Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria
Abstract
:1. Introduction
2. Biosynthesis of SA: Overview
2.1. Biosynthesis of SA in Plants
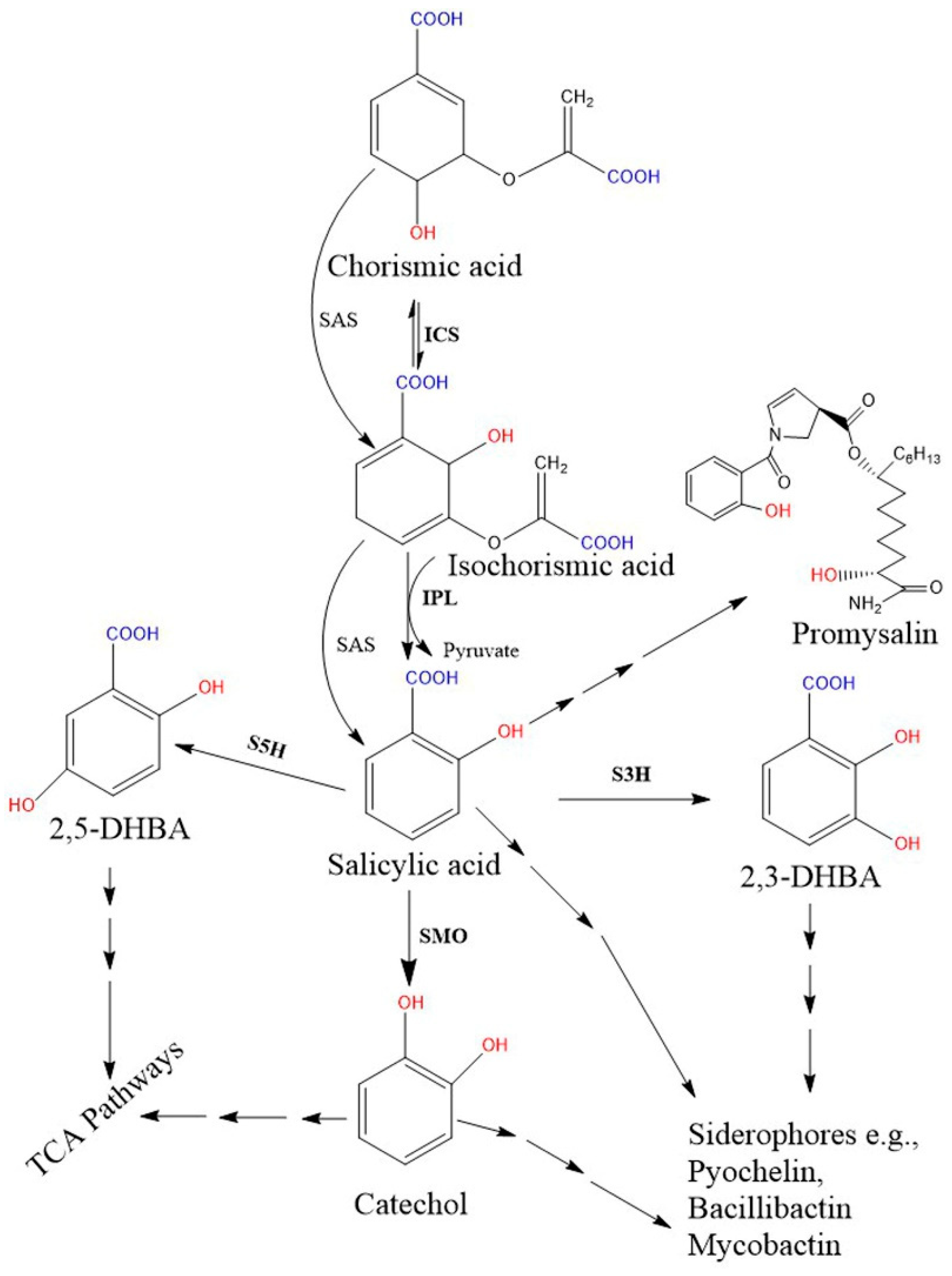
2.2. Biosynthesis of SA in Bacteria
3. SA-Derived (Catecholate) Siderophores in Bacteria
4. Quantification of Salicylic Acid or Salicylate Siderophore
4.1. Quantification of Salicylic Acid in Plant
4.2. Quantification of Bacterial Salicylic Acid and Detection of Salicylate Siderophore
4.2.1. Quantification of Bacterial Salicylic Acid
4.2.2. Detection of Salicylate Siderophore
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Hadacek, F.; Bachmann, G.; Engelmeier, D.; Chobot, V. Hormesis and a chemical raison d’être for secondary plant metabolites. Dose Response 2011, 9, 79–116. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, C.M.; Galarneau, E.R.-A. Phenolic Compound induction in plant-microbe and plant-insect interactions: A meta-analysis. Front. Plant Sci. 2020, 11, 2034. [Google Scholar] [CrossRef] [PubMed]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef] [Green Version]
- Buchner, A. Ueber das rigatellische fiebermittel und über eine in der weidenrinde entdeckte alcaloidische substanz. Repert. Pharm. 1828, 29, 405–420. [Google Scholar]
- Raskin, I.; Ehmann, A.; Melander, W.R.; Meeuse, B.J.D. Salicylic acid: A natural inducer of heat production in Arum lilies. Science 1987, 237, 1601–1602. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [Green Version]
- Malamy, J.; Carr, J.P.; Klessig, D.F.; Raskin, I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990, 250, 1002–1004. [Google Scholar] [CrossRef] [Green Version]
- White, R.F. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology 1979, 99, 410–412. [Google Scholar] [CrossRef]
- Antoniw, J.F.; White, R.F. The effects of aspirin and polyacrylic acid on soluble leaf proteins and resistance to virus infection in five cultivars of Tobacco. J. Phytopathol. 1980, 98, 331–341. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef] [Green Version]
- Métraux, J.P.; Signer, H.; Ryals, J.; Ward, E.; Wyss-Benz, M.; Gaudin, J.; Raschdorf, K.; Schmid, E.; Blum, W.; Inverardi, B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 1990, 250, 1004–1006. [Google Scholar] [CrossRef] [Green Version]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef]
- Raskin, I. Salicylate, A New Plant Hormone 1. Plant Physiol. 1992, 99, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, D.A.; Klessig, D.F. How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 2017, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Malamy, J.; Klessig, D.F. Salicylic acid and plant disease resistance. Plant J. 1992, 2, 643–654. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Gu, X.Y.; Liu, Y.; Liu, L.J. Progress on the biosynthesis and signal transduction of phytohormone salicylic acid. Yi Chuan Hered. 2020, 42, 858–869. [Google Scholar]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Alazem, M.; Lin, N.-S. Roles of plant hormones in the regulation of host–virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef]
- Freeman, J.L.; Garcia, D.; Kim, D.; Hopf, A.; Salt, D.E. Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol. 2005, 137, 1082–1091. [Google Scholar] [CrossRef] [Green Version]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [Green Version]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V. V Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Park, C. Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol. 2010, 188, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Van Butselaar, T.; Van den Ackerveken, G. Salicylic acid steers the growth-immunity tradeoff. Trends Plant Sci. 2020, 25, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, G. Aspirin. Sci. Am. 1991, 264, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Thorat, M.A.; Bosetti, C.; Brown, P.H.; Burn, J.; Cook, N.R.; Ford, L.G.; Jacobs, E.J.; Jankowski, J.A.; La Vecchia, C.; et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 47–57. [Google Scholar] [CrossRef]
