nyamundanda, G.; Patil, Y.; Cheang, M.C.U.; Sadanandam, A. Heterocellular gene signatures reveal luminal-A breast cancer heterogeneity and differential therapeutic responses. NPJ Breast Cancer 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghalvad, M.; Mohammadi-Motlagh, H.-R.; Rezaei, N. Immune microenvironment in different molecular subtypes of ductal breast carcinoma. Breast Cancer Res. Treat. 2021, 185, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Di, G. Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chin. J. Cancer Res. 2017, 29, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abramovitz, M.; Ordanic-Kodani, M.; Wang, Y.; Li, Z.; Catzavelos, C.; Bouzyk, M.; Sledge, G.W.; Moreno, C.S.; Leyland-Jones, B. Optimization of RNA extraction from FFPE tissues for expression profiling in the DASL assay. BioTechniques 2008, 44, 417–423. [Google Scholar] [CrossRef]
- Turnbull, A.K.; Selli, C.; Martinez-Perez, C.; Fernando, A.; Renshaw, L.; Keys, J.; Figueroa, J.D.; He, X.; Tanioka, M.; Munro, A.F.; et al. Unlocking the transcriptomic potential of formalin-fixed paraffin embedded clinical tissues: Comparison of gene expression profiling approaches. BMC Bioinform. 2020, 21, 30. [Google Scholar] [CrossRef]
- Veldman-Jones, M.H.; Brant, R.; Rooney, C.; Geh, C.; Emery, H.; Harbron, C.G.; Wappett, M.; Sharpe, A.; Dymond, M.; Barrett, J.C.; et al. Evaluating Robustness and Sensitivity of the NanoString Technologies nCounter Platform to Enable Multiplexed Gene Expression Analysis of Clinical Samples. Cancer Res. 2015, 75, 2587–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locy, H.; Correa, R.J.M.; Autaers, D.; Schiettecatte, A.; Jonckheere, J.; Waelput, W.; Cras, L.; Brock, S.; Verhulst, S.; Kwan, K.; et al. Overcoming the Challenges of High Quality RNA Extraction from Core Needle Biopsy. Biomolecules 2021, 11, 621. https://doi.org/10.3390/biom11050621
Locy H, Correa RJM, Autaers D, Schiettecatte A, Jonckheere J, Waelput W, Cras L, Brock S, Verhulst S, Kwan K, et al. Overcoming the Challenges of High Quality RNA Extraction from Core Needle Biopsy. Biomolecules. 2021; 11(5):621. https://doi.org/10.3390/biom11050621
Chicago/Turabian StyleLocy, Hanne, Rohann J.M. Correa, Dorien Autaers, Ann Schiettecatte, Jan Jonckheere, Wim Waelput, Louise Cras, Stefanie Brock, Stefaan Verhulst, Keith Kwan, and et al. 2021. "Overcoming the Challenges of High Quality RNA Extraction from Core Needle Biopsy" Biomolecules 11, no. 5: 621. https://doi.org/10.3390/biom11050621








