Tight Junction Protein Claudin-12 Is Involved in Cell Migration during Metastasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Reagents and Antibodies
2.3. Immunocytochemistry and Western Blotting
2.4. Cell Migration Assays
2.5. Cell Proliferation Assay
2.6. Flow Cytometric (FACS) Analysis
2.7. Cell Migration Assay with Competitive Inhibition
2.8. Statistical Analysis
3. Results
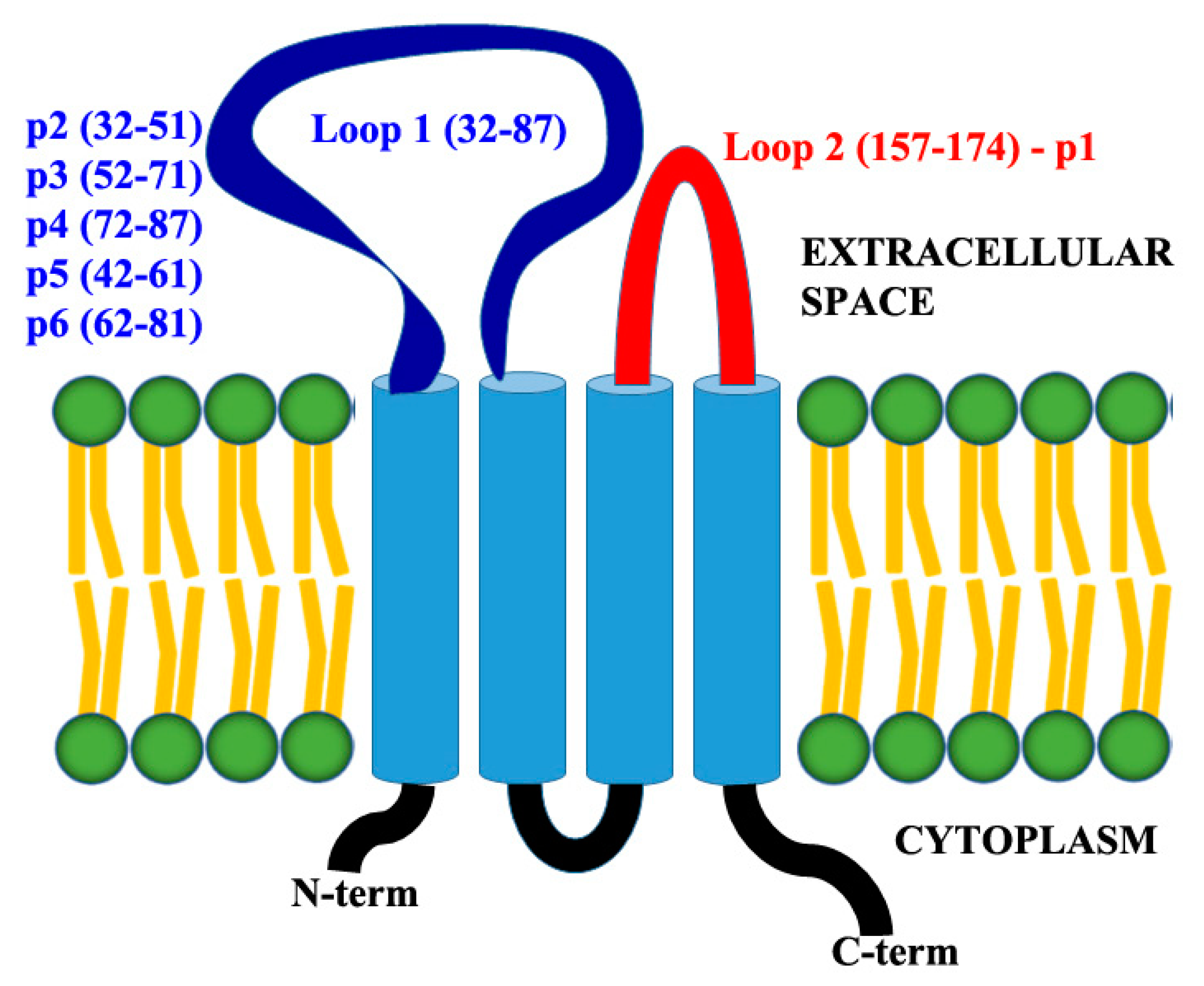
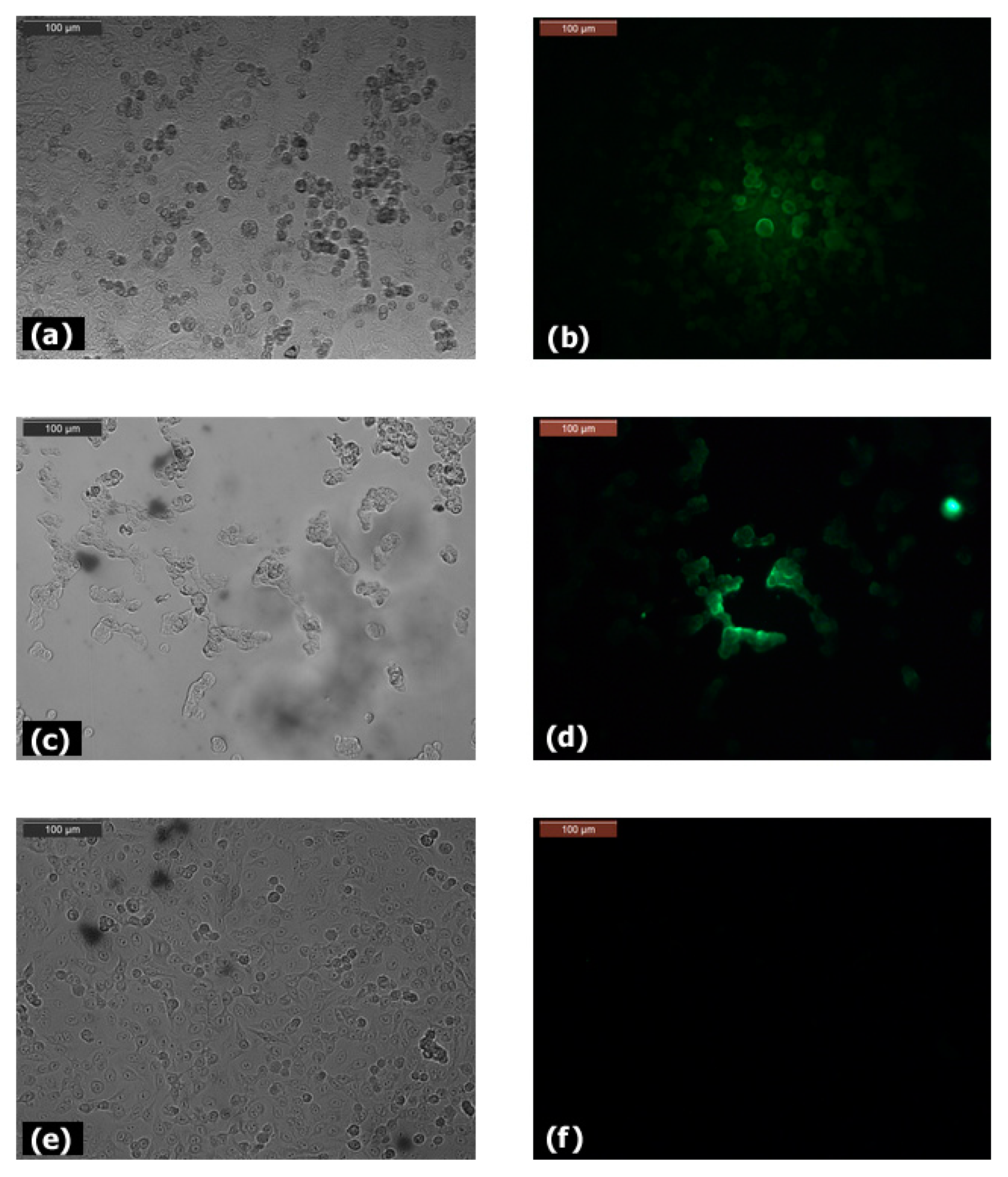
3.1. Expression of Claudin-12
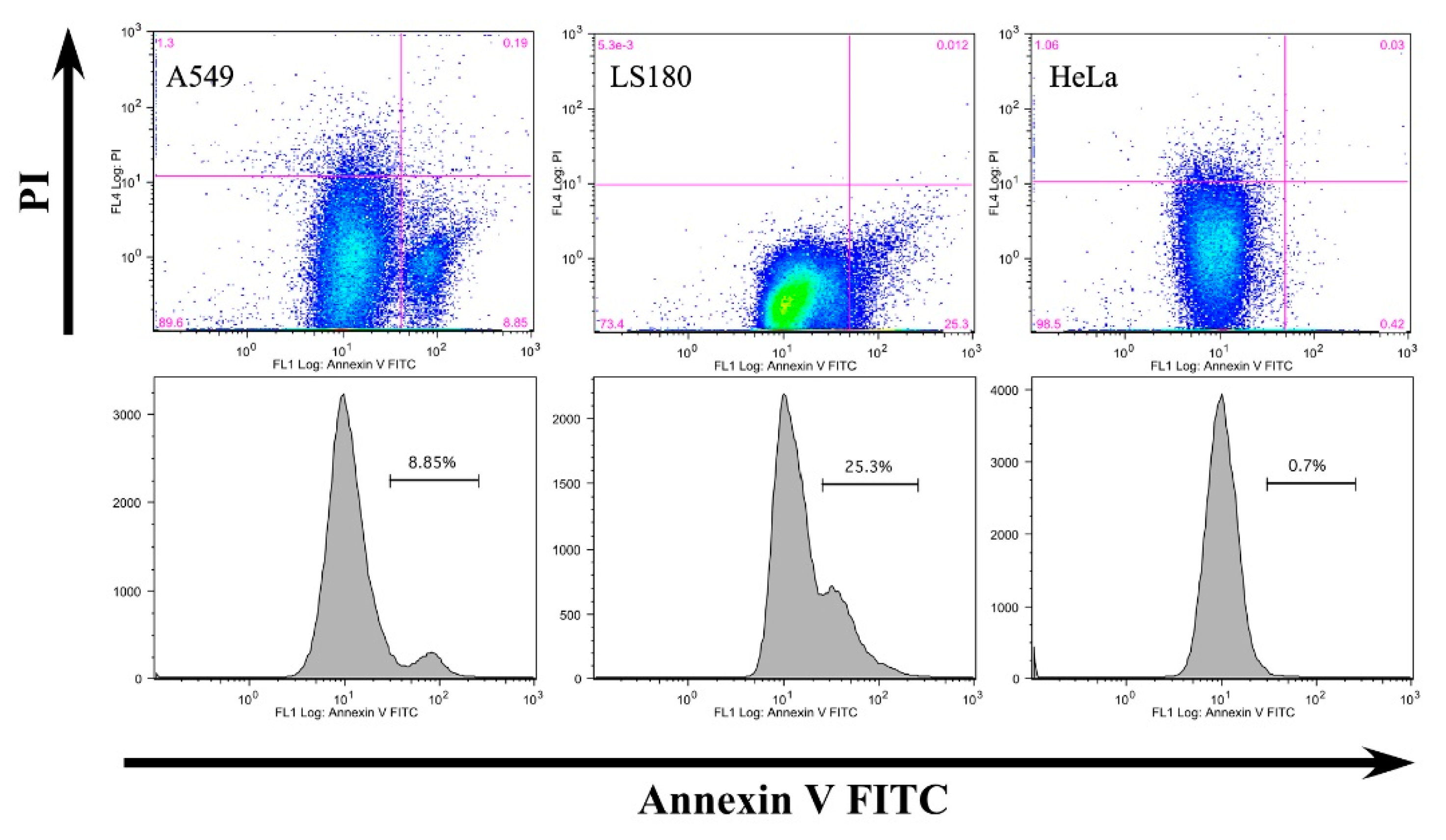
3.2. Anti-Claudin-12 Antibodies Suppress the Migration and Proliferation of Claudin-12 Expressing Cancer Cells, Inducing Apoptosis
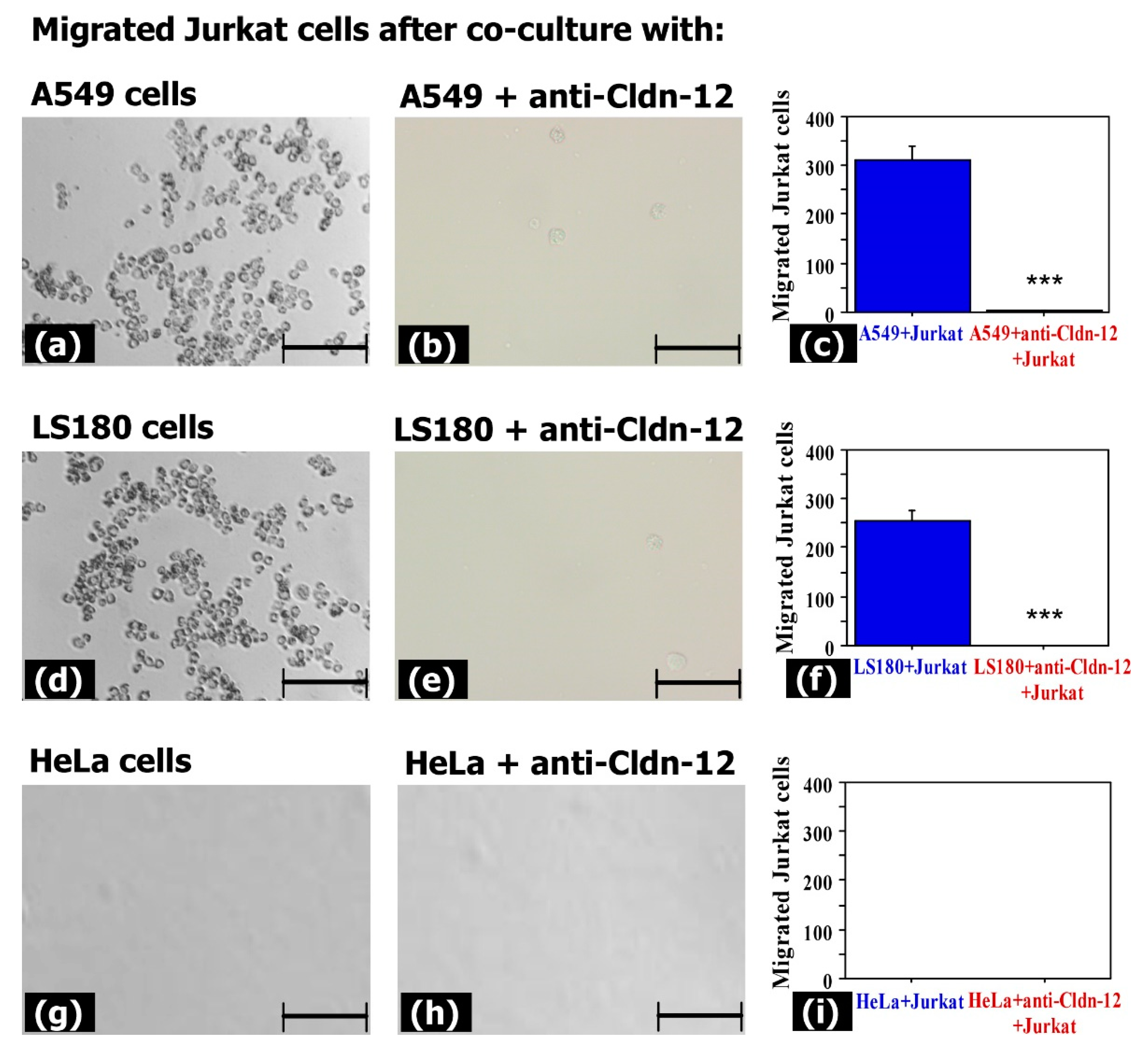
3.3. Claudin-12 Is Involved in the Migration of Jurkat Cells through the Tight Junctions
3.4. Migrating Jurkat Cells Express Lymphocyte Function-Associated Antigen-1 (LFA-1 Integrin) and L-Selectin (CD62L)
3.5. Claudin-12 Peptides Can Block the Migration of Jurkat Cells Through the Tight Junctions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta 2008, 1778, 631–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal-Nag, M.; Morin, P.J. The claudins. Genome Biol. 2009, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, M.J. Emerging roles