- Dachineni, R.; Kumar, D.R.; Callegari, E.; Kesharwani, S.S.; Sankaranarayanan, R.; Seefeldt, T.; Tummala, H.; Bhat, G.J. Salicylic acid metabolites and derivatives inhibit CDK activity: Novel insights into aspirin’s chemopreventive effects against colorectal cancer. Int. J. Oncol. 2017, 51, 1661–1673. [Google Scholar] [CrossRef] [Green Version]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, K.M. The shikimate pathway: Early steps in the biosynthesis of aromatic compounds. Plant Cell 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Averesch, N.J.H.; Krömer, J.O. Metabolic Engineering of the Shikimate Pathway for Production of Aromatics and Derived Compounds—Present and Future Strain Construction Strategies. Front. Bioeng. Biotechnol. 2018, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Kayser, N.A.O.; Averesch, N. Technische Biochemie: Die Biochemie und Industrielle Nutzung von Naturstoffen; Springer: Wiesbaden, Germany, 2015; ISBN 3658055480. [Google Scholar]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A. Shikimate and phenylalanine biosynthesis in the green lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Hubrich, F.; Müller, M.; Andexer, J.N. Chorismate- and isochorismate converting enzymes: Versatile catalysts acting on an important metabolic node. Chem. Commun. 2021, 57, 2441–2463. [Google Scholar] [CrossRef] [PubMed]
- Dosselaere, F.; Vanderleyden, J. A Metabolic Node in Action: Chorismate-utilizing enzymes in microorganisms. Crit. Rev. Microbiol. 2001, 27, 75–131. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562. [Google Scholar] [CrossRef] [PubMed]
- Shelton, C.L.; Lamb, A.L. Unraveling the structure and mechanism of the MST(ery) enzymes. Trends Biochem. Sci. 2018, 43, 342–357. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.-K. PBS3 and EPS1 complete salicylic acid biosynthesis from isochorismate in Arabidopsis. Mol. Plant 2019, S1674-2052, 30369–30377. [Google Scholar] [CrossRef] [Green Version]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Grobelak, A.; Hiller, J. Bacterial siderophores promote plant growth: Screening of catechol and hydroxamate siderophores. Int. J. Phytoremediation 2017, 19, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef]
- Kaduskar, R.D.; Della Scala, G.; Al Jabri, Z.J.; Arioli, S.; Musso, L.; Oggioni, M.R.; Dallavalle, S.; Mora, D. Promysalin is a salicylate-containing antimicrobial with a cell-membrane-disrupting mechanism of action on Gram-positive bacteria. Sci. Rep. 2017, 7, 8861. [Google Scholar] [CrossRef] [Green Version]
- Bakker, P.A.H.M.; Ran, L.; Mercado-Blanco, J. Rhizobacterial salicylate production provokes headaches! Plant Soil 2014, 382, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in iron metabolism: From mechanism to therapy potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef] [Green Version]
- Kjærbølling, I.; Mortensen, U.H.; Vesth, T.; Andersen, M.R. Strategies to establish the link between biosynthetic gene clusters and secondary metabolites. Fungal Genet. Biol. 2019, 130, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.N.; Yen, M.-R.; Chiang, C.-Y.; Lin, H.-C.; Chen, P.-Y. Detecting and prioritizing biosynthetic gene clusters for bioactive compounds in bacteria and fungi. Appl. Microbiol. Biotechnol. 2019, 103, 3277–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehroz, M.; Aslam, M.; Ali Khan, M.; Aiman, S.; Gul Afridi, S.; Khan, A. The in silico characterization of a salicylic acid analogue coding fene clusters in selected Pseudomonas fluorescens strains. Iran. J. Biotechnol. 2019, 17, e2250. [Google Scholar] [PubMed]
- Weber, T.; Kim, H.U. The secondary metabolite bioinformatics portal: Computational tools to facilitate synthetic biology of secondary metabolite production. Synth. Syst. Biotechnol. 2016, 1, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Kim, H.U.; Medema, M.H.; Weber, T. Recent development of antiSMASH and other computational approaches to mine secondary metabolite biosynthetic gene clusters. Brief. Bioinform. 2019, 20, 1103–1113. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef]
