RT-QuIC Using C-Terminally Truncated α-Synuclein Forms Detects Differences in Seeding Propensity of Different Brain Regions from Synucleinopathies
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Recombinant αSyn Proteins
2.2. Circular Dichroism Spectra
2.3. Isolation of TBS (aqueous)-Soluble and Detergent-Soluble Fractions from Brain Tissue
2.4. RT-QuIC Assay
2.5. Proteinase K Digestion and Western Blotting
2.6. Transmission Electron Microscopy (TEM)
2.7. Statistical Analysis
3. Results
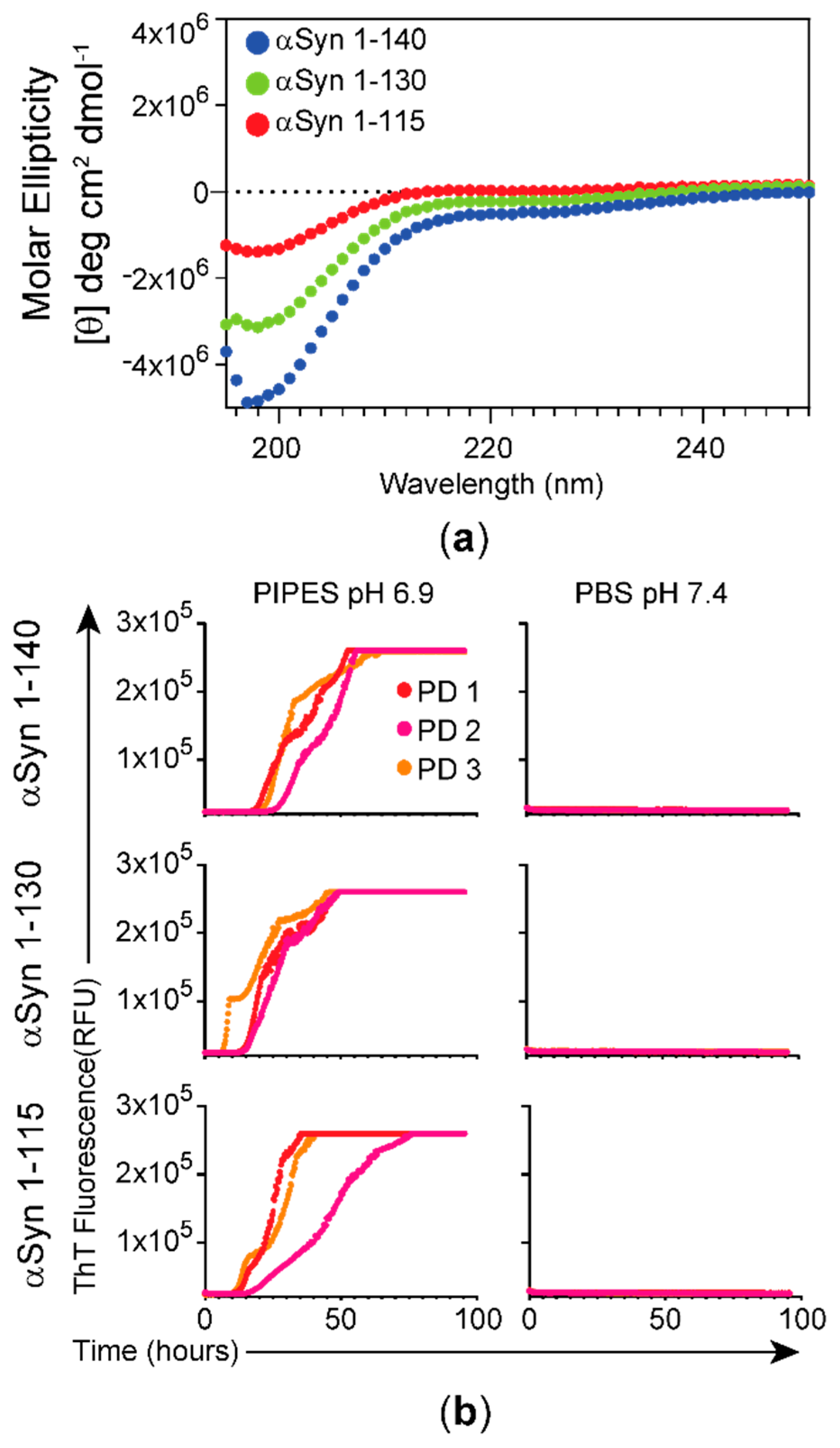
3.1. Characterization of αSyn Forms Used as Substrates and Effect of Buffer Composition on the RT-QuIC Assay
3.2. Seeding RT-QuIC Reactions with Brain Homogenates from PD and DLB Cases Using Different Forms of αSyn
3.3. Detection of Proteinase K (PK)-Resistant Fragments of C-Terminal Truncated αSyn in the RT-QuIC End Products
3.4. Morphological Characterization of RT-QuIC End Products by TEM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCann, H.; Stevens, C.H.; Cartwright, H.; Halliday, G.M. alpha-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 2014, 20, S62–S67. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef]
- Halliday, G.M.; Song, Y.J.C.; Harding, A.J. Striatal beta-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J. Neural Transm. 2011, 118, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Peelaerts, W.; Bousset, L.; Van der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van den Haute, C.; Melki, R.; Baekelandt, V. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Masliah, E. Neurodegeneration: Aggregates feel the strain. Nature 2015, 522, 296–297. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; McLean, C.A.; Culvenor, J.G.; Gai, W.P.; Blumbergs, P.C.; Jäkälä, P.; Beyreuther, K.; Masters, C.L.; Li, Q.-X. The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J. Neurochem. 2001, 76, 87–96. [Google Scholar] [CrossRef]
- Van der Perren, A.; Gelders, G.; Fenyi, A.; Bousset, L.; Brito, F.; Peelaerts, W.; Van den Haute, C.; Gentleman, S.; Melki, R.; Baekelandt, V. The structural differences between patient-derived alpha-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020, 139, 977–1000. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, Z.A.; Goodwin, M.S.; Riffe, C.J.; Dhillon, J.-K.S.; Xia, Y.; Gorion, K.-M.; Vijayaraghavan, N.; McFarland, K.N.; Golbe, L.I.; Yachnis, A.T.; et al. Unique alpha-synuclein pathology within the amygdala in Lewy body dementia: Implications for disease initiation and progression. Acta Neuropathol. Commun. 2019, 7, 142. [Google Scholar] [CrossRef]
- Candelise, N.; Schmitz, M.; Llorens, F.; Villar-Piqué, A.; Cramm, M.; Thom, T.; da Silva Correia, S.M.; da Cunha, J.E.G.; Möbius, W.; Outeiro, T.F.; et al. Seeding variability of different alpha synuclein strains in synucleinopathies. Ann. Neurol. 2019, 85, 691–703. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating alpha-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef]