of claudins in human cancer. Int. J. Mol. Sci. 2013, 14, 18148–18180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niessen, C.M. Tight Junctions/Adherens Junctions: Basic Structure and Function. J. Investig. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Aijaz, S.; Tsapara, A.; Balda, M.S. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell Biol. 2005, 17, 453–458. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Fujita, H.; Chiba, H.; Yokozaki, H.; Sakai, N.; Sugimoto, K.; Wada, T.; Kojima, T.; Yamashita, T.; Sawada, N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2006, 54, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [Green Version]
- Kitajiri, S.I.; Furuse, M.; Morita, K.; Saishin-Kiuchi, Y.; Kido, H.; Ito, J.; Tsukita, S. Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear. Res. 2004, 187, 25–34. [Google Scholar] [CrossRef]
- Castro Dias, M.; Coisne, C.; Baden, P.; Enzmann, G.; Garrett, L.; Becker, L.; Holter, S.M.; Hrabe de Angelis, M.; Deutsch, U.; Engelhardt, B. Claudin-12 is not required for blood-brain barrier tight junction function. Fluids Barriers CNS 2019, 16, 30. [Google Scholar] [CrossRef]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 2008, 19, 1912–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osanai, M.; Takasawa, A.; Murata, M.; Sawada, N. Claudins in cancer: Bench to bedside. Pflug. Arch. Eur. J. Physiol. 