lncRNA PVT1 in the Pathogenesis and Clinical Management of Renal Cell Carcinoma
Abstract
:1. Introduction
2. General Characterization of Long Non-Coding RNAs
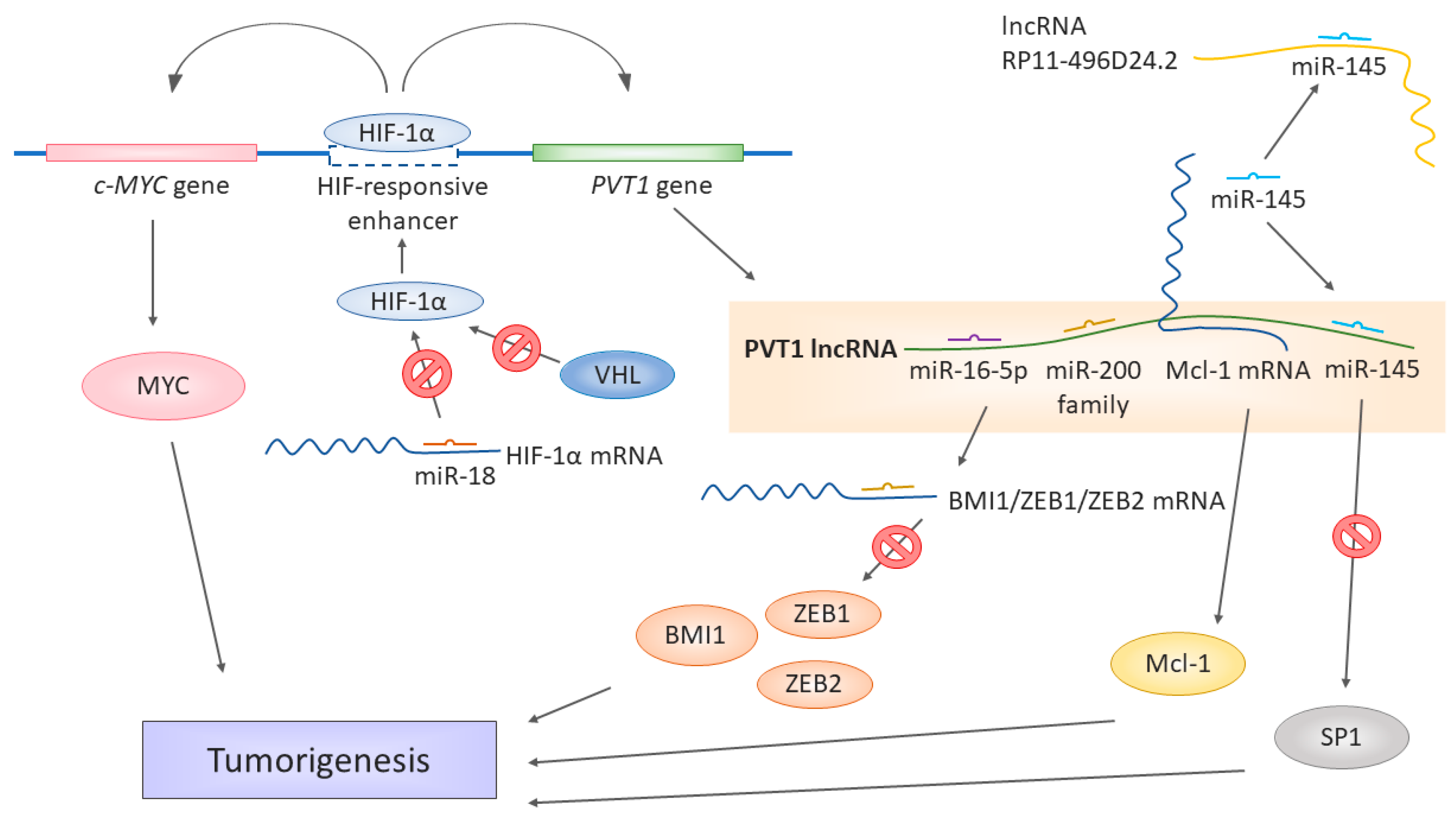
3. Role of PVT1 in Development of Renal Cell Carcinoma
4. PVT1 as a Diagnostic and Prognostic Biomarker
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Athanazio, D.A.; Amorim, L.S.; da Cunha, I.W.; Leite, K.R.M.; da Paz, A.R.; de Paula Xavier Gomes, R.; Tavora, F.R.F.; Faraj, S.F.; Cavalcanti, M.S.; Bezerra, S.M. Classification of Renal Cell Tumors–Current Concepts and Use of Ancillary Tests: Recommendations of the Brazilian Society of Pathology. Surg. Exp. Pathol. 2021, 4, 4. [Google Scholar] [CrossRef]
- Warren, A.Y.; Harrison, D. WHO/ISUP Classification, Grading and Pathological Staging of Renal Cell Carcinoma: Standards and Controversies. World J. Urol. 2018, 36, 1913–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A. ESMO Guidelines Committee Renal Cell Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Beltran, A.; Henriques, V.; Cimadamore, A.; Santoni, M.; Cheng, L.; Gevaert, T.; Blanca, A.; Massari, F.; Scarpelli, M.; Montironi, R. The Identification of Immunological Biomarkers in Kidney Cancers. Front. Oncol. 2018, 8, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabandziev, P.; Kakisaka, T.; Bohosova, J.; Pinkasova, T.; Kunovsky, L.; Slaby, O.; Goel, A. MicroRNAs in Colon Tissue of Pediatric Ulcerative Pancolitis Patients Allow Detection and Prognostic Stratification. J. Clin. Med. 2021, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Brito, G.C.; Fachel, A.A.; Vettore, A.L.; Vignal, G.M.; Gimba, E.R.P.; Campos, F.S.; Barcinski, M.A.; Verjovski-Almeida, S.; Reis, E.M. Identification of Protein-Coding and Intronic Noncoding RNAs down-Regulated in Clear Cell Renal Carcinoma. Mol. Carcinog. 2008, 47, 757–767. [Google Scholar] [CrossRef]
- Yu, G.; Yao, W.; Wang, J.; Ma, X.; Xiao, W.; Li, H.; Xia, D.; Yang, Y.; Deng, K.; Xiao, H.; et al. LncRNAs Expression Signatures of Renal Clear Cell Carcinoma Revealed by Microarray. PLoS ONE 2012, 7, e42377. [Google Scholar] [CrossRef] [Green Version]
- Zou, B.; Wang, D.; Xu, K.; Liu, J.; Yuan, D.; Meng, Z.; Zhang, B. Prognostic Value of Long Non-Coding RNA Plasmacytoma Variant Translocation1 in Human Solid Tumors. Medicine 2019, 98, e16087. [Google Scholar] [CrossRef]
- International Human Genome Sequencing Consortium Finishing the Euchromatic Sequence of the Human Genome. Nature 2004, 431, 931–945. [CrossRef] [PubMed]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The Reference Human Genome Annotation for The ENCODE Project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of Transcription in Human Cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frith, M.C.; Pheasant, M.; Mattick, J.S. The Amazing Complexity of the Human Transcriptome. Eur. J. Hum. Genet. 2005, 13, 894–897. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, L.; Guo, J.; Niu, Y.; Wu, Y.; Li, H.; Zhao, L.; Li, X.; Teng, X.; Sun, X.; et al. NONCODEV5: A Comprehensive Annotation Database for Long Non-Coding RNAs. Nucleic Acids Res. 2018, 46, D308–D314. [Google Scholar] [CrossRef]