- Yokoo, S.; Inoue, S.; Suzuki, N.; Amakawa, N.; Matsui, H.; Nakagami, H.; Takahashi, A.; Arai, R.; Katou, S. Comparative analysis of plant isochorismate synthases reveals structural mechanisms underlying their distinct biochemical properties. Biosci. Rep. 2018, 38, BSR20171457. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, K.; Motomura, Y.; Yagi, Y.; Nomura, H.; Kikuchi, S.; Nakai, M.; Shiina, T. Chloroplast envelope localization of EDS5, an essential factor for salicylic acid biosynthesis in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e23603. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.-J.; Shang, Q.-M. Genome-wide characterization of phenylalanine ammonia-lyase gene family in watermelon (Citrullus lanatus). Planta 2013, 238, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.V.; Shah, R.P.; Mohammed, L.A. Formation and vacuolar localization of salicylic acid glucose conjugates in soybean cell suspension cultures. Physiol. Plant. 2003, 118, 328–336. [Google Scholar] [CrossRef]
- Snoeren, T.A.L.; Mumm, R.; Poelman, E.H.; Yang, Y.; Pichersky, E.; Dicke, M. The herbivore-induced plant volatile methyl salicylate negatively affects attraction of the parasitoid Diadegma semiclausum. J. Chem. Ecol. 2010, 36, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Halitschke, R.; Yin, C.; Liu, C.-J.; Gan, S.-S. Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc. Natl. Acad. Sci. USA 2013, 110, 14807–14812. [Google Scholar] [CrossRef] [Green Version]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; De Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C. Minimum information about a biosynthetic gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.; Fischbach, M. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Kumar, L.; Meena, N.L.; Singh, U. Role of phytosiderophores in acquisition of iron and other micronutrients in food legumes. In Biofortification of Food Crops; Singh, U., Praharaj, C.S., Singh, S.S., Singh, N.P., Eds.; Springer India: New Delhi, India, 2016; pp. 291–302. ISBN 978-81-322-2716-8. [Google Scholar]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator–Siderophore: A review. Microbiol. Res. 2018, 212, 103–111. [Google Scholar] [CrossRef]
- Das, A.; Prasad, R.; Srivastava, A.; Giang, P.H.; Bhatnagar, K.; Varma, A. Fungal Siderophores: Structure, Functions and Regulation BT—Microbial Siderophores; Varma, A., Chincholkar, S.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–42. ISBN 978-3-540-71160-5. [Google Scholar]
- Plach, M.G.; Löffler, P.; Merkl, R.; Sterner, R. Conversion of anthranilate synthase into isochorismate synthase: Implications for the Evolution of chorismate-utilizing enzymes. Angew. Chem. Int. Ed. 2015, 54, 11270–11274. [Google Scholar] [CrossRef] [PubMed]
- Beiderbeck, H.; Taraz, K.; Budzikiewicz, H.; Walsby, A.E. Anachelin, the siderophore of the cyanobacterium Anabaena cylindrica CCAP 1403/2A. Z. Nat. C 2000, 55, 681–687. [Google Scholar] [CrossRef]
- Gademann, K.; Kobylinska, J.; Wach, J.-Y.; Woods, T.M. Surface modifications based on the cyanobacterial siderophore anachelin: From structure to functional biomaterials design. BioMetals 2009, 22, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Vallenet, D.; Nordmann, P.; Barbe, V.; Poirel, L.; Mangenot, S.; Bataille, E.; Dossat, C.; Gas, S.; Kreimeyer, A.; Lenoble, P.; et al. Comparative analysis of Acinetobacters: Three genomes for three lifestyles. PLoS ONE 2008, 3, e1805. [Google Scholar] [CrossRef]
- Song, W.Y.; Kim, H.J. Current biochemical understanding regarding the metabolism of acinetobactin, the major siderophore of the human pathogen Acinetobacter baumannii, and outlook for discovery of novel anti-infectious agents based thereon. Nat. Prod. Rep. 2020, 37, 477–487. [Google Scholar] [CrossRef]
