From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues
Abstract
:1. Introduction
2. The ECM, Focal Adhesions and Adherens Junctions in Periodontal Health and Disease
3. Mechanotransduction to the Core: YAP/TAZ in the Periodontium
4. The Gist of the Matter: Nuclear Mechanotransduction
5. Porphyromonas gingivalis-Derived Proteases: A “Heavy Load” for the Periodontium
6. May the Force Be with You: MT and Its Implications for Periodontal Regeneration
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vogel, V. Unraveling the mechanobiology of extracellular matrix. Annu. Rev. Physiol. 2018, 80, 353–387. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Hillege, M.M.; Wüst, R.C.; Wu, G.; Jaspers, R.T. Synergistic short-term and long-term effects of TGF-β1 and 3 on collagen production in differentiating myoblasts. Biochem. Biophys. Res. Commun. 2021, 547, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, N.B.; Jurj, A.; Sorițău, O.; Lucaciu, O.P.; Dirzu, N.; Raduly, L.; Berindan-Neagoe, I.; Cenariu, M.; Boșca, B.A.; Campian, R.S. Cannabidiol and Vitamin D3 Impact on Osteogenic Differentiation of Human Dental Mesenchymal Stem Cells. Medicina 2020, 56, 607. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Debbi, L.; Zohar, B.; Samuel, R.; Arzi, R.S.; Fried, A.I.; Carmon, T.; Shevach, D.; Redenski, I.; Schlachet, I. Stimulating Extracellular Vesicles Production from Engineered Tissues by Mechanical Forces. Nano Lett. 2021, 21, 2497–2504. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G.; Habal, M.B. BMP Application as Grafting Materials for Bone Regeneration in the Craniofacial Surgery: Current Application and Future Directions by an RCT Analysis. J. Craniofac. Surg. 2020, 32, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-T.; Heuer, R.A.; Oleksijew, A.M.; Coots, K.S.; Roque, C.B.; Nella, K.T.; McGuire, T.L.; Matsuoka, A.J. An engineered three-dimensional stem cell niche in the inner ear by applying a nanofibrillar cellulose hydrogel with a sustained-release neurotrophic factor delivery system. Acta Biomater. 2020, 108, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Danish, A.; Gedschold, R.; Hinz, S.; Schiedel, A.C.; Thimm, D.; Bedner, P.; Steinhäuser, C.; Müller, C.E. A Cellular Assay for the Identification and Characterization of Connexin Gap Junction Modulators. Int. J. Mol. Sci. 2021, 22, 1417. [Google Scholar] [CrossRef]
- Imafuku, K.; Kamaguchi, M.; Natsuga, K.; Nakamura, H.; Shimizu, H.; Iwata, H. Zonula occludens-1 demonstrates a unique appearance in buccal mucosa over several layers. Cell Tissue Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Patil, R.; Kale, A.D.; Mane, D.R.; Patil, D. Isolation, culture and characterization of primary cell lines of human buccal mucosal fibroblasts: A combination of explant enzamytic technique. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 68. [Google Scholar] [CrossRef]
- Fagalde, P.; Reininger, D. Oral tissues regeneration using intraoral mesenchymal stem cells. J. Clin. Exp. Dent. 2021, 13, e268. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Lele, T.P. Periodontal cell mechanotransduction. Open Biol. 2018, 8, 180053. [Google Scholar] [CrossRef] [Green Version]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Zuidema, A.; Wang, W.; Sonnenberg, A. Crosstalk between Cell Adhesion Complexes in Regulation of Mechanotransduction. BioEssays 2020, 42, 2000119. [Google Scholar] [CrossRef]
- Naqvi, S.M.; McNamara, L.M. Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 1375. [Google Scholar] [CrossRef]
- Case, L.B.; Waterman, C.M. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 2015, 17, 955–963. [Google Scholar] [CrossRef]
- Hatte, G.; Prigent, C.; Tassan, J.-P. Adherens junctions are involved in polarized contractile ring formation in dividing epithelial cells of Xenopus laevis embryos. Exp. Cell Res. 2021, 402, 112525. [Google Scholar] [CrossRef]
- Jasuja, H.; Kar, S.; Katti, D.R.; Katti, K. Perfusion bioreactor enabled fluid-derived shear stress conditions for novel bone metastatic prostate cancer testbed. Biofabrication 2021, 13, 035004. [Google Scholar] [CrossRef]
- Jaumouillé, V.; Cartagena-Rivera, A.X.; Waterman, C.M. Coupling of β 2 integrins to actin by a mechanosensitive molecular clutch drives complement receptor-mediated phagocytosis. Nat. Cell Biol. 2019, 21, 1357–1369. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Qian, K.-j.; Yu, J.; Zhu, H.-y. Effects of nanofibers on mesenchymal stem cells: Environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms. J. Zhejiang Univ. Sci. B 2020, 21, 871–884. [Google Scholar] [CrossRef]
- Liu, M.; Banerjee, R.; Rossa Jr, C.; D’Silva, N. RAP1-RAC1 signaling has an important role in adhesion and migration in HNSCC. J. Dent. Res. 2020, 99, 959–968. [Google Scholar] [CrossRef]
- Jacob, A.E.; Amack, J.D.; Turner, C.E. Paxillin genes and actomyosin contractility regulate myotome morphogenesis in zebrafish. Dev. Biol. 2017, 425, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Pence, L.J.; Kourtidis, A.; Feathers, R.W.; Haddad, M.T.; Sotiriou, S.; Decker, P.A.; Nassar, A.; Ocal, I.T.; Shah, S.S.; Anastasiadis, P.Z. PLEKHA7, an Apical Adherens Junction Protein, Suppresses Inflammatory Breast Cancer in the Context of High E-Cadherin and p120-Catenin Expression. Int. J. Mol. Sci. 2021, 22, 1275. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.E.; Sotomayor, M. Crystal structure of the nonclassical cadherin-17 N-terminus and implications for its adhesive binding mechanism. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2021, 77, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ilina, O.; Gritsenko, P.G.; Syga, S.; Lippoldt, J.; La Porta, C.A.; Chepizhko, O.; Grosser, S.; Vullings, M.; Bakker, G.-J.; Starruß, J. Cell–cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat. Cell Biol. 2020, 22, 1103–1115. [Google Scholar] [CrossRef]