- Ravasi, T.; Suzuki, H.; Pang, K.C.; Katayama, S.; Furuno, M.; Okunishi, R.; Fukuda, S.; Ru, K.; Frith, M.C.; Gongora, M.M.; et al. Experimental Validation of the Regulated Expression of Large Numbers of Non-Coding RNAs from the Mouse Genome. Genome Res. 2006, 16, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Mercer, T.R.; Dinger, M.E.; Sunkin, S.M.; Mehler, M.F.; Mattick, J.S. Specific Expression of Long Noncoding RNAs in the Mouse Brain. Proc. Natl. Acad. Sci. USA 2008, 105, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Cabili, M.N.; Trapnell, C.; Goff, L.; Koziol, M.; Tazon-Vega, B.; Regev, A.; Rinn, J.L. Integrative Annotation of Human Large Intergenic Noncoding RNAs Reveals Global Properties and Specific Subclasses. Genes Dev. 2011, 25, 1915–1927. [Google Scholar] [CrossRef] [Green Version]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 Catalog of Human Long Noncoding RNAs: Analysis of Their Gene Structure, Evolution, and Expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Bánfai, B.; Jia, H.; Khatun, J.; Wood, E.; Risk, B.; Gundling, W.E.; Kundaje, A.; Gunawardena, H.P.; Yu, Y.; Xie, L.; et al. Long Noncoding RNAs Are Rarely Translated in Two Human Cell Lines. Genome Res. 2012, 22, 1646–1657. [Google Scholar] [CrossRef] [Green Version]
- Chooniedass-Kothari, S.; Emberley, E.; Hamedani, M.K.; Troup, S.; Wang, X.; Czosnek, A.; Hube, F.; Mutawe, M.; Watson, P.H.; Leygue, E. The Steroid Receptor RNA Activator Is the First Functional RNA Encoding a Protein. FEBS Lett. 2004, 566, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Plaza, S.; Zanet, J.; Benrabah, E.; Valenti, P.; Hashimoto, Y.; Kobayashi, S.; Payre, F.; Kageyama, Y. Small Peptides Switch the Transcriptional Activity of Shavenbaby during Drosophila Embryogenesis. Science 2010, 329, 336–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin Signature Reveals over a Thousand Highly Conserved Large Non-Coding RNAs in Mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef]
- Yang, L.; Duff, M.O.; Graveley, B.R.; Carmichael, G.G.; Chen, L.-L. Genomewide Characterization of Non-Polyadenylated RNAs. Genome Biol. 2011, 12, R16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Yang, L.; Chen, L.-L. The Diversity of Long Noncoding RNAs and Their Generation. Trends Genet. 2017, 33, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Zheng, H.; Li, J.; Liu, D.; Li, H.; Samudrala, R.; Yu, J.; Wong, G.K.-S. Mouse Transcriptome: Neutral Evolution of “non-Coding” Complementary DNAs. Nature 2004, 14, 431. [Google Scholar]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long Non-Coding RNAs: Insights into Functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Carninci, P.; Kasukawa, T.; Katayama, S.; Gough, J.; Frith, M.C.; Maeda, N.; Oyama, R.; Ravasi, T.; Lenhard, B.; Wells, C.; et al. The Transcriptional Landscape of the Mammalian Genome. Science 2005, 309, 1559–1563. [Google Scholar] [CrossRef] [Green Version]
- Ponjavic, J.; Ponting, C.P.; Lunter, G. Functionality or Transcriptional Noise? Evidence for Selection within Long Noncoding RNAs. Genome Res. 2007, 17, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.C.; Ponting, C.P. Catalogues of Mammalian Long Noncoding RNAs: Modest Conservation and Incompleteness. Genome Biol. 2009, 10, R124. [Google Scholar] [CrossRef] [Green Version]
