The Effect of the New Lupeol Derivatives on Human Skin Cells as Potential Agents in the Treatment of Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Antioxidant Activity
2.1.1. Free Radical Scavenging Ability
2.1.2. Capability to Prevent Structural Damage of Proteins
2.2. The Effect on the Proliferation and Migration of Human Skin Cells In Vitro
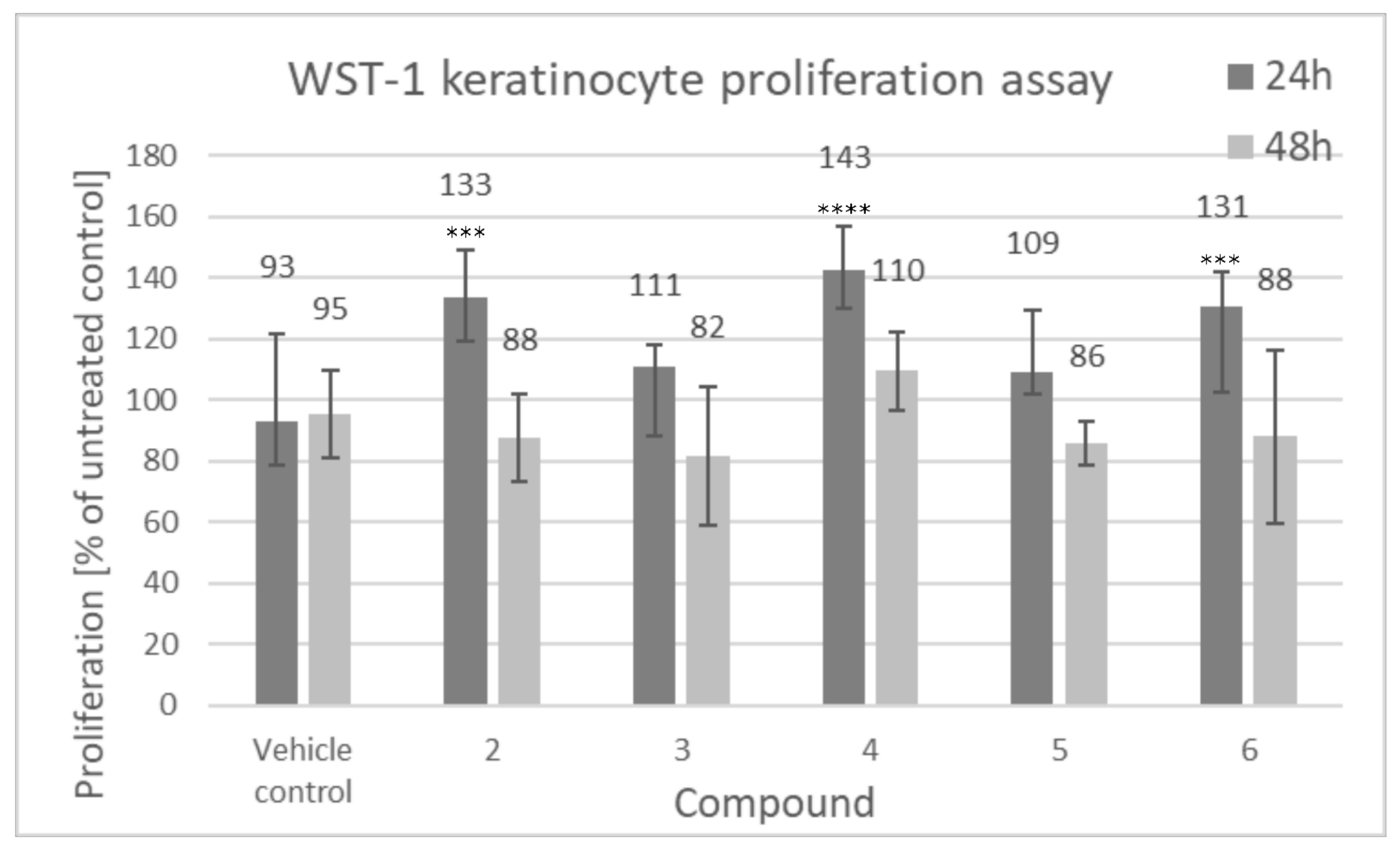
2.2.1. Cell Proliferation
2.2.2. Time Lapse Analysis of Movement of Individual Cells
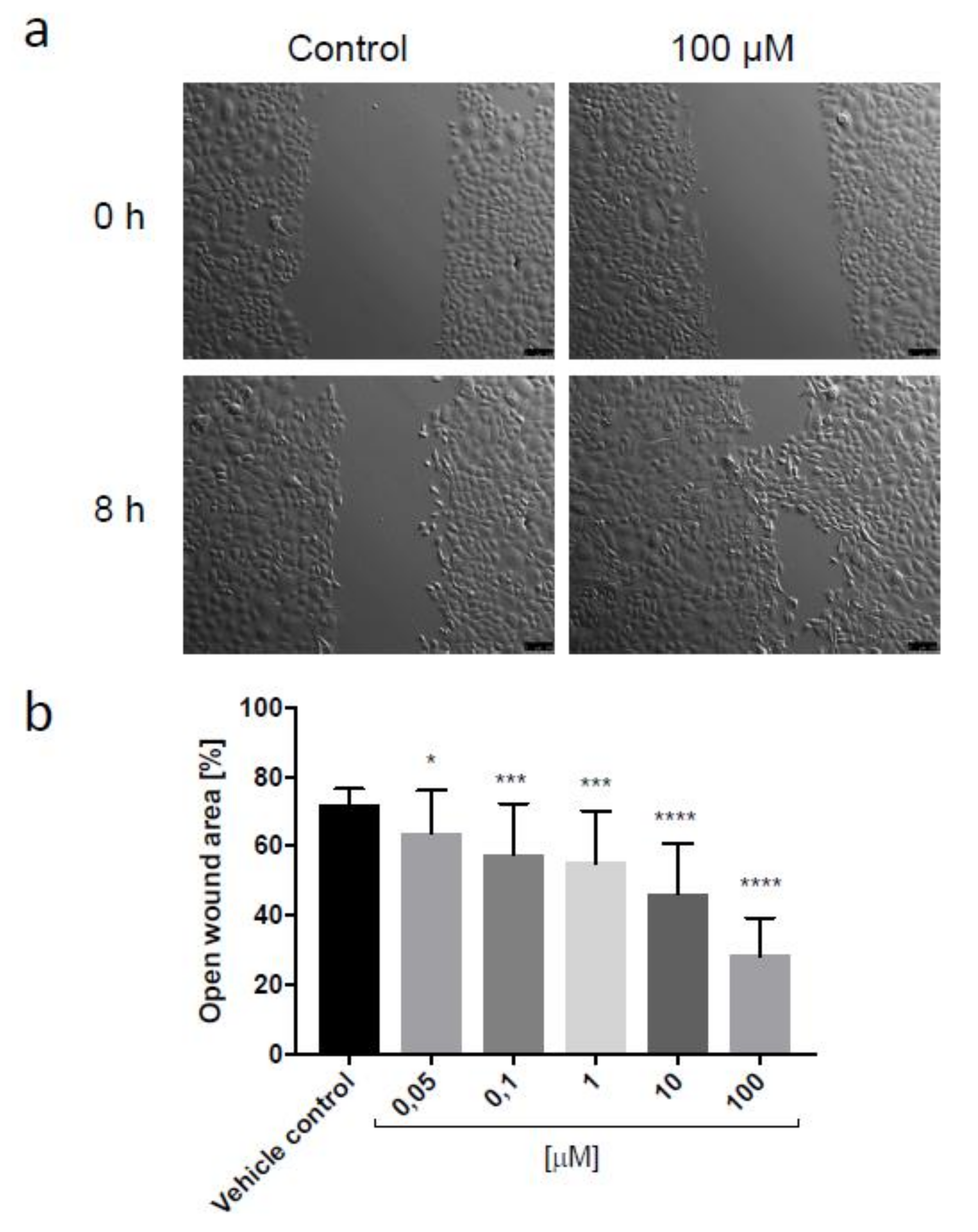
2.2.3. Scratch Assay
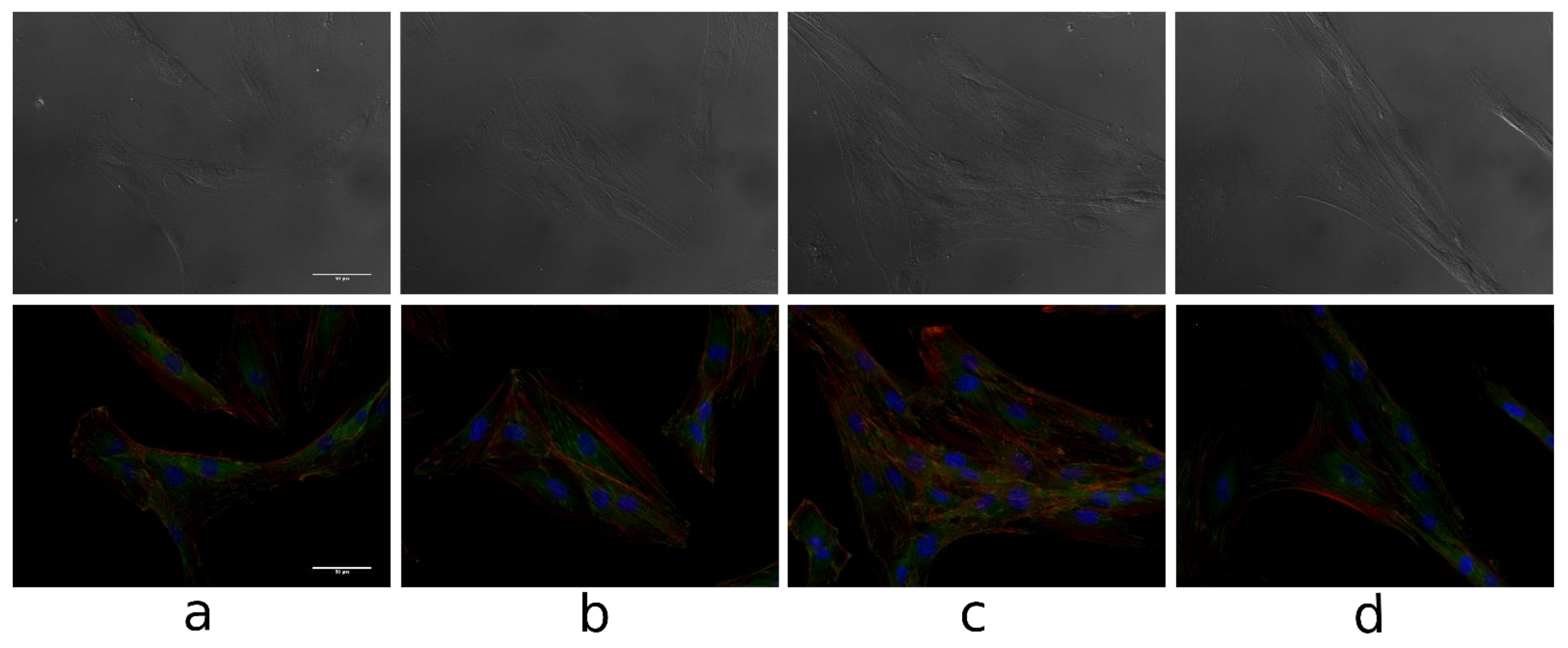
2.2.4. The Cell Morphology and Cytoskeleton
3. Results and Discussion
3.1. Antioxidant Activity
3.1.1. Free Radical Scavenging Ability
3.1.2. Capability to Prevent Structural Damage of Proteins
3.2. The Effect on the Proliferation and Migration of Human Skin Cells In Vitro
3.2.1. Cell Proliferation
3.2.2. Time Lapse Analysis of Movement of Individual Cells
3.2.3. Scratch Assay
3.2.4. Cell Morphology and Cytoskeleton
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal-Y-Sy, S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a traditional medicinal plant to a rational drug: Understanding the clinically proven wound healing efficacy of birch bark extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk–Cwynar, B.; Zaprutko, L. Triterpenoids in cosmetics and cosmetology. Pol. J. Cosmetol. 2003, 4, 218–240. [Google Scholar]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef]