2017, 469, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; He, Y.; Han, Z.; Su, H.; Chu, C. The Cytoplasmic Expression of CLDN12 Predicts an Unfavorable Prognosis and Promotes Proliferation and Migration of Osteosarcoma. Cancer Manag. Res. 2019, 11, 9339–9351. [Google Scholar] [CrossRef] [Green Version]
- Morin, P.J. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005, 65, 9603–9606. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.B.; Sharma, A.; Dhawan, P. Claudin family of proteins and cancer: An overview. J. Oncol. 2010, 2010, 541957. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Feng, L.; Cui, J. Increased expression of claudin-12 promotes the metastatic phenotype of human bronchial epithelial cells and is associated with poor prognosis in lung squamous cell carcinoma. Exp. Ther. Med. 2019, 17, 165–174. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Ouban, A.; Ahmed, A.A. Claudins in human cancer: A review. Histol. Histopathol. 2010, 25, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Usami, Y.; Chiba, H.; Nakayama, F.; Ueda, J.; Matsuda, Y.; Sawada, N.; Komori, T.; Ito, A.; Yokozaki, H. Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Human Pathol. 2006, 37, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Rangel, L.B.; Agarwal, R.; D’Souza, T.; Pizer, E.S.; Alo, P.L.; Lancaster, W.D.; Gregoire, L.; Schwartz, D.R.; Cho, K.R.; Morin, P.J. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 2567–2575. [Google Scholar]
- Lee, J.W.; Lee, S.J.; Seo, J.; Song, S.Y.; Ahn, G.; Park, C.S.; Lee, J.H.; Kim, B.G.; Bae, D.S. Increased expressions of claudin-1 and claudin-7 during the progression of cervical neoplasia. Gynecol. Oncol. 2005, 97, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; D’Souza, T.; Morin, P.J. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005, 65, 7378–7385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Feng, L.; Cui, J. Increased expression of claudin-17 promotes a malignant phenotype in hepatocyte via Tyk2/Stat3 signaling and is associated with poor prognosis in patients with hepatocellular carcinoma. Diagn. Pathol. 2018, 13, 72. [Google Scholar] [CrossRef]
- Yamada, G.; Murata, M.; Takasawa, A.; Nojima, M.; Mori, Y.; Sawada, N.; Takahashi, H. Increased expressions of claudin 4 and 7 in atypical adenomatous hyperplasia and adenocarcinoma of the lung. Med. Mol. Morphol. 2016, 49, 163–169. [Google Scholar] [CrossRef]