- Ørom, U.A.; Derrien, T.; Beringer, M.; Gumireddy, K.; Gardini, A.; Bussotti, G.; Lai, F.; Zytnicki, M.; Notredame, C.; Huang, Q.; et al. Long Noncoding RNAs with Enhancer-like Function in Human Cells. Cell 2010, 143, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2020, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.C.; Oliveira, L.C.; Mathias, C.; Pedroso, G.A.; Lemos, D.S.; Salviano-Silva, A.; Jucoski, T.S.; Lobo-Alves, S.C.; Zambalde, E.P.; Cipolla, G.A.; et al. Long Non-Coding RNAs in Cancer: Another Layer of Complexity. J. Gene Med. 2019, 21, e3065. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-L. Linking Long Noncoding RNA Localization and Function. Trends Biochem. Sci. 2016, 41, 761–772. [Google Scholar] [CrossRef]
- Schmitz, K.-M.; Mayer, C.; Postepska, A.; Grummt, I. Interaction of Noncoding RNA with the RDNA Promoter Mediates Recruitment of DNMT3b and Silencing of RRNA Genes. Genes Dev. 2010, 24, 2264–2269. [Google Scholar] [CrossRef] [Green Version]
- Di Ruscio, A.; Ebralidze, A.K.; Benoukraf, T.; Amabile, G.; Goff, L.A.; Terragni, J.; Figueroa, M.E.; De Figueiredo Pontes, L.L.; Alberich-Jorda, M.; Zhang, P.; et al. DNMT1-Interacting RNAs Block Gene-Specific DNA Methylation. Nature 2013, 503, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.M.; Guttman, M.; Huarte, M.; Garber, M.; Raj, A.; Rivea Morales, D.; Thomas, K.; Presser, A.; Bernstein, B.E.; van Oudenaarden, A.; et al. Many Human Large Intergenic Noncoding RNAs Associate with Chromatin-Modifying Complexes and Affect Gene Expression. Proc. Natl. Acad. Sci. USA 2009, 106, 11667–11672. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Hao, Q.; Prasanth, K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. 2018, 34, 142–157. [Google Scholar] [CrossRef]
- Lam, M.T.Y.; Li, W.; Rosenfeld, M.G.; Glass, C.K. Enhancer RNAs and Regulated Transcriptional Programs. Trends Biochem. Sci. 2014, 39, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Bi, C.; Clark, B.S.; Mady, R.; Shah, P.; Kohtz, J.D. The Evf-2 Noncoding RNA Is Transcribed from the Dlx-5/6 Ultraconserved Region and Functions as a Dlx-2 Transcriptional Coactivator. Genes Dev. 2006, 20, 1470–1484. [Google Scholar] [CrossRef] [Green Version]
- Shamovsky, I.; Ivannikov, M.; Kandel, E.S.; Gershon, D.; Nudler, E. RNA-Mediated Response to Heat Shock in Mammalian Cells. Nature 2006, 440, 556–560. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Jin, C.; Yang, J.C.; Tanasa, B.; Li, W.; Merkurjev, D.; Ohgi, K.A.; Meng, D.; Zhang, J.; et al. LncRNA-Dependent Mechanisms of Androgen-Receptor-Regulated Gene Activation Programs. Nature 2013, 500, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Lai, F.; Orom, U.A.; Cesaroni, M.; Beringer, M.; Taatjes, D.J.; Blobel, G.A.; Shiekhattar, R. Activating RNAs Associate with Mediator to Enhance Chromatin Architecture and Transcription. Nature 2013, 494, 497–501. [Google Scholar] [CrossRef]
- Tian, D.; Sun, S.; Lee, J.T. The Long Noncoding RNA, Jpx, Is a Molecular Switch for X Chromosome Inactivation. Cell 2010, 143, 390–403. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Del Rosario, B.C.; Szanto, A.; Ogawa, Y.; Jeon, Y.; Lee, J.T. Jpx RNA Activates Xist by Evicting CTCF. Cell 2013, 153, 1537–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Arai, S.; Song, X.; Reichart, D.; Du, K.; Pascual, G.; Tempst, P.; Rosenfeld, M.G.; Glass, C.K.; Kurokawa, R. Induced NcRNAs Allosterically Modify RNA-Binding Proteins in Cis to Inhibit Transcription. Nature 2008, 454, 126–130. [Google Scholar] [CrossRef]