- Abyshey, A.Z.; Agaev, E.M.; Guseinov, A.B. Studies of the chemical composition of birch bark extracts (Cortex betula) from the Betulaceae family. Pharm. Chem. J. 2007, 41, 22–26. [Google Scholar] [CrossRef]
- Ekman, R. The suberin monomers and triterpenoids from the outer bark of Betula verrucose, Ehrh. Holzforschung 1983, 37, 205–211. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Msika, P.; Piccirilli, A.; Piccardi, N. Use of a Cosmetic of Pharmaceutical Composition, Comprising a Lupeol-Rich Extract as an Active Ingredient for Stimulating the Synthesis of Heat Shock Proteins. U.S. Patent US2006/216249A1, 28 September 2006. [Google Scholar]
- Naaimi, D. Lupeol stimulates the production of high-quality type I collagen in human skin through HSP47 induction. J. Am. Acad. Dermatol. 2008, 58, AB62. [Google Scholar]
- Muffler, K.; Leipold, D.; Scheller, M.C.; Haas, C.; Steingroewer, J.; Bley, T.; Neuhaus, H.E.; Mirata, M.A.; Schrader, J.; Ulber, R. Biotransformation of triterpenes. Proc. Biochem. 2011, 46, 1–15. [Google Scholar] [CrossRef]