- Stutz, E.W.; Défago, G.; Kern, H. Naturally occurring fluorescent pseudomonads involved in suppression of black root rot of tobacco. Phytopathology 1986, 76, 181–185. [Google Scholar] [CrossRef]
- De Meyer, G.; Höfte, M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology 1997, 87, 588–593. [Google Scholar] [CrossRef] [Green Version]
- Maurhofer, M. Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHA0: Influence of the gacA gene and of pyoverdine production. Phytopathology 1994, 84, 139–146. [Google Scholar] [CrossRef]
- Leeman, M.; Van Pelt, J.A.; Den Ouden, F.M.; Heinsbroek, M.; Bakker, P.A.; Schippers, B. Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharides of Pseudomonas fluorescens. Phytopathology 1995, 85, 1021–1027. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; van der Drift, K.M.G.M.; Olsson, P.E.; Thomas-Oates, J.E.; van Loon, L.C.; Bakker, P.A.H.M. Analysis of the pmsCEAB gene cluster involved in biosynthesis of salicylic acid and the siderophore pseudomonine in the biocontrol strain Pseudomonas fluorescens WCS374. J. Bacteriol. 2001, 183, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Van Wees, S.C.M.; Pieterse, C.M.J.; Trijssenaar, A.; Van ’t Westende, Y.A.M.; Hartog, F.; Van Loon, L.C. Differential induction of systemic resistance in Arabidopsis by biocontrol bacteria. Mol. Plant Microbe Interact. 1997, 10, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Ran, L.X.; Liu, C.Y.; Wu, G.J.; van Loon, L.C.; Bakker, P.A.H.M. Suppression of bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. in China. Biol. Control 2005, 32, 111–120. [Google Scholar] [CrossRef]
- Press, C.M.; Wilson, M.; Tuzun, S.; Kloepper, J.W. Salicylic acid produced by Serratia marcescens 90-166 is not the primary determinant of induced systemic resistance in cucumber or tobacco. Mol. Plant Microbe Interact. 1997, 10, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Khilyas, I.V.; Tursunov, K.A.; Shirshikova, T.V.; Kamaletdinova, L.K.; Matrosova, L.E.; Desai, P.T.; McClelland, M.; Bogomolnaya, L.M. Genome sequence of pigmented siderophore-producing strain Serratia marcescens SM6. Microbiol. Resour. Announc. 2019, 8, e00247-19. [Google Scholar] [CrossRef] [Green Version]
- Buysens, S.; Heungens, K.; Poppe, J.; Hofte, M. Involvement of pyochelin and pyoverdin in suppression of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Appl. Environ. Microbiol. 1996, 62, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Gaille, C.; Kast, P.; Haas, D. Salicylate biosynthesis in Pseudomonas aeruginosa: Purification and characterization of PchB, a novel bifunctional enzyme displaying isochorismate pyruvate-lyase and chorismate mutase activities. J. Biol. Chem. 2002, 277, 21768–21775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneely, K.M.; Luo, Q.; Riley, A.P.; Taylor, B.; Roy, A.; Stein, R.L.; Prisinzano, T.E.; Lamb, A.L. Expanding the results of a high throughput screen against an isochorismate-pyruvate lyase to enzymes of a similar scaffold or mechanism. Bioorg. Med. Chem. 2014, 22, 5961–5969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Bélanger, R.R.; Benhamou, N.; Paulitz, T.C. Role of salicylic acid in systemic resistance induced by Pseudomonas spp. against Pythium aphanidermatum in cucumber roots. Eur. J. Plant Pathol. 1999, 105, 477–486. [Google Scholar] [CrossRef]
- Saikia, R.; Singh, T.; Kumar, R.; Srivastava, J.; Srivastava, A.K.; Singh, K.; Arora, D.K. Role of salicylic acid in systemic resistance induced by Pseudomonas fluorescens against Fusarium oxysporum f. sp. ciceri in chickpea. Microbiol. Res. 2003, 158, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Saikia, R.; Srivastava, A.K.; Singh, K.; Arora, D.K.; Lee, M.-W. Effect of iron availability on induction of systemic resistance to fusarium wilt of chickpea by Pseudomonas spp. Mycobiology 2005, 33, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, R.; Kumar, R.; Arora, D.K.; Gogoi, D.K.; Azad, P. Pseudomonas aeruginosa inducing rice resistance against Rhizoctonia solani: Production of salicylic acid and peroxidases. Folia Microbiol. 2006, 51, 375–380. [Google Scholar] [CrossRef]