- Martianov, I.; Ramadass, A.; Serra Barros, A.; Chow, N.; Akoulitchev, A. Repression of the Human Dihydrofolate Reductase Gene by a Non-Coding Interfering Transcript. Nature 2007, 445, 666–670. [Google Scholar] [CrossRef]
- Ramos, A.D.; Andersen, R.E.; Liu, S.J.; Nowakowski, T.J.; Hong, S.J.; Gertz, C.; Salinas, R.D.; Zarabi, H.; Kriegstein, A.R.; Lim, D.A. The Long Noncoding RNA Pnky Regulates Neuronal Differentiation of Embryonic and Postnatal Neural Stem Cells. Cell Stem Cell 2015, 16, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, I.; Munita, R.; Agirre, E.; Dittmer, T.A.; Gysling, K.; Misteli, T.; Luco, R.F. A LncRNA Regulates Alternative Splicing via Establishment of a Splicing-Specific Chromatin Signature. Nat. Struct. Mol. Biol. 2015, 22, 370–376. [Google Scholar] [CrossRef]
- Gong, C.; Maquat, L.E. LncRNAs Transactivate STAU1-Mediated MRNA Decay by Duplexing with 3’ UTRs via Alu Elements. Nature 2011, 470, 284–288. [Google Scholar] [CrossRef] [Green Version]
- Damas, N.D.; Marcatti, M.; Côme, C.; Christensen, L.L.; Nielsen, M.M.; Baumgartner, R.; Gylling, H.M.; Maglieri, G.; Rundsten, C.F.; Seemann, S.E.; et al. SNHG5 Promotes Colorectal Cancer Cell Survival by Counteracting STAU1-Mediated MRNA Destabilization. Nat. Commun. 2016, 7, 13875. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Lou, Z.; Gupta, M. The Long Non-Coding RNA GAS5 Cooperates with the Eukaryotic Translation Initiation Factor 4E to Regulate c-Myc Translation. PLoS ONE 2014, 9, e107016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrieri, C.; Cimatti, L.; Biagioli, M.; Beugnet, A.; Zucchelli, S.; Fedele, S.; Pesce, E.; Ferrer, I.; Collavin, L.; Santoro, C.; et al. Long Non-Coding Antisense RNA Controls Uchl1 Translation through an Embedded SINEB2 Repeat. Nature 2012, 491, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A CeRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA Gas5 Is a Growth Arrest- and Starvation-Associated Repressor of the Glucocorticoid Receptor. Sci. Signal. 2010, 3, ra8. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kopp, F.; Chang, T.-C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.-H.; Abdelmohsen, K.; Kim, J.; Yang, X.; Martindale, J.L.; Tominaga-Yamanaka, K.; White, E.J.; Orjalo, A.V.; Rinn, J.L.; Kreft, S.G.; et al. Scaffold Function of Long Non-Coding RNA HOTAIR in Protein Ubiquitination. Nat. Commun. 2013, 4, 2939. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.; Mei, Y.; Wu, M. Reciprocal Regulation of HIF-1α and LincRNA-P21 Modulates the Warburg Effect. Mol. Cell 2014, 53, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Willingham, A.T.; Orth, A.P.; Batalov, S.; Peters, E.C.; Wen, B.G.; Aza-Blanc, P.; Hogenesch, J.B.; Schultz, P.G. A Strategy for Probing the Function of Noncoding RNAs Finds a Repressor of NFAT. Science 2005, 309, 1570–1573. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, K.M.; Abdelmohsen, K.; Yoon, J.-H.; Panda, A.C.; Munk, R.; Kim, J.; Curtis, J.; Moad, C.A.; Wohler, C.M.; et al. HuR and GRSF1 Modulate the Nuclear Export and Mitochondrial Localization of the LncRNA RMRP. Genes Dev. 2016, 30, 1224–1239. [Google Scholar] [CrossRef] [Green Version]