- Malinowska, M.; Miroslaw, B.; Sikora, E.; Wojtkiewicz, A.M.; Szaleniec, M.; Ogonowski, J.; Pasikowska-Piwko, M.; Eris, I. New lupeol esters as active substances in the treatment of skin damage. PLoS ONE 2019, 14, e0214216. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Cybul, M.; Nowak, R. Review of the methods applied to measuring of antioxidant activity of plant extracts. Herba Pol. 2008, 54, 68–78. [Google Scholar]
- Rong, T.; Zeyuan, D. Separation procedures for naturally occurring antioxidants phytochemicals. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812, 85–89. [Google Scholar] [CrossRef]
- De la Torre, A.A.B.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Bartosz, G. Druga Twarz Tlenu; Wydawnictwo PWN: Warszawa, Poland, 2013; pp. 365–368. [Google Scholar]
- Zuber, A.; Borowczyk, J.; Zimolag, J.; Krok, M.; Madeja, Z.; Pamula, E.; Drukala, J. Poly(L-lactide-coglycolide) thin films can act as autologous cell carriers for skin tissue engineering. Cell. Moll. Biol. Lett. 2014, 19, 297–314. [Google Scholar] [CrossRef]
- Wójcik-Pszczoła, K.; Stalinska, J.; Koczurkiewicz, P.; Chlon-Rzepa, G.; Drukala, J.; Wyszkowska-Kolatko, M.; Pekala, E. Studies of new purine derivatives with acetic acid moiety in human keratinocytes. Acta Poloniae Pharm. 2017, 74, 111–117. [Google Scholar] [PubMed]