- Sokol, P.A.; Lewis, C.J.; Dennis, J.J. Isolation of a novel siderophore from Pseudomonas cepacia. J. Med. Microbiol. 1992, 36, 184–189. [Google Scholar] [CrossRef]
- Islam, M.; Ali, M.; Choi, S.-J.; Park, Y.-I.; Baek, K.-H. Salicylic acid-producing endophytic bacteria increase nicotine accumulation and resistance against wildfire disease in tobacco plants. Microorganisms 2020, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertlein, G.; Müller, S.; Garcia-Gonzalez, E.; Poppinga, L.; Süssmuth, R.D.; Genersch, E. Production of the catechol type siderophore bacillibactin by the honey bee pathogen Paenibacillus larvae. PLoS ONE 2014, 9, e108272. [Google Scholar] [CrossRef] [Green Version]
- Saxena, B.; Modi, M.; Modi, V. V Isolation and characterization of siderophores from Azospirillum lipoferum D-2. Microbiology 1986, 132, 2219–2224. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Passalacqua, K.D.; Hanna, P.C.; Sherman, D.H. Regulation of petrobactin and bacillibactin biosynthesis in Bacillus anthracis under iron and oxygen variation. PLoS ONE 2011, 6, e20777. [Google Scholar] [CrossRef] [Green Version]
- Barbeau, K.; Zhang, G.; Live, D.H.; Butler, A. Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. J. Am. Chem. Soc. 2002, 124, 378–379. [Google Scholar] [CrossRef]
- Forchetti, G.; Masciarelli, O.; Izaguirre, M.J.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria improve seedling growth of sunflower under water stress, produce salicylic acid, and inhibit growth of pathogenic fungi. Curr. Microbiol. 2010, 61, 485–493. [Google Scholar] [CrossRef]
- Kesaulya, H.; Hasinu, J.; Tuhumury, G. Potential of Bacillus spp. produces siderophores insuppressing thewilt disease of banana plants. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 12016. [Google Scholar] [CrossRef]
- Nandhini, S.; Sendhilvel, V.; Babu, S. Endophytic bacteria from tomato and their efficacy against Fusarium oxysporum f.sp. lycopersici, the wilt pathogen. J. Biopestic. 2012, 5, 178–185. [Google Scholar]
- Ciche, T.A.; Blackburn, M.; Carney, J.R.; Ensign, J.C. Photobactin: A catechol siderophore produced by Photorhabdus luminescens, an entomopathogen mutually associated with Heterorhabditis bacteriophora NC1 nematodes. Appl. Environ. Microbiol. 2003, 69, 4706–4713. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Dai, S.; Shen, J.; Ren, B.; Huang, P.; Wang, Q.; Liu, X.; Zhang, B.; Dai, H.; Zhang, L. A new salicylate synthase AmS is identified for siderophores biosynthesis in Amycolatopsis methanolica 239T. Appl. Microbiol. Biotechnol. 2015, 99, 5895–5905. [Google Scholar] [CrossRef]
- Müller, S.I.; Valdebenito, M.; Hantke, K. Salmochelin, the long-overlooked catecholate siderophore of Salmonella. BioMetals 2009, 22, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Crouch, M.-L.V.; Castor, M.; Karlinsey, J.E.; Kalhorn, T.; Fang, F.C. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2008, 67, 971–983. [Google Scholar] [CrossRef]
- Hantke, K.; Nicholson, G.; Rabsch, W.; Winkelmann, G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 2003, 100, 3677–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, G.L.; Sigel, S.P.; Payne, S.M.; Neilands, J.B. Vibriobactin, a siderophore from Vibrio cholerae. J. Biol. Chem. 1984, 259, 383–385. [Google Scholar] [CrossRef]
- Thode, S.K.; Rojek, E.; Kozlowski, M.; Ahmad, R.; Haugen, P. Distribution of siderophore gene systems on a Vibrionaceae phylogeny: Database searches, phylogenetic analyses and evolutionary perspectives. PLoS ONE 2018, 13, e0191860. [Google Scholar] [CrossRef] [Green Version]
- Simpson, L.M.; Oliver, J.D. Siderophore production by Vibrio vulnificus. Infect. Immun. 1983, 41, 644–649. [Google Scholar] [CrossRef] [Green Version]
- Okujo, N.; Saito, M.; Yamamoto, S.; Yoshida, T.; Miyoshi, S.; Shinoda, S. Structure of vulnibactin, a new polyamine-containing siderophore from Vibrio vulnificus. Biometals 1994, 7, 109–116. [Google Scholar] [CrossRef]