- Vučićević, D.; Schrewe, H.; Orom, U.A. Molecular Mechanisms of Long NcRNAs in Neurological Disorders. Front. Genet. 2014, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faghihi, M.A.; Modarresi, F.; Khalil, A.M.; Wood, D.E.; Sahagan, B.G.; Morgan, T.E.; Finch, C.E.; St Laurent, G.; Kenny, P.J.; Wahlestedt, C. Expression of a Noncoding RNA Is Elevated in Alzheimer’s Disease and Drives Rapid Feed-Forward Regulation of Beta-Secretase. Nat. Med. 2008, 14, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Goyal, N.; Kesharwani, D.; Datta, M. Lnc-Ing Non-Coding RNAs with Metabolism and Diabetes: Roles of LncRNAs. Cell. Mol. Life Sci. 2018, 75, 1827–1837. [Google Scholar] [CrossRef]
- Xu, F.; Jin, L.; Jin, Y.; Nie, Z.; Zheng, H. Long Noncoding RNAs in Autoimmune Diseases. J. Biomed. Mater. Res. A 2019, 107, 468–475. [Google Scholar] [CrossRef]
- Jabandziev, P.; Bohosova, J.; Pinkasova, T.; Kunovsky, L.; Slaby, O.; Goel, A. The Emerging Role of Noncoding RNAs in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 985–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennessy, E.J. Cardiovascular Disease and Long Noncoding RNAs: Tools for Unraveling the Mystery Lnc-Ing RNA and Phenotype. Circ. Cardiovasc. Genet. 2017, 10, e001556. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Gutschner, T.; Diederichs, S. The Hallmarks of Cancer: A Long Non-Coding RNA Point of View. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Zhou, H.; Liu, P.; Yan, L.; Yao, W.; Chen, K.; Zeng, J.; Li, H.; Hu, J.; Xu, H.; et al. LncRNA PVT1 and Its Splicing Variant Function as Competing Endogenous RNA to Regulate Clear Cell Renal Cell Carcinoma Progression. Oncotarget 2017, 8, 85353–85367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Luo, P.; Wang, Q.; Ye, Y.; Wang, B. LncRNA PVT1 in Cancer: A Review and Meta-Analysis. Clin. Chim Acta 2017, 474, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Z.; Chen, H.; Cai, Y.; Xie, W. Knockdown of Long Non-Coding RNA PVT1 Induces Apoptosis and Cell Cycle Arrest in Clear Cell Renal Cell Carcinoma through the Epidermal Growth Factor Receptor Pathway. Oncol. Lett. 2018, 15, 7855–7863. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tan, Y.; Wang, H.; Xu, M.; Xu, L. Long Non-Coding RNA Plasmacytoma Variant Translocation 1 (PVT1) Enhances Proliferation, Migration, and Epithelial-Mesenchymal Transition (EMT) of Pituitary Adenoma Cells by Activating β-Catenin, c-Myc, and Cyclin D1 Expression. Med. Sci. Monit. 2019, 25, 7652–7659. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, W.; Weng, G.; Cui, P.; Liang, H.; Li, Y. LncRNA PVT1 Promotes Proliferation, Invasion and Epithelial-Mesenchymal Transition of Renal Cell Carcinoma Cells through Downregulation of MiR-16-5p. OncoTargets Ther. 2019, 12, 2563–2575. [Google Scholar] [CrossRef]
- Zeidler, R.; Joos, S.; Delecluse, H.J.; Klobeck, G.; Vuillaume, M.; Lenoir, G.M.; Bornkamm, G.W.; Lipp, M. Breakpoints of Burkitt’s Lymphoma t(8;22) Translocations Map within a Distance of 300 Kb Downstream of MYC. Genes Chromosomes Cancer 1994, 9, 282–287. [Google Scholar] [CrossRef]