- Young, L.C.; Nanah, C.N.; Held, R.A.; Clark, A.R.; Huynh, G.T.; Maraskine, M.C.; Uzarski, R.L.; McCracken, J.; Sharma, A. Effect of electron donating groups on polyphenol-based antioxidant dendrimers. Biochimie 2015, 111, 125–134. [Google Scholar] [CrossRef]
- Prieto, M.A.; Curran, T.P.; Gowen, A.; Vazquez, J.A. An efficient methodology for quantification of synergy and antagonism in single electron transfer antioxidant assays. Food Res. Int. 2015, 67, 284–298. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed]
- Shelly, C.; Lu, M.D. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Hirose, T.; Suzuki, T.; Masami, K.; Kazuhiro, C.; Shigeo, O.; Motomu, M.; Shin-Ichi, O. Atypical protein kinase C isoforms differentially regulate directional keratinocyte migration during would healing. J. Dermatol. Sci. 2019, 93, 101–108. [Google Scholar] [CrossRef]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Actin and microtubules in cell motility: Which one is in control. Traffic 2004, 5, 470–477. [Google Scholar] [CrossRef] [PubMed]





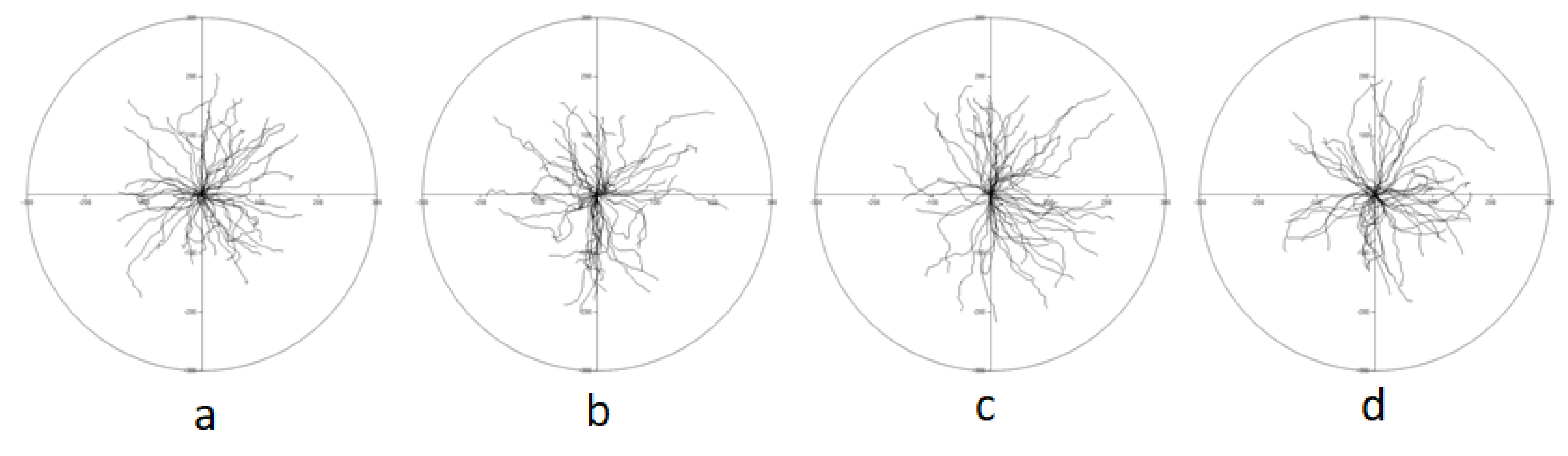


| Condition: | Vehicle Control | 2 | 4 | 6 |
| Speed [µm/min]: | 1867 ± 0.060 | 1893 ± 0.074 | 2104 ± 0.067 | 1947 ± 0.061 |
| Distance [µm]: | 168,004 ± 5364 | 170,403 ± 6681 | 189,395 ± 6006 | 175,209 ± 5515 |
| Displacement [µm]: | 135,875 ± 6157 | 132,519 ± 7250 | 159,458 ± 6136 | 140,483 ± 6639 |