- Dahl, K.N.; Kahn, S.M.; Wilson, K.L.; Discher, D.E. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 2004, 117, 4779–4786. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.; Lammerding, J. The driving force: Nuclear mechanotransduction in cellular function, fate, and disease. Annu. Rev. Biomed. Eng. 2019, 21, 443–468. [Google Scholar] [CrossRef]
- Reynolds, N.; McEvoy, E.; Ghosh, S.; Pérez, J.A.P.; Neu, C.P.; McGarry, P. Image-derived modeling of nucleus strain amplification associated with chromatin heterogeneity. Biophys. J. 2021, 120, 1323–1332. [Google Scholar] [CrossRef]
- Owens, D.J.; Messéant, J.; Moog, S.; Viggars, M.; Ferry, A.; Mamchaoui, K.; Lacène, E.; Roméro, N.; Brull, A.; Bonne, G. Lamin-related congenital muscular dystrophy alters mechanical signaling and skeletal muscle growth. Int. J. Mol. Sci. 2021, 22, 306. [Google Scholar]
- Lin, J.D.; Ryder, M.; Kang, M.; Ho, S.P. Biomechanical pathways of dentoalveolar fibrous joints in health and disease. Periodontology 2000 2020, 82, 238–256. [Google Scholar] [CrossRef]
- Connizzo, B.; Sun, L.; Lacin, N.; Gendelman, A.; Solomonov, I.; Sagi, I.; Grodzinsky, A.; Naveh, G. Nonuniformity in Periodontal Ligament: Mechanics and Matrix Composition. J. Dent. Res. 2020, 100, 179–186. [Google Scholar] [CrossRef]
- Li, Z.; Yu, M.; Jin, S.; Wang, Y.; Luo, R.; Huo, B.; Liu, D.; He, D.; Zhou, Y.; Liu, Y. Stress distribution and collagen remodeling of periodontal ligament during orthodontic tooth movement. Front. Pharmacol. 2019, 10, 1263. [Google Scholar] [CrossRef]
- Takewaki, M.; Kajiya, M.; Takeda, K.; Sasaki, S.; Motoike, S.; Komatsu, N.; Matsuda, S.; Ouhara, K.; Mizuno, N.; Fujita, T. MSC/ECM cellular complexes induce periodontal tissue regeneration. J. Dent. Res. 2017, 96, 984–991. [Google Scholar] [CrossRef]
- Lundmark, A.; Johannsen, G.; Eriksson, K.; Kats, A.; Jansson, L.; Tervahartiala, T.; Rathnayake, N.; Åkerman, S.; Klinge, B.; Sorsa, T. Mucin 4 and matrix metalloproteinase 7 as novel salivary biomarkers for periodontitis. J. Clin. Periodontol. 2017, 44, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, M.; Shirasugi, M.; Yamamoto, T.; Nakaya, T.; Kanamura, N. Long-term exposure to butyric acid induces excessive production of matrix metalloproteases in human gingival fibroblasts. Arch. Oral Biol. 2021, 123, 105035. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, Y.; Kim, M.-K.; Park, H.-R.; Kim, H.-J.; Bae, S.-K.; Bae, M.-K. Infection of Porphyromonas gingivalis Increases Phosphate-Induced Calcification of Vascular Smooth Muscle Cells. Cells 2020, 9, 2694. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Blufstein, A.; Schubert, M.; Rausch-Fan, X.; Andrukhov, O.; Jonke, E. Interleukin-1β Induced Matrix Metalloproteinase Expression in Human Periodontal Ligament-Derived Mesenchymal Stromal Cells under In Vitro Simulated Static Orthodontic Forces. Int. J. Mol. Sci. 2021, 22, 1027. [Google Scholar] [CrossRef]
- Kamarajan, P.; Ateia, I.; Shin, J.M.; Fenno, J.C.; Le, C.; Zhan, L.; Chang, A.; Darveau, R.; Kapila, Y.L. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 2020, 16, e1008881. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Zhao, X.; Xu, Z.; Dai, W.; Duan, W.; Huang, S.; Zhang, E.; Liu, J.; Zhang, S. Composition and function of oral microbiota between gingival squamous cell carcinoma and periodontitis. Oral Oncol. 2020, 107, 104710. [Google Scholar] [CrossRef]
- Batool, H.; Nadeem, A.; Kashif, M.; Shahzad, F.; Tahir, R.; Afzal, N. Salivary levels of IL-6 and IL-17 could be an indicator of disease severity in patients with calculus associated chronic periodontitis. BioMed Res. Int. 2018, 2018, 8531961. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, R.; López, S.; Romo, E.; Montoya, G.; Hoz, L.; Pedraza, C.; Garfias, Y.; Arzate, H. Carboxy-Terminal Cementum Protein 1-Derived Peptide 4 (cemp1-p4) Promotes Mineralization through wnt/β-catenin Signaling in Human Oral Mucosa Stem Cells. Int. J. Mol. Sci. 2020, 21, 1307. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.; Amorim, B.R.; Salmon, C.R.; Leme, A.F.P.; Kantovitz, K.R.; Nociti Jr, F.H. Novel LRAP-binding partner revealing the plasminogen activation system as a regulator of cementoblast differentiation and mineral nodule formation in vitro. J. Cell. Physiol. 2020, 235, 4545–4558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Xu, M.; Zheng, J.; Xu, Y.; Chen, G.; Guo, Q.; Tian, W.; Guo, W. The Dual Effects of Reactive Oxygen Species on the Mandibular Alveolar Bone Formation in SOD1 Knockout Mice: Promotion or Inhibition. Oxidative Med. Cell. Longev. 2021, 2021, 8847140. [Google Scholar] [CrossRef] [PubMed]
- Denes, B.J.; Ait-Lounis, A.; Wehrle-Haller, B.; Kiliaridis, S. Core matrisome protein signature during periodontal ligament maturation from pre-occlusal eruption to occlusal function. Front. Physiol. 2020, 11, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zvackova, I.; Matalova, E.; Lesot, H. Regulators of collagen fibrillogenesis during molar development in the mouse. Front. Physiol. 2017, 8, 554. [Google Scholar] [CrossRef] [Green Version]