- Jin, K.; Wang, S.; Zhang, Y.; Xia, M.; Mo, Y.; Li, X.; Li, G.; Zeng, Z.; Xiong, W.; He, Y. Long Non-Coding RNA PVT1 Interacts with MYC and Its Downstream Molecules to Synergistically Promote Tumorigenesis. Cell Mol. Life Sci. 2019, 76, 4275–4289. [Google Scholar] [CrossRef] [Green Version]
- Perciavalle, R.M.; Opferman, J.T. Delving Deeper: MCL-1’s Contributions to Normal and Cancer Biology. Trends Cell Biol. 2013, 23, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Yang, F.; Yang, Z.; Fang, Z.; Fu, W.; Chen, W.; Liu, X.; Zhao, J.; Wang, Q.; Hu, X.; et al. Long Noncoding RNA PVT1 Inhibits Renal Cancer Cell Apoptosis by Up-Regulating Mcl-1. Oncotarget 2017, 8, 101865–101875. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.-Y.; Xu, Z.-H.; Chen, Y.-L.; Lu, Z.-Y.; Shen, D.-Y.; Lu, J.-Y.; Zheng, Q.-M.; Wang, L.-Y.; Xu, L.-W.; et al. The Prognostic Value of MiRNA-18a-5p in Clear Cell Renal Cell Carcinoma and Its Function via the MiRNA-18a-5p/HIF1A/PVT1 Pathway. J. Cancer 2020, 11, 2737–2748. [Google Scholar] [CrossRef]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L. Identification of the von Hippel-Lindau Disease Tumor Suppressor Gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef]
- Gnarra, J.R.; Tory, K.; Weng, Y.; Schmidt, L.; Wei, M.H.; Li, H.; Latif, F.; Liu, S.; Chen, F.; Duh, F.M. Mutations of the VHL Tumour Suppressor Gene in Renal Carcinoma. Nat. Genet. 1994, 7, 85–90. [Google Scholar] [CrossRef]
- Shuin, T.; Kondo, K.; Torigoe, S.; Kishida, T.; Kubota, Y.; Hosaka, M.; Nagashima, Y.; Kitamura, H.; Latif, F.; Zbar, B. Frequent Somatic Mutations and Loss of Heterozygosity of the von Hippel-Lindau Tumor Suppressor Gene in Primary Human Renal Cell Carcinomas. Cancer Res. 1994, 54, 2852–2855. [Google Scholar]
- Herman, J.G.; Latif, F.; Weng, Y.; Lerman, M.I.; Zbar, B.; Liu, S.; Samid, D.; Duan, D.S.; Gnarra, J.R.; Linehan, W.M. Silencing of the VHL Tumor-Suppressor Gene by DNA Methylation in Renal Carcinoma. Proc. Natl. Acad. Sci. USA 1994, 91, 9700–9704. [Google Scholar] [CrossRef] [Green Version]
- Kaelin, W.G. Molecular Basis of the VHL Hereditary Cancer Syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef]
- Kaelin, W.G. Von Hippel-Lindau Disease. Annu. Rev. Pathol. 2007, 2, 145–173. [Google Scholar] [CrossRef]
- Grampp, S.; Platt, J.L.; Lauer, V.; Salama, R.; Kranz, F.; Neumann, V.K.; Wach, S.; Stöhr, C.; Hartmann, A.; Eckardt, K.-U.; et al. Genetic Variation at the 8q24.21 Renal Cancer Susceptibility Locus Affects HIF Binding to a MYC Enhancer. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Schödel, J.; Bardella, C.; Sciesielski, L.K.; Brown, J.M.; Pugh, C.W.; Buckle, V.; Tomlinson, I.P.; Ratcliffe, P.J.; Mole, D.R. Common Genetic Variants at the 11q13.3 Renal Cancer Susceptibility Locus Influence Binding of HIF to an Enhancer of Cyclin D1 Expression. Nat. Genet. 2012, 44, 420–425. [Google Scholar] [CrossRef]
- Vaishnave, S. BMI1 and PTEN Are Key Determinants of Breast Cancer Therapy: A Plausible Therapeutic Target in Breast Cancer. Gene 2018, 678, 302–311. [Google Scholar] [CrossRef]