- Tan, W.; Verma, V.; Jeong, K.; Kim, S.Y.; Jung, C.-H.; Lee, S.E.; Rhee, J.H. Molecular characterization of vulnibactin biosynthesis in Vibrio vulnificus indicates the existence of an alternative siderophore. Front. Microbiol. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, B.A.; Wang, C.C.C.; Walsh, C.T.; Khosla, C. Biosynthesis of yersiniabactin, a complex polyketide-nonribosomal peptide, using Escherichia coli as a heterologous host. Appl. Environ. Microbiol. 2003, 69, 6698–6702. [Google Scholar] [CrossRef] [Green Version]
- Kerbarh, O.; Ciulli, A.; Howard, N.I.; Abell, C. Salicylate biosynthesis: Overexpression, purification, and characterization of Irp9, a bifunctional salicylate synthase from Yersinia enterocolitica. J. Bacteriol. 2005, 187, 5061–5066. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.D.; Fetherston, J.D. Yersiniabactin iron uptake: Mechanisms and role in Yersinia pestis pathogenesis. Microbes Infect. 2011, 13, 808–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paauw, A.; Leverstein-van Hall, M.A.; van Kessel, K.P.M.; Verhoef, J.; Fluit, A.C. Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells. PLoS ONE 2009, 4, e8240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quadri, L.E.N.; Sello, J.; Keating, T.A.; Weinreb, P.H.; Walsh, C.T. Identification of a Mycobacterium tuberculosis gene cluster encoding the biosynthetic enzymes for assembly of the virulence-conferring siderophore mycobactin. Chem. Biol. 1998, 5, 631–645. [Google Scholar] [CrossRef] [Green Version]
- De Voss, J.J.; Rutter, K.; Schroeder, B.G.; Su, H.; Zhu, Y.; Barry, C.E., 3rd. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 2000, 97, 1252–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, A.J.; Yu, M.; Gårdenborg, T.; Middleditch, M.; Ramsay, R.J.; Baker, E.N.; Lott, J.S. The structure of MbtI from Mycobacterium tuberculosis, the first enzyme in the biosynthesis of the siderophore mycobactin, reveals it to be a salicylate synthase. J. Bacteriol. 2006, 188, 6081–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sritharan, M. Iron homeostasis in Mycobacterium tuberculosis: Mechanistic insights into siderophore-mediated Iron uptake. J. Bacteriol. 2016, 198, 2399–2409. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Butala, S.; Khan, T.; Suvarna, V.; Sherje, A.; Dravyakar, B. Mycobacterial siderophore: A review on chemistry and biology of siderophore and its potential as a target for tuberculosis. Eur. J. Med. Chem. 2018, 157, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Shyam, M.; Shilkar, D.; Verma, H.; Dev, A.; Sinha, B.N.; Brucoli, F.; Bhakta, S.; Jayaprakash, V. The Mycobactin biosynthesis pathway: A prospective therapeutic target in the battle against tuberculosis. J. Med. Chem. 2020, 64, 71–100. [Google Scholar] [CrossRef]
- Ho, Y.-N.; Lee, H.-J.; Hsieh, C.-T.; Peng, C.-C.; Yang, Y.-L. Chemistry and biology of salicyl-capped siderophores. In Studies in Natural Products Chemistry; Atta-ur-Rahman, B.T.-S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 431–490. ISBN 1572-5995. [Google Scholar]
- Masschelein, J.; Mattheus, W.; Gao, L.-J.; Moons, P.; Van Houdt, R.; Uytterhoeven, B.; Lamberigts, C.; Lescrinier, E.; Rozenski, J.; Herdewijn, P.; et al. A PKS/NRPS/FAS hybrid gene cluster from Serratia plymuthica RVH1 encoding the biosynthesis of three broad spectrum, Zeamine-related antibiotics. PLoS ONE 2013, 8, e54143. [Google Scholar] [CrossRef] [Green Version]
- Süssmuth, R.D.; Mainz, A. Nonribosomal peptide synthesis—Principles and prospects. Angew. Chem. Int. Ed. 2017, 56, 3770–3821. [Google Scholar] [CrossRef]
- Wang, H.; Fewer, D.P.; Holm, L.; Rouhiainen, L.; Sivonen, K. Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, 9259–9264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaremko, M.J.; Davis, T.D.; Corpuz, J.C.; Burkart, M.D. Type II non-ribosomal peptide synthetase proteins: Structure, mechanism, and protein–protein interactions. Nat. Prod. Rep. 2020, 37, 355–379. [Google Scholar] [CrossRef]