- Kurylo, M.P.; Grandfield, K.; Marshall, G.W.; Altoe, V.; Aloni, S.; Ho, S.P. Effect of proteoglycans at interfaces as related to location, architecture, and mechanical cues. Arch. Oral Biol. 2016, 63, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Chen, Q.; Zheng, Y.; Nan, L.; Liao, N.; Mo, S. Potential Role of Integrin α5β1/Focal Adhesion Kinase (FAK) and Actin Cytoskeleton in the Mechanotransduction and Response of Human Gingival Fibroblasts Cultured on a 3-Dimension Lactide-Co-Glycolide (3D PLGA) Scaffold. Med Sci. Monit. Int. Med J. Exp. Clin. Res. 2020, 26, e921621–e921626. [Google Scholar]
- Hetmanski, J.H.; Jones, M.C.; Chunara, F.; Schwartz, J.-M.; Caswell, P.T. Combinatorial mathematical modelling approaches to interrogate rear retraction dynamics in 3D cell migration. PLoS Comput. Biol. 2021, 17, e1008213. [Google Scholar] [CrossRef]
- Kao, T.-W.; Chiou, A.; Lin, K.-H.; Liu, Y.-S.; Lee, O.K.-S. Alteration of 3D Matrix Stiffness Regulates Viscoelasticity of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 2441. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Hegedűs, O.; Juriga, D.; Sipos, E.; Voniatis, C.; Juhász, Á.; Idrissi, A.; Zrínyi, M.; Varga, G.; Jedlovszky-Hajdú, A.; Nagy, K.S. Free thiol groups on poly (aspartamide) based hydrogels facilitate tooth-derived progenitor cell proliferation and differentiation. PLoS ONE 2019, 14, e0226363. [Google Scholar] [CrossRef]
- Feld, L.; Kellerman, L.; Mukherjee, A.; Livne, A.; Bouchbinder, E.; Wolfenson, H. Cellular contractile forces are nonmechanosensitive. Sci. Adv. 2020, 6, eaaz6997. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Ramos, A.M.; Álvarez-García, Y.R.; Solodin, N.; Almodovar, J.; Alarid, E.T.; Torres-Garcia, W.; Domenech, M. Collagen I Fibrous Substrates Modulate the Proliferation and Secretome of Estrogen Receptor-Positive Breast Tumor Cells in a Hormone-Restricted Microenvironment. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Wei, D.; Li, C.; Ye, J.; Xiang, F.; Liu, J. Extracellular Collagen Mediates Osteosarcoma Progression Through an Integrin α2β1/JAK/STAT3 Signaling Pathway. Cancer Manag. Res. 2020, 12, 12067. [Google Scholar] [CrossRef]
- Xing, Q.; Parvizi, M.; Higuita, M.L.; Griffiths, L.G. Basement membrane proteins modulate cell migration on bovine pericardium extracellular matrix scaffold. Sci. Rep. 2021, 11, 4607. [Google Scholar] [CrossRef]
- Al-Yafeai, Z.; Orr, A.W. Quantification of integrin activation and ligation in adherent cells. In The Integrin Interactome; Springer: New York, NY, USA, 2021; pp. 17–25. [Google Scholar]
- Ye, Y.; Zhang, R.; Feng, H. Fibronectin promotes tumor cells growth and drugs resistance through a CDC42-YAP-dependent signaling pathway in colorectal cancer. Cell Biol. Int. 2020, 44, 1840–1849. [Google Scholar] [CrossRef]
- Roy, S.; Spinali, K.; Schmuck, E.G.; Kink, J.A.; Hematti, P.; Raval, A.N. Cardiac fibroblast derived matrix-educated macrophages express VEGF and IL-6, and recruit mesenchymal stromal cells. J. Immunol. Regen. Med. 2020, 10, 100033. [Google Scholar]
- Sugahara, M.; Nakaoki, Y.; Yamaguchi, A.; Hashimoto, K.; Miyamoto, Y. Vitronectin is involved in the morphological transition of neurites in retinoic acid-induced neurogenesis of neuroblastoma cell line neuro2a. Neurochem. Res. 2019, 44, 1621–1635. [Google Scholar] [CrossRef]
- Jakhu, H.; Gill, G.; Singh, A. Role of integrins in wound repair and its periodontal implications. J. Oral Biol. Craniofac. Res. 2018, 8, 122–125. [Google Scholar] [CrossRef]
- Jang, A.; Wang, B.; Ustriyana, P.; Gansky, S.A.; Maslenikov, I.; Useinov, A.; Prevost, R.; Ho, S.P. Functional adaptation of interradicular alveolar bone to reduced chewing loads on dentoalveolar joints in rats. Dent. Mater. 2021, 37, 486–495. [Google Scholar] [CrossRef]
- Husari, A.; Steinberg, T.; Dieterle, M.P.; Prucker, O.; Rühe, J.; Jung, B.; Tomakidi, P. On the relationship of YAP and FAK in hMSCs and osteosarcoma cells: Discrimination of FAK modulation by nuclear YAP depletion or YAP silencing. Cell. Signal. 2019, 63, 109382. [Google Scholar] [CrossRef]
- Belgardt, E.; Steinberg, T.; Husari, A.; Dieterle, M.P.; Hülter-Hassler, D.; Jung, B.; Tomakidi, P. Force-responsive Zyxin modulation in periodontal ligament cells is regulated by YAP rather than TAZ. Cell. Signal. 2020, 72, 109662. [Google Scholar] [CrossRef]
- Gao, W.-J.; Liu, J.-X.; Xie, Y.; Luo, P.; Liu, Z.-Q.; Liu, L.; Zhou, H. Suppression of macrophage migration by down-regulating Src/FAK/P130Cas activation contributed to the anti-inflammatory activity of sinomenine. Pharmacol. Res. 2021, 167, 105513. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, H.; Tai, Y.; Xue, Y.; Wei, Y.; Wang, K.; Zhao, Q.; Wang, S.; Kong, D.; Midgley, A.C. Design and Evaluation of a Polypeptide that Mimics the Integrin Binding Site for EDA Fibronectin to Block Profibrotic Cell Activity. Int. J. Mol. Sci. 2021, 22, 1575. [Google Scholar] [CrossRef]
- Boujemaa-Paterski, R.; Martins, B.; Eibauer, M.; Beales, C.T.; Geiger, B.; Medalia, O. Talin-activated vinculin interacts with branched actin networks to initiate bundles. eLife 2020, 9, e53990. [Google Scholar] [CrossRef]
- Damiano-Guercio, J.; Kurzawa, L.; Mueller, J.; Dimchev, G.; Schaks, M.; Nemethova, M.; Pokrant, T.; Brühmann, S.; Linkner, J.; Blanchoin, L. Loss of Ena/VASP interferes with lamellipodium architecture, motility and integrin-dependent adhesion. eLife 2020, 9, e55351. [Google Scholar] [CrossRef]