- Lewis, K.A.; Su, Y.; Jou, O.; Ritchie, C.; Foong, C.; Hynan, L.S.; White, C.L., III; Thomas, P.J.; Hatanpaa, K.J. Abnormal neurites containing C-terminally truncated alpha-synuclein are present in Alzheimer’s disease without conventional Lewy body pathology. Am. J. Pathol. 2010, 177, 3037–3050. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orru, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Giasson, B.I.; Lewis, K.A.; Lee, V.M.; Demartino, G.N.; Thomas, P.J. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: Implications for pathogenesis of Parkinson disease. J. Biol. Chem. 2005, 280, 22670–22678. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.V.; Giasson, B.I.; Quinn, S.M.; Koppaka, V.; Axelsen, P.H.; Ischiropoulos, H.; Trojanowski, J.Q.; Lee, V.M. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry 2003, 42, 8530–8540. [Google Scholar] [CrossRef] [PubMed]
- Periquet, M.; Fulga, T.; Myllykangas, L.; Schlossmacher, M.G.; Feany, B.M. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J. Neurosci. 2007, 27, 3338–3346. [Google Scholar] [CrossRef] [PubMed]
- Michell, A.W.; Tofaris, G.K.; Gossage, H.; Tyers, P.; Spillantini, M.G.; Barker, R.A. The effect of truncated human alpha-synuclein (1-120) on dopaminergic cells in a transgenic mouse model of Parkinson’s disease. Cell Transpl. 2007, 16, 461–474. [Google Scholar] [CrossRef]
- Baba, M.; Nakajo, S.; Tu, P.H.; Tomita, T.; Nakaya, K.; Lee, V.M.; Trojanowski, J.Q.; Iwatsubo, T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998, 152, 879–884. [Google Scholar]
- Sorrentino, Z.A.; Vijayaraghavan, N.; Gorion, K.M.; Riffe, C.J.; Strang, K.H.; Caldwell, J.; Giasson, B.I. Physiological C-terminal truncation of alpha-synuclein potentiates the prion-like formation of pathological inclusions. J. Biol. Chem. 2018, 293, 18914–18932. [Google Scholar] [CrossRef]
- Chakroun, T.; Evsyukov, V.; Nykanen, N.P.; Hollerhage, M.; Schmidt, A.; Kamp, F.; Ruf, V.C.; Wurst, W.; Rosler, T.W.; Hoglinger, G.U. Alpha-synuclein fragments trigger distinct aggregation pathways. Cell Death Dis. 2020, 11, 84. [Google Scholar] [CrossRef]
- Vaikath, N.N.; Erskine, D.; Morris, C.M.; Majbour, N.K.; Vekrellis, K.; Li, J.Y.; El-Agnaf, O.M.A. Heterogeneity in alpha-synuclein subtypes and their expression in cortical brain tissue lysates from Lewy body diseases and Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2019, 45, 597–608. [Google Scholar] [CrossRef]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef]