- Bloudoff, K.; Schmeing, T.M. Structural and functional aspects of the nonribosomal peptide synthetase condensation domain superfamily: Discovery, dissection and diversity. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1587–1604. [Google Scholar] [CrossRef]
- Sayari, M.; van der Nest, M.A.; Steenkamp, E.T.; Soal, N.C.; Wilken, P.M.; Wingfield, B.D. Distribution and evolution of nonribosomal peptide synthetase gene clusters in the Ceratocystidaceae. Genes 2019, 10, 328. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.T.; Chen, H.; Keating, T.A.; Hubbard, B.K.; Losey, H.C.; Luo, L.; Marshall, C.G.; Miller, D.A.; Patel, H.M. Tailoring enzymes that modify nonribosomal peptides during and after chain elongation on NRPS assembly lines. Curr. Opin. Chem. Biol. 2001, 5, 525–534. [Google Scholar] [CrossRef]
- White-Stevens, R.H.; Kamin, H. Studies of a flavoprotein, salicylate hydroxylase: I. Preparation, properties, and the uncoupling of oxygen reduction from hydroxylation. J. Biol. Chem. 1972, 247, 2358–2370. [Google Scholar] [CrossRef]
- Costa, D.M.A.; Gómez, S.V.; de Araújo, S.S.; Pereira, M.S.; Alves, R.B.; Favaro, D.C.; Hengge, A.C.; Nagem, R.A.P.; Brandão, T.A.S. Catalytic mechanism for the conversion of salicylate into catechol by the flavin-dependent monooxygenase salicylate hydroxylase. Int. J. Biol. Macromol. 2019, 129, 588–600. [Google Scholar] [CrossRef] [Green Version]
- You, I.S.; Ghosal, D.; Gunsalus, I.C. Nucleotide sequence analysis of the Pseudomonas putida PpG7 salicylate hydroxylase gene (nahG) and its 3′-flanking region. Biochemistry 1991, 30, 1635–1641. [Google Scholar] [CrossRef]
- Bosch, R.; Moore, E.R.B.; García-Valdés, E.; Pieper, D.H. NahW, a novel, inducible salicylate hydroxylase involved in mineralization of naphthalene by Pseudomonas stutzeri AN10. J. Bacteriol. 1999, 181, 2315–2322. [Google Scholar] [CrossRef] [Green Version]
- Pozdnyakova-Filatova, I.; Petrikov, K.; Vetrova, A.; Frolova, A.; Streletskii, R.; Zakharova, M. The naphthalene catabolic genes of Pseudomonas putida BS3701: Additional regulatory control. Front. Microbiol. 2020, 11, 1217. [Google Scholar] [CrossRef]
- Maspoli, A.; Wenner, N.; Mislin, G.L.A.; Reimmann, C. Functional analysis of pyochelin-/enantiopyochelin-related genes from a pathogenicity island of Pseudomonas aeruginosa strain PA14. BioMetals 2014, 27, 559–573. [Google Scholar] [CrossRef]
- Ronnebaum, T.A.; Lamb, A.L. Nonribosomal peptides for iron acquisition: Pyochelin biosynthesis as a case study. Curr. Opin. Struct. Biol. 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Schalk, I.J.; Rigouin, C.; Godet, J. An overview of siderophore biosynthesis among fluorescent Pseudomonads and new insights into their complex cellular organization. Environ. Microbiol. 2020, 22, 1447–1466. [Google Scholar] [CrossRef]
- Wyckoff, E.E.; Smith, S.L.; Payne, S.M. VibD and VibH are required for late steps in vibriobactin biosynthesis in Vibrio cholerae. J. Bacteriol. 2001, 183, 1830–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawlor, M.S.; O’connor, C.; Miller, V.L. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect. Immun. 2007, 75, 1463–1472. [Google Scholar] [CrossRef] [Green Version]
- Manos-Turvey, A.; Bulloch, E.M.M.; Rutledge, P.J.; Baker, E.N.; Lott, J.S.; Payne, R.J. Inhibition studies of Mycobacterium tuberculosis salicylate synthase (MbtI). ChemMedChem 2010, 5, 1067–1079. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef] [Green Version]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Golonka, R.; San Yeoh, B.; Vijay-Kumar, M. The iron tug-of-war between bacterial siderophores and innate immunity. J. Innate Immun. 2019, 11, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Loba, V.C.; Pollmann, S. Highly sensitive salicylic acid quantification in milligram amounts of plant tissue. In Plant Hormones: Methods and Protocols; Kleine-Vehn, J., Sauer, M., Eds.; Springer: New York, NY, USA, 2017; pp. 221–229. ISBN 978-1-4939-6469-7. [Google Scholar]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; O’ Donnell, P.; Sammons, M.; Toshima, H.; Tumlinson, J.H. Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10552–10557. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, Z.; Shi, B.; Wei, D.; Chen, J.; Wang, S.; Gao, B. Simultaneous determination of salicylic acid, jasmonic acid, methyl salicylate, and methyl jasmonate from Ulmus pumila Leaves by GC-MS. Int. J. Anal. Chem. 2015, 2015, 698630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allasia, V.; Ponchet, M.; Quentin, M.; Favery, B.; Keller, H. Quantification of salicylic acid (SA) and SA-glucosides in Arabidopsis thaliana. Bio-Protocol 2018, 8, e2844. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.B.; Zuberer, D.A. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertil. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Arnow, L.E. Colorimetric determination of the components of 3, 4-dihydroxyphenylalanine-tyrosine mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar] [CrossRef]


| Bacteria Species | Salicylate-Siderophore | Bacterial Source | NRPS Biosynthetic Genes | References |
|---|---|---|---|---|
| Anabaena cylindrical # | Anachelin | Pond | ICS-IPL | [73,74] |
| Acinetobacter baumannii | Acinetobactin * | Human | ICS-IPL | [75,76] |
| Pseudomonas fluorescens CHA0 | Pyochelin | Tobacco rhizosphere | ICS-IPL | [77,78,79] |
| P. fluorescens WCS374 | Pyochelin | Potato rhizosphere | ICS-IPL | [80,81] |
| P. fluorescens WCS417 | Pyochelin | Wheat rhizosphere | ICS-IPL | [80,82,83] |
| Serratia marcescens | Pyochelin | Cucumber/Tobacco rhizosphere | ICS-IPL | [84,85] |
| P. aeruginosa 7NSK2 | Pyochelin | Barley roots | ICS-IPL | [78,86,87,88] |
| P. aureofaciens 63-28 | Pyochelin | Cucumber roots | ICS-IPL | [89] |
| P. corrugata 13 | Pyochelin | Cucumber roots | ICS-IPL | [89] |
| P. fluorescens Pf4–92 | Pseudobactin * | Chickpea rhizosphere | ICS-IPL | [90,91] |
| P. fluorescens PICF3 | Pyochelin | Olive root | ICS-IPL | [81] |
| P. aeruginosa RsG18 and P. aeruginosa RsG27 | Pseudobactin * | Rhizosphere soil | ICS-IPL | [92] |
| P. cepacia | Azurechelin * | Human | ICS-IPL | [93] |
| P.tremae | N/A | Leaves of Salix babylonica | N/A | [94] |
| Paenibacillus larvae | Bacillibactin | Larvae of honeybees | ICS-IPL | [95] |
| Azospirillum iipoferurn D-2 | N/A | Digireria roots | ICS-IPL | [96] |
| Bacillus anthracis | Petrobactin | Sunflower soil | ICS-IPL | [97] |
| Marinobacter hydrocarbonoclasticus | Petrobactin | N/A | ICS-IPL | [98] |
| B. pumilus SF3 | Bacillibactin | Sunflower plant | ICS-IPL | [95,99,100] |
| B. subtilis | Bacillibactin | Banana plant | [100] | |
| Citrobacter | Enterobactin | Tomato roots | ICS-IPL | [101] |
| Klebsiella pneumoniae | Enterobactin | Tomato leaves | ICS-IPL | [101] |
| Photorhabdus luminescens | Photobactin | Nematode(Heterorhabditis bacteriophora) | SAS | [102] |
| Amycolatopsis methanolica 239T | Amychelin | Soil | SAS | [103] |
| Salmonella enterica serotype Typhimurium | Salmochelin | Human | SAS | [104,105,106] |
| Vibrio cholerae | Vibriobactin | Human | SAS | [107,108] |
| V. vulnificus | Vulnibactin | Human | SAS | [108,109,110,111] |
| Yersinia enterocolitica | Yersiniabactin * | Human | SAS | [112,113,114] |
| Y. pestis | Yersiniabactin * | Human | SAS | [115] |
| Mycobacterium tuberculosis | Mycobactin | Human | SAS | [116,117,118,119,120,121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.K.; Baek, K.-H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules 2021, 11, 705. https://doi.org/10.3390/biom11050705
Mishra AK, Baek K-H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules. 2021; 11(5):705. https://doi.org/10.3390/biom11050705
Chicago/Turabian StyleMishra, Awdhesh Kumar, and Kwang-Hyun Baek. 2021. "Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria" Biomolecules 11, no. 5: 705. https://doi.org/10.3390/biom11050705
APA StyleMishra, A. K., & Baek, K.-H. (2021). Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules, 11(5), 705. https://doi.org/10.3390/biom11050705