- Hsiao, B.-Y.; Chen, C.-H.; Chi, H.-Y.; Yen, P.-R.; Yu, Y.-Z.; Lin, C.-H.; Pang, T.-L.; Lin, W.-C.; Li, M.-L.; Yeh, Y.-C. Human Costars Family Protein ABRACL Modulates Actin Dynamics and Cell Migration and Associates with Tumorigenic Growth. Int. J. Mol. Sci. 2021, 22, 2037. [Google Scholar] [CrossRef]
- Roopnarine, O.; Thomas, D.D. Mechanistic analysis of actin-binding compounds that affect the kinetics of cardiac myosin-actin interaction. J. Biol. Chem. 2021, 196, 100471. [Google Scholar] [CrossRef]
- Mani, S.; Katkar, H.H.; Voth, G.A. Compressive and Tensile Deformations Alter ATP Hydrolysis and Phosphate Release Rates in Actin Filaments. J. Chem. Theory Comput. 2021, 17, 1900–1913. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, Y.; Liu, L.; Zhao, J.; Zhang, T.; Xiao, L.; Jia, M.; Tian, Q.; Yu, H.; Chen, S. SEPT9_i1 regulates human breast cancer cell motility through cytoskeletal and RhoA/FAK signaling pathway regulation. Cell Death Dis. 2019, 10, 720. [Google Scholar] [CrossRef] [Green Version]
- Kurotsu, S.; Sadahiro, T.; Fujita, R.; Tani, H.; Yamakawa, H.; Tamura, F.; Isomi, M.; Kojima, H.; Yamada, Y.; Abe, Y. Soft Matrix Promotes Cardiac Reprogramming via Inhibition of YAP/TAZ and Suppression of Fibroblast Signatures. Stem Cell Rep. 2020, 15, 612–628. [Google Scholar] [CrossRef]
- Nikoloudaki, G.; Snider, P.; Simmons, O.; Conway, S.J.; Hamilton, D.W. Periostin and matrix stiffness combine to regulate myofibroblast differentiation and fibronectin synthesis during palatal healing. Matrix Biol. 2020, 94, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rens, E.G.; Edelstein-Keshet, L. Spots, stripes, and spiral waves in models for static and motile cells. J. Math. Biol. 2021, 82, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Ugawa, Y.; Yamamoto, T.; Kawamura, M.; Yamashiro, K.; Shimoe, M.; Tomikawa, K.; Hongo, S.; Maeda, H.; Takashiba, S. Rho-kinase regulates extracellular matrix-mediated osteogenic differentiation of periodontal ligament cells. Cell Biol. Int. 2017, 41, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ugawa, Y.; Yamashiro, K.; Shimoe, M.; Tomikawa, K.; Hongo, S.; Kochi, S.; Ideguchi, H.; Maeda, H.; Takashiba, S. Osteogenic differentiation regulated by Rho-kinase in periodontal ligament cells. Differentiation 2014, 88, 33–41. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ren, H. Profilin promotes formin-mediated actin filament assembly and vesicle transport during polarity formation in pollen. Plant Cell 2021. [Google Scholar] [CrossRef]
- Matarrese, P.; Vona, R.; Ascione, B.; Paggi, M.G.; Mileo, A.M. Physical Interaction between HPV16E7 and the Actin-Binding Protein Gelsolin Regulates Epithelial-Mesenchymal Transition via HIPPO-YAP Axis. Cancers 2021, 13, 353. [Google Scholar] [CrossRef]
- Choi, C.K.; Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.A.; Mogilner, A.; Horwitz, A.R. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008, 10, 1039–1050. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ugawa, Y.; Kawamura, M.; Yamashiro, K.; Kochi, S.; Ideguchi, H.; Takashiba, S. Modulation of microenvironment for controlling the fate of periodontal ligament cells: The role of Rho/ROCK signaling and cytoskeletal dynamics. J. Cell Commun. Signal. 2018, 12, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Romero, S.; Le Clainche, C.; Gautreau, A.M. Actin polymerization downstream of integrins: Signaling pathways and mechanotransduction. Biochem. J. 2020, 477, 1–21. [Google Scholar] [CrossRef]
- Ramirez, I.; Gholkar, A.A.; Velasquez, E.F.; Guo, X.; Tofig, B.; Damoiseaux, R.; Torres, J.Z. The myosin regulatory light chain Myl5 localizes to mitotic spindle poles and is required for proper cell division. Cytoskeleton 2021, 78, 23–35. [Google Scholar] [CrossRef]
- Jarvis, K.J.; Bell, K.M.; Loya, A.K.; Swank, D.M.; Walcott, S. Force-velocity and tension transient measurements from Drosophila jump muscle reveal the necessity of both weakly-bound cross-bridges and series elasticity in models of muscle contraction. Arch. Biochem. Biophys. 2021, 701, 108809. [Google Scholar] [CrossRef]
- Salomon, J.; Gaston, C.; Magescas, J.; Duvauchelle, B.; Canioni, D.; Sengmanivong, L.; Mayeux, A.; Michaux, G.; Campeotto, F.; Lemale, J. Contractile forces at tricellular contacts modulate epithelial organization and monolayer integrity. Nat. Commun. 2017, 8, 13998. [Google Scholar] [CrossRef]
- Schreiber, C.; Amiri, B.; Heyn, J.C.; Rädler, J.O.; Falcke, M. On the adhesion–velocity relation and length adaptation of motile cells on stepped fibronectin lanes. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Santa-Cruz Mateos, C.; Valencia-Expósito, A.; Palacios, I.M.; Martín-Bermudo, M.D. Integrins regulate epithelial cell shape by controlling the architecture and mechanical properties of basal actomyosin networks. PLoS Genet. 2020, 16, e1008717. [Google Scholar] [CrossRef]
- Uçar, M.C.; Lipowsky, R. Collective force generation by molecular motors is determined by strain-induced unbinding. Nano Lett. 2019, 20, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Cong, J.; Fang, B.; Wang, Q.; Su, Y.; Gu, T.; Luo, T. The mechanobiology of actin cytoskeletal proteins during cell–cell fusion. J. R. Soc. Interface 2019, 16, 20190022. [Google Scholar] [CrossRef] [Green Version]
- Van Helvert, S.; Friedl, P. Strain stiffening of fibrillar collagen during individual and collective cell migration identified by AFM nanoindentation. ACS Appl. Mater. Interfaces 2016, 8, 21946–21955. [Google Scholar] [CrossRef]