- Che, J.; Yang, Y.; Xiao, J.; Zhao, P.; Yan, B.; Dong, S.; Cao, B. Decreased expression of claudin-3 is associated with a poor prognosis and EMT in completely resected squamous cell lung carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 6559–6568. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kusumi, T.; Sato, F.; Kawasaki, H.; Shibata, S.; Ohashi, M.; Hakamada, K.; Sasaki, M.; Kijima, H. Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed. Res. 2008, 29, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Morohashi, S.; Kusumi, T.; Sato, F.; Odagiri, H.; Chiba, H.; Yoshihara, S.; Hakamada, K.; Sasaki, M.; Kijima, H. Decreased expression of claudin-1 correlates with recurrence status in breast cancer. Int. J. Mol. Med. 2007, 20, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, B.L.; Ward, C.; Smith, T.; Ritzenthaler, J.D.; Koval, M. Regulation of heterotypic claudin compatibility. J. Biol. Chem. 2007, 282, 30005–30013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunniffe, C.; Brankin, B.; Lambkin, H.; Ryan, F. The role of claudin-1 and claudin-7 in cervical tumorigenesis. Anticancer Res. 2014, 34, 2851–2857. [Google Scholar]
- Sigal, A.; Bleijs, D.A.; Grabovsky, V.; van Vliet, S.J.; Dwir, O.; Figdor, C.G.; van Kooyk, Y.; Alon, R. The LFA-1 Integrin Supports Rolling Adhesions on ICAM-1 Under Physiological Shear Flow in a Permissive Cellular Environment. J. Immunol. 2000, 165, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walling, B.L.; Kim, M. LFA-1 in T Cell Migration and Differentiation. Front. Immunol. 2018, 9, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grailer, J.J.; Kodera, M.; Steeber, D.A. L-selectin: Role in regulating homeostasis and cutaneous inflammation. J. Dermatol. Sci. 2009, 56, 141–147. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolchakova, D.; Moten, D.; Batsalova, T.; Dzhambazov, B. Tight Junction Protein Claudin-12 Is Involved in Cell Migration during Metastasis. Biomolecules 2021, 11, 636. https://doi.org/10.3390/biom11050636
Kolchakova D, Moten D, Batsalova T, Dzhambazov B. Tight Junction Protein Claudin-12 Is Involved in Cell Migration during Metastasis. Biomolecules. 2021; 11(5):636. https://doi.org/10.3390/biom11050636
Chicago/Turabian StyleKolchakova, Desislava, Dzhemal Moten, Tsvetelina Batsalova, and Balik Dzhambazov. 2021. "Tight Junction Protein Claudin-12 Is Involved in Cell Migration during Metastasis" Biomolecules 11, no. 5: 636. https://doi.org/10.3390/biom11050636