| Speed displacement [µm/min] | 1510 ± 0.068 | 1472 ± 0.081 | 1772 ± 0.068 | 1561 ± 0.074 |
| CDE: | 0.797 ± 0.025 | 0.763 ± 0.026 | 0.836 ± 0.013 | 0.792 ± 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowska, M.A.; Sikora, E.; Stalińska, J.; Ogonowski, J.; Drukała, J. The Effect of the New Lupeol Derivatives on Human Skin Cells as Potential Agents in the Treatment of Wound Healing. Biomolecules 2021, 11, 774. https://doi.org/10.3390/biom11060774
Malinowska MA, Sikora E, Stalińska J, Ogonowski J, Drukała J. The Effect of the New Lupeol Derivatives on Human Skin Cells as Potential Agents in the Treatment of Wound Healing. Biomolecules. 2021; 11(6):774. https://doi.org/10.3390/biom11060774
Chicago/Turabian StyleMalinowska, Magdalena Anna, Elżbieta Sikora, Joanna Stalińska, Jan Ogonowski, and Justyna Drukała. 2021. "The Effect of the New Lupeol Derivatives on Human Skin Cells as Potential Agents in the Treatment of Wound Healing" Biomolecules 11, no. 6: 774. https://doi.org/10.3390/biom11060774
APA StyleMalinowska, M. A., Sikora, E., Stalińska, J., Ogonowski, J., & Drukała, J. (2021). The Effect of the New Lupeol Derivatives on Human Skin Cells as Potential Agents in the Treatment of Wound Healing. Biomolecules, 11(6), 774. https://doi.org/10.3390/biom11060774