- Wu, C.; Bauer, J.; Juliano, R.; McDonald, J. The alpha 5 beta 1 integrin fibronectin receptor, but not the alpha 5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J. Biol. Chem. 1993, 268, 21883–21888. [Google Scholar] [CrossRef]
- Attia, M.S.; Alblowi, J.A. Effect of Subantimicrobial Dose Doxycycline Treatment on Gingival Crevicular Fluid Levels of MMP-9 and MMP-13 in Periodontitis Stage 2, Grade B in Subjects with Type 2 Diabetes Mellitus. J. Immunol. Res. 2020, 2020, 2807259. [Google Scholar] [CrossRef]
- Sato, T.; Verma, S.; Andrade, C.D.C.; Omeara, M.; Campbell, N.; Wang, J.S.; Cetinbas, M.; Lang, A.; Ausk, B.J.; Brooks, D.J. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. Nat. Commun. 2020, 11, 3282. [Google Scholar] [CrossRef]
- Li, X.; Ominsky, M.S.; Niu, Q.T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef]
- Muhamed, I.; Wu, J.; Sehgal, P.; Kong, X.; Tajik, A.; Wang, N.; Leckband, D.E. E-cadherin-mediated force transduction signals regulate global cell mechanics. J. Cell Sci. 2016, 129, 1843–1854. [Google Scholar] [CrossRef] [Green Version]
- Krischak, A.; Kowaliuk, J.; Sarsarshahi, S.; Dörr, W.; Kleiter, M. Effect of irradiation on the expression of E-cadherin and β-catenin in early and late radiation sequelae of the urinary bladder and its modulation by NF-κB inhibitor thalidomide. Strahlenther. Onkol. 2021, 197, 537–546. [Google Scholar] [CrossRef]
- Kluger, C.; Braun, L.; Sedlak, S.M.; Pippig, D.A.; Bauer, M.S.; Miller, K.; Milles, L.F.; Gaub, H.E.; Vogel, V. Different vinculin binding sites use the same mechanism to regulate directional force transduction. Biophys. J. 2020, 118, 1344–1356. [Google Scholar] [CrossRef] [Green Version]
- Chandran, R.; Kale, G.; Philippe, J.-M.; Lecuit, T.; Mayor, S. Distinct actin-dependent nanoscale assemblies underlie the dynamic and hierarchical organization of E-cadherin. Curr. Biol. 2021, 31, 1726–1736.e4. [Google Scholar] [CrossRef]
- Huang, S.-C.; Liang, J.Y.; Vu, L.V.; Faye, H.Y.; Ou, A.C.; Ou, J.P.; Zhang, H.S.; Burnett, K.M.; Benz, E.J., Jr. Epithelial-specific isoforms of protein 4.1 R promote adherens junction assembly in maturing epithelia. J. Biol. Chem. 2020, 295, 191–211. [Google Scholar] [CrossRef]
- Ishiyama, N.; Sarpal, R.; Wood, M.N.; Barrick, S.K.; Nishikawa, T.; Hayashi, H.; Kobb, A.B.; Flozak, A.S.; Yemelyanov, A.; Fernandez-Gonzalez, R. Force-dependent allostery of the α-catenin actin-binding domain controls adherens junction dynamics and functions. Nat. Commun. 2018, 9, 5121. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Chen, C.-C.; Hsueh, Y.-J.; Hung, L.-M.; Ma, D.H.-K.; Chen, H.-C.; Len, W.-B.; Meir, Y.-J.J. Extraneous E-Cadherin Engages the Deterministic Process of Somatic Reprogramming through Modulating STAT3 and Erk1/2 Activity. Cells 2021, 10, 284. [Google Scholar] [CrossRef]
- Ma, Y.-C.; Yang, Z.-S.; Ma, L.-Q.; Shu, R.; Zou, C.-G.; Zhang, K.-Q. YAP in epithelium senses gut barrier loss to deploy defenses against pathogens. PLoS Pathog. 2020, 16, e1008766. [Google Scholar] [CrossRef]
- Monster, J.L.; Donker, L.; Vliem, M.J.; Win, Z.; Matthews, H.K.; Cheah, J.S.; Yamada, S.; de Rooij, J.; Baum, B.; Gloerich, M. An asymmetric junctional mechanoresponse coordinates mitotic rounding with epithelial integrity. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef]
- Lim, J.C.; Bae, S.H.; Lee, G.; Ryu, C.J.; Jang, Y.J. Activation of β-catenin by TGF-β1 promotes ligament-fibroblastic differentiation and inhibits cementoblastic differentiation of human periodontal ligament cells. STEM CELLS 2020, 38, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.; Hemalatha, R.; Arun, K.; Kumar, T. E-cadherin and CD1a expression in gingival epithelium in periodontal health, disease and post-treatment. Indian J. Dent. Res. 2010, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, S.; Batra, M.; Abidullah, M.; Bhuvinder, S.; Katragadda, P. Role of E-cadherin in Progression of Oral Squamous Cell Carcinoma: A Retrospective Immunohistochemical Study. J. Contemp. Dent. Pract. 2018, 19, 1105–1110. [Google Scholar] [PubMed]
- Abe-Yutori, M.; Chikazawa, T.; Shibasaki, K.; Murakami, S. Decreased expression of E-cadherin by Porphyromonas gingivalis-lipopolysaccharide attenuates epithelial barrier function. J. Periodontal Res. 2017, 52, 42–50. [Google Scholar] [CrossRef]
- Sowmya, S.; Rao, R.S.; Prasad, K. Development of clinico-histopathological predictive model for the assessment of metastatic risk of oral squamous cell carcinoma. J. Carcinog. 2020, 19, 2. [Google Scholar] [CrossRef]
- Johnson, C.L.; Merryman, W.D. Side-specific valvular endothelial-interstitial cell mechano-communication via cadherin-11. J. Biomech. 2021, 119, 110253. [Google Scholar] [CrossRef]
- Piao, S.; Inglehart, R.C.; Scanlon, C.S.; Russo, N.; Banerjee, R.; D’Silva, N.J. CDH 11 inhibits proliferation and invasion in head and neck cancer. J. Oral Pathol. Med. 2017, 46, 89–97. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Kou, X.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Cao, H.; He, D.; Gan, Y. Cadherin-11 modulates cell morphology and collagen synthesis in periodontal ligament cells under mechanical stress. Angle Orthod. 2017, 87, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Row, S.; Liu, Y.; Alimperti, S.; Agarwal, S.K.; Andreadis, S.T. Cadherin-11 is a novel regulator of extracellular matrix synthesis and tissue mechanics. J. Cell Sci. 2016, 129, 2950–2961. [Google Scholar] [CrossRef] [Green Version]