- Koss, D.J.; Dubini, M.; Buchanan, H.; Hull, C.; Platt, B. Distinctive temporal profiles of detergent-soluble and -insoluble tau and Abeta species in human Alzheimer’s disease. Brain Res. 2018, 1699, 121–134. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.M.G.; Elia, A.E.; Portaleone, S.M.; Cazzaniga, F.A.; Rossi, M.; Bistaffa, E.; De Cecco, E.; Narkiewicz, J.; Salzano, G.; Carletta, O.; et al. Efficient RT-QuIC seeding activity for alpha-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl. Neurodegener. 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Manne, S.; Kondru, N.; Jin, H.; Anantharam, V.; Huang, X.; Kanthasamy, A.; Kanthasamy, A.G. alpha-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov. Disord. 2020, 35, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Candelise, N.; Schmitz, M.; Thune, K.; Cramm, M.; Rabano, A.; Zafar, S.; Stoops, E.; Vanderstichele, H.; Villar-Pique, A.; Llorens, F.; et al. Effect of the micro-environment on alpha-synuclein conversion and implication in seeded conversion assays. Transl. Neurodegener. 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Koob, A.O.; Shaked, G.M.; Bender, A.; Bisquertt, A.; Rockenstein, E.; Masliah, E. Neurogranin binds alpha-synuclein in the human superior temporal cortex and interaction is decreased in Parkinson’s disease. Brain. Res. 2014, 1591, 102–110. [Google Scholar] [CrossRef]
- Kellie, J.F.; Higgs, R.E.; Ryder, J.W.; Major, A.; Beach, T.G.; Adler, C.H.; Merchant, K.; Knierman, M.D. Quantitative measurement of intact alpha-synuclein proteoforms from post-mortem control and Parkinson’s disease brain tissue by intact protein mass spectrometry. Sci. Rep. 2014, 4, 5797. [Google Scholar] [CrossRef]
- Dufty, B.M.; Warner, L.R.; Hou, S.T.; Jiang, S.X.; Gomez-Isla, T.; Leenhouts, K.M.; Oxford, J.T.; Feany, M.B.; Masliah, E.; Rohn, T.T. Calpain-cleavage of alpha-synuclein: Connecting proteolytic processing to disease-linked aggregation. Am. J. Pathol. 2007, 170, 1725–1738. [Google Scholar]





| a | TBS-Soluble Fraction | Detergent-Soluble Fraction | ||||||
| Temporal | Sens. | Spec. | p * | Cutoff | Sens. | Spec. | p * | Cutoff |
| (RT-QuIC AUC) | (RT-QuIC AUC) | |||||||
| αSyn 1-140 | ||||||||
| Cases vs. HC | 100% | 100% | 0.006 | >5,686,027 | 100% | 100% | 0.007 | >4,010,801 |
| PD vs. HC | 100% | 100% | 0.02 | >5,686,027 | 100% | 100% | 0.02 | >5,343,731 |
| DLB vs. HC | 100% | 100% | 0.02 | >5,889,846 | 100% | 100% | 0.02 | >4,010,801 |
| PD vs. DLB | 75% | 50% | 0.563 | <14,474,992 | 100% | 75% | 0.043 | >10,077,548 |
| αSyn 1-130 | ||||||||
| Cases vs. HC | 100% | 100% | 0.003 | >1,957,147 | 94% | 100% | 0.003 | >1,367,850 |
| PD vs. HC | 100% | 100% | 0.007 | >2,775,490 | 100% | 100% | 0.007 | >700,526 |
| DLB vs. HC | 100% | 100% | 0.007 | >1,957,147 | 88% | 100% | 0.007 | >1,404,507 |
| PD vs. DLB | 75% | 50% | 0.834 | <16,198,128 | 88% | 50% | 0.401 | >3,158,315 |
| αSyn 1-115 | ||||||||
| Cases vs. HC | 100% | 100% | 0.003 | >5,198,217 | 94% | 100% | 0.003 | >6,530,860 |