- Caramel, J.; Ligier, M.; Puisieux, A. Pleiotropic Roles for ZEB1 in Cancer. Cancer Res. 2018, 78, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Wang, W.; Lin, X.; Zheng, X.; Yang, A. Roles of ZEB2 and RBM38 in Liver Cancer Stem Cell Proliferation. J. BUON 2020, 25, 1390–1394. [Google Scholar]
- Wu, H.; Wei, M.; Jiang, X.; Tan, J.; Xu, W.; Fan, X.; Zhang, R.; Ding, C.; Zhao, F.; Shao, X.; et al. LncRNA PVT1 Promotes Tumorigenesis of Colorectal Cancer by Stabilizing MiR-16-5p and Interacting with the VEGFA/VEGFR1/AKT Axis. Mol. Ther. Nucleic Acids 2020, 20, 438–450. [Google Scholar] [CrossRef]
- Huang, C.; Yuan, N.; Wu, L.; Wang, X.; Dai, J.; Song, P.; Li, F.; Xu, C.; Zhao, X. An Integrated Analysis for Long Noncoding RNAs and MicroRNAs with the Mediated Competing Endogenous RNA Network in Papillary Renal Cell Carcinoma. OncoTargets Ther. 2017, 10, 4037–4050. [Google Scholar] [CrossRef] [Green Version]
- Chu, S. Transcriptional Regulation by Post-Transcriptional Modification--Role of Phosphorylation in Sp1 Transcriptional Activity. Gene 2012, 508, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, R.; Wu, Y.; Liu, Y.; Su, W.; Xiong, W.; Zeng, Z. PVT1 Promotes Cancer Progression via MicroRNAs. Front. Oncol. 2019, 9, 609. [Google Scholar] [CrossRef] [Green Version]
- Posa, I.; Carvalho, S.; Tavares, J.; Grosso, A.R. A Pan-Cancer Analysis of MYC-PVT1 Reveals CNV-Unmediated Deregulation and Poor Prognosis in Renal Carcinoma. Oncotarget 2016, 7, 47033–47041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Ye, T.; Yang, X.; Lv, P.; Wu, X.; Zhou, H.; Zeng, J.; Tang, K.; Ye, Z. A Panel of Four-LncRNA Signature as a Potential Biomarker for Predicting Survival in Clear Cell Renal Cell Carcinoma. J. Cancer 2020, 11, 4274–4283. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; He, W.; Gou, X. Construction and Comprehensive Analysis of Dysregulated Long Non-Coding RNA-Associated Competing Endogenous RNA Network in Clear Cell Renal Cell Carcinoma. J. Cell. Biochem. 2018, 120, 2576–2593. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.-Q.; Weng, W.-W.; Zhang, Q.-Y.; Yang, X.-Q.; Gan, H.-L.; Yang, Y.-S.; Zhang, P.-P.; Sun, M.-H.; Xu, M.-D.; et al. A Serum-Circulating Long Noncoding RNA Signature Can Discriminate between Patients with Clear Cell Renal Cell Carcinoma and Healthy Controls. Oncogenesis 2016, 5, e192. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Xu, Y.; Shi, C.; Wang, B.; Yu, X.; Zou, Y.; Hu, T. A Genome-Wide Comprehensively Analyses of Long Noncoding RNA Profiling and Metastasis Associated LncRNAs in Renal Cell Carcinoma. Oncotarget 2017, 8, 87773–87781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, X.; Duan, J.; Yan, Y.; Ma, X.; Zhang, Y.; Wang, H.; Ni, D.; Wu, S.; Peng, C.; Fan, Y.; et al. Upregulation of Long Noncoding RNA PVT1 Predicts Unfavorable Prognosis in Patients with Clear Cell Renal Cell Carcinoma. Cancer Biomark. 2017, 21, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fachel, A.A.; Tahira, A.C.; Vilella-Arias, S.A.; Maracaja-Coutinho, V.; Gimba, E.R.P.; Vignal, G.M.; Campos, F.S.; Reis, E.M.; Verjovski-Almeida, S. Expression Analysis and in Silico Characterization of Intronic Long Noncoding RNAs in Renal Cell Carcinoma: Emerging Functional Associations. Mol. Cancer 2013, 12, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogure, T.; Yan, I.K.; Lin, W.-L.; Patel, T. Extracellular Vesicle–Mediated Transfer of a Novel Long Noncoding RNA TUC339. Genes Cancer 2013, 4, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Hasselmann, D.O.; Rappl, G.; Tilgen, W.; Reinhold, U. Extracellular Tyrosinase MRNA within Apoptotic Bodies Is Protected from Degradation in Human Serum. Clin. Chem. 2001, 47, 1488–1489. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [Green Version]