- Alimperti, S.; You, H.; George, T.; Agarwal, S.K.; Andreadis, S.T. Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo. J. Cell Sci. 2014, 127, 2627–2638. [Google Scholar]
- Steinmetz, E.L.; Dewald, D.N.; Walldorf, U. Drosophila Homeodomain-Interacting Protein Kinase (Hipk) Phosphorylates the Hippo/Warts Signalling Effector Yorkie. Int. J. Mol. Sci. 2021, 22, 1862. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef]
- Dobrokhotov, O.; Samsonov, M.; Sokabe, M.; Hirata, H. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin. Transl. Med. 2018, 7, 23. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Dong, J.; Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003, 114, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.; Antenos, M.; Madan, P. A Comparative Analysis of Hippo Signaling Pathway Components during Murine and Bovine Early Mammalian Embryogenesis. Genes 2021, 12, 281. [Google Scholar] [CrossRef]
- Oh, J.-E.; Kim, H.; Kang, H.K.; Chung, C.-P.; Park, W.H.; Min, B.-M. α3β1 integrin promotes cell survival via multiple interactions between 14-3-3 isoforms and proapoptotic proteins. Exp. Cell Res. 2009, 315, 3187–3200. [Google Scholar] [CrossRef]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Spencer-Dene, B.; Stone, R.K.; Boeing, S.; Wculek, S.K.; Cordero, J.; Tan, E.H.; Ridgway, R.; Brunton, V.G. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 2016, 143, 1674–1687. [Google Scholar]
- Park, J.; Kim, J.S.; Nahm, J.H.; Kim, S.-K.; Lee, D.-H.; Lim, D.-S. WWC1 and NF2 Prevent the Development of Intrahepatic Cholangiocarcinoma by Regulating YAP/TAZ Activity through LATS in Mice. Mol. Cells 2020, 43, 491. [Google Scholar]
- Li, T.; Guo, T.; Liu, H.; Jiang, H.; Wang, Y. Platelet-derived growth factor-BB mediates pancreatic cancer malignancy via regulation of the Hippo/Yes-associated protein signaling pathway. Oncol. Rep. 2021, 45, 83–94. [Google Scholar] [CrossRef]
- Maziarz, M.; Federico, A.; Zhao, J.; Dujmusic, L.; Zhao, Z.; Monti, S.; Varelas, X.; Garcia-Marcos, M. Naturally occurring hotspot cancer mutations in Gα13 promote oncogenic signaling. J. Biol. Chem. 2020, 295, 16897–16904. [Google Scholar] [CrossRef]
- Strippoli, R.; Sandoval, P.; Moreno-Vicente, R.; Rossi, L.; Battistelli, C.; Terri, M.; Pascual-Antón, L.; Loureiro, M.; Matteini, F.; Calvo, E. Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis. Cell Death Dis. 2020, 11, 647. [Google Scholar] [CrossRef]
- Kang, P.H.; Schaffer, D.V.; Kumar, S. Angiomotin links ROCK and YAP signaling in mechanosensitive differentiation of neural stem cells. Mol. Biol. Cell 2020, 31, 386–396. [Google Scholar] [CrossRef]
- Pagliari, S.; Vinarsky, V.; Martino, F.; Perestrelo, A.R.; De La Cruz, J.O.; Caluori, G.; Vrbsky, J.; Mozetic, P.; Pompeiano, A.; Zancla, A. YAP–TEAD1 control of cytoskeleton dynamics and intracellular tension guides human pluripotent stem cell mesoderm specification. Cell Death Differ. 2021, 28, 1193–1207. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Xie, Z.; Chen, L.; Sinkemani, A.; Yu, H.; Wu, X. AMOT 130 linking F-actin to YAP is involved in intervertebral disc degeneration. Cell Prolif. 2018, 51, e12492. [Google Scholar] [CrossRef] [Green Version]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.K.-H.; Du, W.; Shelton, S.J.; Oldham, M.C.; DiPersio, C.M.; Klein, O.D. An FAK-YAP-mTOR signaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell 2017, 21, 91–106.e106. [Google Scholar] [CrossRef] [Green Version]
- Marikawa, Y.; Alarcon, V.B. RHOA activity in expanding blastocysts is essential to regulate HIPPO-YAP signaling and to maintain the trophectoderm-specific gene expression program in a ROCK/actin filament-independent manner. MHR Basic Sci. Reprod. Med. 2019, 25, 43–60. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, Q.; Wang, M.; Irani, S.; Li, X.; Zhang, Q.; Meng, F.; Liu, S.; Zhang, F.; Wu, L. The protein phosphatase PPM1A dephosphorylates and activates YAP to govern mammalian intestinal and liver regeneration. PLoS Biol. 2021, 19, e3001122. [Google Scholar] [CrossRef]
- Samarakoon, R.; Chitnis, S.S.; Higgins, S.P.; Higgins, C.E.; Krepinsky, J.C.; Higgins, P.J. Redox-induced Src kinase and caveolin-1 signaling in TGF-β1-initiated SMAD2/3 activation and PAI-1 expression. PLoS ONE 2011, 6, e22896. [Google Scholar] [CrossRef]
- Li, C.-Y.; Hu, J.; Lu, H.; Lan, J.; Du, W.; Galicia, N.; Klein, O.D. αE-catenin inhibits YAP/TAZ activity to regulate signalling centre formation during tooth development. Nat. Commun. 2016, 7, 12133. [Google Scholar] [CrossRef] [Green Version]
- Alisafaei, F.; Jokhun, D.S.; Shivashankar, G.; Shenoy, V.B. Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proc. Natl. Acad. Sci. USA 2019, 116, 13200–13209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Li, M.; Lin, J.; Hu, C. Hippo/TEAD4 signaling pathway as a potential target for the treatment of breast cancer. Oncol. Lett. 2021, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, W.; Wang, Q.; Fu, X.; Wang, Y.; Xu, X.; Wen, Y. C-MYC and BCL-2 mediate YAP-regulated tumorigenesis in OSCC. Oncotarget 2018, 9, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, T.; Remue, E.; Meerschaert, K.; Vanloo, B.; Boucherie, C.; Gfeller, D.; Bader, G.D.; Sidhu, S.S.; Vandekerckhove, J.; Gettemans, J. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem. J. 2010, 432, 461–478. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Schmitt, A.; Sudol, M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene 2012, 31, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Calderón, A.; Ávila-Flores, A.; Ponce, A.; López-Bayghen, E.; Calderón-Salinas, J.-V.; Luis Reyes, J.; Chávez-Munguía, B.; Segovia, J.; Angulo, C.; Ramírez, L. ZO-2 silencing induces renal hypertrophy through a cell cycle mechanism and the activation of YAP and the mTOR pathway. Mol. Biol. Cell 2016, 27, 1581–1595. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Lin, Q.; Wang, X.-P. YAP regulates the expression of Hoxa1 and Hoxc13 in mouse and human oral and skin epithelial tissues. Mol. Cell. Biol. 2015, 35, 1449–1461. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xiao, Y.; Hsu, C.-W.; Martinez-Traverso, I.M.; Zhang, M.; Bai, Y.; Ishii, M.; Maxson, R.E.; Olson, E.N.; Dickinson, M.E. Yap and Taz play a crucial role in neural crest-derived craniofacial development. Development 2016, 143, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Kaku, M.; Komatsu, Y.; Mochida, Y.; Yamauchi, M.; Mishina, Y.; Ko, C.-C. Identification and characterization of neural crest-derived cells in adult periodontal ligament of mice. Arch. Oral Biol. 2012, 57, 1668–1675. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Sun, X.; Jin, H. Role of YAP1 gene in proliferation, osteogenic differentiation, and apoptosis of human periodontal ligament stem cells induced by TNF-α. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Komatsu, N.; Kajiya, M.; Motoike, S.; Takewaki, M.; Horikoshi, S.; Iwata, T.; Ouhara, K.; Takeda, K.; Matsuda, S.; Fujita, T. Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes. Stem Cell Res. Ther. 2018, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, B.-K.; Chang, M.-L.; Wan, Z.-Q.; Han, G.-L. Cyclic stretch enhances osteogenic differentiation of human periodontal ligament cells via YAP activation. BioMed Res. Int. 2018, 2018, 2174824. [Google Scholar] [CrossRef]
- Wang, C.; Gu, W.; Sun, B.; Zhang, Y.; Ji, Y.; Xu, X.; Wen, Y. CTHRC1 promotes osteogenic differentiation of periodontal ligament stem cells by regulating TAZ. J. Mol. Histol. 2017, 48, 311–319. [Google Scholar] [CrossRef]
- Sun, B.; Wen, Y.; Wu, X.; Zhang, Y.; Qiao, X.; Xu, X. Expression pattern of YAP and TAZ during orthodontic tooth movement in rats. J. Mol. Histol. 2018, 49, 123–131. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, B.; Hu, R.; Tong, X.; Zhang, M.; Xu, C.; He, Z.; Zhao, Y.; Deng, H. TAZ contributes to osteogenic differentiation of periodontal ligament cells under tensile stress. J. Periodontal Res. 2020, 55, 152–160. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, X.N.; Lu, Y.; Wu, P.; Zhao, H.G.; Li, Q.L.; Xu, Y.H. miR-140 inhibits osteogenic differentiation of human periodontal ligament fibroblasts through ras homolog gene family, member A-transcriptional co-activator with PDZ-binding motif pathway. Kaohsiung J. Med Sci. 2021, 37, 38–46. [Google Scholar] [CrossRef]
- Hu, P.; Gao, Q.; Zheng, H.; Tian, Y.; Zheng, G.; Yao, X.; Zhang, J.; Wu, X.; Sui, L. The Role and Activation Mechanism of TAZ in Hierarchical Microgroove/Nanopore Topography-Mediated Regulation of Stem Cell Differentiation. Int. J. Nanomed. 2021, 16, 1021. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Chen, L.; Jiang, J.; Zhou, X.; Wang, M.; Fan, Y. Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2029–2040. [Google Scholar] [CrossRef]
- Kim, S.C.; Im, W.; Shim, J.Y.; Kim, S.-K.; Kim, B.J. Static magnetic field controls cell cycle in cultured human glioblastoma cells. Cytotechnology 2016, 68, 2745–2751. [Google Scholar] [CrossRef] [Green Version]
- Jouni, F.J.; Abdolmaleki, P.; Behmanesh, M.; Movahedin, M. An in vitro study of the impact of 4mT static magnetic field to modify the differentiation rate of rat bone marrow stem cells into primordial germ cells. Differentiation 2014, 87, 230–237. [Google Scholar] [CrossRef]
- Izzo, L.; Tunesi, M.; Boeri, L.; Laganà, M.; Giordano, C.; Raimondi, M.T. Influence of the static magnetic field on cell response in a miniaturized optically accessible bioreactor for 3D cell culture. Biomed. Microdevices 2019, 21, 531. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Gong, Y.; Bian, Z.; Zhang, X.; Xu, B.; Zhang, J.; Shi, X.; Yu, Y.; Song, L. Comparison of three different types of two-implant-supported magnetic attachments on the stress distribution in edentulous mandible. Comput. Math. Methods Med. 2019, 2019, 6839517. [Google Scholar] [CrossRef]
- Alfarsi, M.A.; Shaik, S. Oral rehabilitation of a cleft palate patient with tooth-supported, telescopic magnetic overdenture. BMJ Case Rep. CP 2020, 13, e233777. [Google Scholar] [CrossRef]
- He, Y.; Xu, H.; Xiang, Z.; Yu, H.; Xu, L.; Guo, Y.; Tian, Y.; Shu, R.; Yang, X.; Xue, C. YAP regulates periodontal ligament cell differentiation into myofibroblast interacted with RhoA/ROCK pathway. J. Cell. Physiol. 2019, 234, 5086–5096. [Google Scholar] [CrossRef]
- Jia, L.; Gu, W.; Zhang, Y.; Jiang, B.; Qiao, X.; Wen, Y. Activated Yes-associated protein accelerates cell cycle, inhibits apoptosis, and delays senescence in human periodontal ligament stem cells. Int. J. Med Sci. 2018, 15, 1241. [Google Scholar] [CrossRef] [Green Version]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.-J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef] [Green Version]