| PD vs. HC | 100% | 100% | 0.007 | >5,198,217 | 100% | 100% | 0.007 | >4,395,742 |
| DLB vs. HC | 100% | 100% | 0.007 | >6,335,727 | 100% | 100% | 0.007 | >2,164,070 |
| PD vs. DLB | 63% | 75% | 0.142 | <14,687,906 | 63% | 63% | 0.207 | <13,688,774 |
| b | TBS-soluble fraction | Detergent-soluble fraction | ||||||
| Frontal | Sens. | Spec. | p * | Cutoff | Sens. | Spec. | p * | Cutoff |
| (RT-QuIC AUC) | (RT-QuIC AUC) | |||||||
| αSyn 1-140 | ||||||||
| Cases vs. HC | 100% | 100% | 0.006 | <35,870 | 100% | 100% | 0.006 | >64.66 |
| PD vs. HC | 100% | 100% | 0.02 | >515,271 | 100% | 100% | 0.02 | >72.59 |
| DLB vs. HC | 100% | 100% | 0.02 | >35,870 | 100% | 100% | 0.006 | >64.66 |
| PD vs. DLB | 75% | 100% | 0.25 | <893,180 | 100% | 75% | 0.15 | <200,163 |
| αSyn 1-130 | ||||||||
| Cases vs. HC | 100% | 100% | 0.001 | >5,142,617 | 100% | 50% | 0.024 | 0.7891 |
| PD vs. HC | 100% | 100% | 0.001 | >5,142,617 | 88% | 88% | 0.003 | 0.9375 |
| DLB vs. HC | 88% | 88% | 0.005 | >4,666,272 | 88% | 50% | 0.345 | >318,073 |
| PD vs. DLB | 75% | 88% | 0.093 | >10,642,878 | 88% | 100% | 0.001 | <957,841 |
| αSyn 1-115 | ||||||||
| Cases vs. HC | 88% | 75% | 0.006 | >11,421,461 | 87% | 88% | 0.001 | >673,667 |
| PD vs. HC | 75% | 88% | 0.021 | >13,701,264 | 88% | 88% | 0.002 | >1,205,104 |
| DLB vs. HC | 88% | 75% | 0.016 | >11,421,461 | 88% | 88% | 0.009 | >673,667 |
| PD vs. DLB | 88% | 50% | 0.528 | <15,596,073 | 75% | 75% | 0.248 | <4,442,097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poggiolini, I.; Erskine, D.; Vaikath, N.N.; Ponraj, J.; Mansour, S.; Morris, C.M.; El-Agnaf, O.M.A. RT-QuIC Using C-Terminally Truncated α-Synuclein Forms Detects Differences in Seeding Propensity of Different Brain Regions from Synucleinopathies. Biomolecules 2021, 11, 820. https://doi.org/10.3390/biom11060820
Poggiolini I, Erskine D, Vaikath NN, Ponraj J, Mansour S, Morris CM, El-Agnaf OMA. RT-QuIC Using C-Terminally Truncated α-Synuclein Forms Detects Differences in Seeding Propensity of Different Brain Regions from Synucleinopathies. Biomolecules. 2021; 11(6):820. https://doi.org/10.3390/biom11060820
Chicago/Turabian StylePoggiolini, Ilaria, Daniel Erskine, Nishant N. Vaikath, Janarthanan Ponraj, Said Mansour, Christopher M. Morris, and Omar M. A. El-Agnaf. 2021. "RT-QuIC Using C-Terminally Truncated α-Synuclein Forms Detects Differences in Seeding Propensity of Different Brain Regions from Synucleinopathies" Biomolecules 11, no. 6: 820. https://doi.org/10.3390/biom11060820
APA StylePoggiolini, I., Erskine, D., Vaikath, N. N., Ponraj, J., Mansour, S., Morris, C. M., & El-Agnaf, O. M. A. (2021). RT-QuIC Using C-Terminally Truncated α-Synuclein Forms Detects Differences in Seeding Propensity of Different Brain Regions from Synucleinopathies. Biomolecules, 11(6), 820. https://doi.org/10.3390/biom11060820