- Batagov, A.O.; Kuznetsov, V.A.; Kurochkin, I.V. Identification of Nucleotide Patterns Enriched in Secreted RNAs as Putative Cis-Acting Elements Targeting Them to Exosome Nano-Vesicles. BMC Genom. 2011, 12 (Suppl. 3), S18. [Google Scholar] [CrossRef] [Green Version]
- Irion, U.; St Johnston, D. Bicoid RNA Localization Requires Specific Binding of an Endosomal Sorting Complex. Nature 2007, 445, 554–558. [Google Scholar] [CrossRef]
| Reference | Primary Endpoint | N of Patients/Controls (Source) | Diagnostic Properties | Prognostic Properties | |
|---|---|---|---|---|---|
| p-Value, AUC | p-Value | Risk Ratio, 95% CI | |||
| Studies focused on the diagnostic properties of PVT1 only | |||||
| Wu et al., 2016 ** [101] | - | 71/62 healthy + 8 benign (patient samples) *** | 0.007, 0.900 | - | - |
| Grampp et al., 2016 ** [89] | - | 453/453 (patient samples) 138/138 (TCGA) | <0.05 | - | - |
| Studies focused on the prognostic properties of PVT1 only | |||||
| Posa et al., 2016 [98] | OS | -/- (TCGA) | - | <0.0001 | - |
| Huang et al., 2017 * [95] | OS | 289/32 (TCGA) | - | 0.024 (panel of 15 lncRNAs) | - |
| Wu et al., 2017 [81] | OS | 55/55 (patient samples) 534/72 (TCGA) | - | 0.00007 | - |
| Xu et al., 2017 [102] | OS | 530/72 (TCGA) 109/100 (microarray) | - | 9.15 × 10−7 | - |
| Wang et al., 2018 [100] | OS | 539/72 (TCGA) | - | 0.0002 (panel of 11 lncRNAs) | 1.47, - |
| Liu et al., 2020 [99] | OS | 525/- (TCGA) 60/60 (patient samples) | - | 00002 | 1.79, 1.32–2.43 |
| Studies showing both the diagnostic and prognostic properties of PVT1 | |||||
| Yang et al., 2017 [73] | OS DFS | 50/50 (patient samples), 534/72 (TCGA) | <0.001 | 0.014 NS | 1.494, 1.081–2.063 1.469, 0.976–2.211 |
| Bao et al., 2018 [103] | OS DFS | 129/129 (patient samples) | <0.01 | 0.012 0.004 | 4.445, 1.515–8.392 3.553, - |
| Li et al., 2018 [75] | OS | 448/67 (TCGA) 40/40 (patient samples) | <0.001 | <0.01 | - |
| Ren et al., 2019 [77] | OS | 25/25 (patient samples) | <0.001 | 0.007 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohosova, J.; Kubickova, A.; Slaby, O. lncRNA PVT1 in the Pathogenesis and Clinical Management of Renal Cell Carcinoma. Biomolecules 2021, 11, 664. https://doi.org/10.3390/biom11050664
Bohosova J, Kubickova A, Slaby O. lncRNA PVT1 in the Pathogenesis and Clinical Management of Renal Cell Carcinoma. Biomolecules. 2021; 11(5):664. https://doi.org/10.3390/biom11050664
Chicago/Turabian StyleBohosova, Julia, Adela Kubickova, and Ondrej Slaby. 2021. "lncRNA PVT1 in the Pathogenesis and Clinical Management of Renal Cell Carcinoma" Biomolecules 11, no. 5: 664. https://doi.org/10.3390/biom11050664
APA StyleBohosova, J., Kubickova, A., & Slaby, O. (2021). lncRNA PVT1 in the Pathogenesis and Clinical Management of Renal Cell Carcinoma. Biomolecules, 11(5), 664. https://doi.org/10.3390/biom11050664