- Morice, S.; Mullard, M.; Brion, R.; Dupuy, M.; Renault, S.; Tesfaye, R.; Royer, B.-L.; Ory, B.; Redini, F.; Verrecchia, F. The YAP/TEAD Axis as a New Therapeutic Target in Osteosarcoma: Effect of Verteporfin and CA3 on Primary Tumor Growth. Cancers 2020, 12, 3847. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Gu, K.; Li, A.; Fu, X.; Wang, Y.; Gu, W.; Wen, Y. Effect of YAP on an immortalized periodontal ligament stem cell line. Stem Cells Int. 2019, 2019, 6804036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, H.Y.; Tang, J.; Casey, P.J.; Wang, M. Evaluating the Epithelial-Mesenchymal Program in Human Breast Epithelial Cells Cultured in Soft Agar Using a Novel Macromolecule Extraction Protocol. Cancers 2021, 13, 807. [Google Scholar] [CrossRef]
- Omori, H.; Nishio, M.; Masuda, M.; Miyachi, Y.; Ueda, F.; Nakano, T.; Sato, K.; Mimori, K.; Taguchi, K.; Hikasa, H. YAP1 is a potent driver of the onset and progression of oral squamous cell carcinoma. Sci. Adv. 2020, 6, eaay3324. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Pan, Y.; Guo, S.; Sun, L.; Zhang, C.; Wang, L. The roles of mechanosensitive ion channels and associated downstream MAPK signaling pathways in PDLC mechanotransduction. Mol. Med. Rep. 2020, 21, 2113–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco-Estevez, M.; Gadalla, K.K.; Liñan-Barba, N.; Cobb, S.; Dev, K.K.; Sheridan, G.K. Inhibition of Piezo1 attenuates demyelination in the central nervous system. Glia 2020, 68, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Fujii, S.; Matsumoto, S.; Tajiri, Y.; Kikuchi, A.; Kiyoshima, T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation. J. Pathol. 2021, 253, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, X.; Jiang, J.; Xiao, B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem. Sci. 2021, 46, 472–488. [Google Scholar] [CrossRef]
- Totaro, A.; Zhuang, Q.; Panciera, T.; Battilana, G.; Azzolin, L.; Brumana, G.; Gandin, A.; Brusatin, G.; Cordenonsi, M.; Piccolo, S. Cell phenotypic plasticity requires autophagic flux driven by YAP/TAZ mechanotransduction. Proc. Natl. Acad. Sci. USA 2019, 116, 17848–17857. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Füllgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef] [Green Version]
- Zainab, H.; Ameena Sultana, S. Stromal desmoplasia as a possible prognostic indicator in different grades of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 338. [Google Scholar]
- Matte, B.F.; Kumar, A.; Placone, J.K.; Zanella, V.G.; Martins, M.D.; Engler, A.J.; Lamers, M.L. Matrix stiffness mechanically conditions EMT and migratory behavior of oral squamous cell carcinoma. J. Cell Sci. 2019, 132, jcs224360. [Google Scholar] [CrossRef] [Green Version]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Dou, C.; Liu, Z.; Tu, K.; Zhang, H.; Chen, C.; Yaqoob, U.; Wang, Y.; Wen, J.; Van Deursen, J.; Sicard, D. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology 2018, 154, 2209–2221.e2214. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, M.B.; Sun, H.; Robichaux, J.; Pfeifer, M.; McDermott, U.; Travers, J.; Diao, L.; Xi, Y.; Tong, P.; Shen, L. A YAP/FOXM1 axis mediates EMT-associated EGFR inhibitor resistance and increased expression of spindle assembly checkpoint components. Sci. Transl. Med. 2020, 12, eaaz4589. [Google Scholar] [CrossRef]
- Wei, W.; Xue, L.; Tan, L.; Liu, J.; Yang, Q.; Wang, J.; Yan, B.; Cai, Q.; Yang, L.; Yue, Y. Inhibition of yes-associated protein dephosphorylation prevents aggravated periodontitis with occlusal trauma. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Pan, W.; Yang, L.; Li, J.; Xue, L.; Wei, W.; Ding, H.; Deng, S.; Tian, Y.; Yue, Y.; Wang, M. Traumatic occlusion aggravates bone loss during periodontitis and activates Hippo-YAP pathway. J. Clin. Periodontol. 2019, 46, 438–447. [Google Scholar] [CrossRef]
- Dupont, S. Regulation of YAP/TAZ activity by mechanical cues: An experimental overview. Hippo Pathw. 2019, 183–202. [Google Scholar] [CrossRef]
- Bautista, M.; Fernandez, A.; Pinaud, F. A Micropatterning Strategy to Study Nuclear Mechanotransduction in Cells. Micromachines 2019, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Stephens, A.D.; Liu, P.Z.; Banigan, E.J.; Almassalha, L.M.; Backman, V.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol. Biol. Cell 2018, 29, 220–233. [Google Scholar] [CrossRef]
- Morel, V.; Lepicard, S.; Rey, A.N.; Parmentier, M.-L.; Schaeffer, L. Drosophila Nesprin-1 controls glutamate receptor density at neuromuscular junctions. Cell. Mol. Life Sci. 2014, 71, 3363–3379. [Google Scholar] [CrossRef]
- Kracklauer, M.P.; Banks, S.M.; Xie, X.; Wu, Y.; Fischer, J.A. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly 2007, 1, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Ulianov, S.V.; Doronin, S.A.; Khrameeva, E.E.; Kos, P.I.; Luzhin, A.V.; Starikov, S.S.; Galitsyna, A.A.; Nenasheva, V.V.; Ilyin, A.A.; Flyamer, I.M. Nuclear lamina integrity is required for proper spatial organization of chromatin in Drosophila. Nat. Commun. 2019, 10, 1176. [Google Scholar] [CrossRef] [Green Version]
- Jahed, Z.; Hao, H.; Thakkar, V.; Vu, U.T.; Valdez, V.A.; Rathish, A.; Tolentino, C.; Kim, S.C.; Fadavi, D.; Starr, D.A. Role of KASH domain lengths in the regulation of LINC complexes. Mol. Biol. Cell 2019, 30, 2076–2086. [Google Scholar] [CrossRef]
- Gurusaran, M.; Davies, O.R. A molecular mechanism for LINC complex branching by structurally diverse SUN-KASH 6: 6 assemblies. eLife 2021, 10, e60175. [Google Scholar] [CrossRef] [PubMed]
- Alena, S.K.; Eva, B.; Aleš, K.; Emilie, L. Spatiotemporal Mislocalization of Nuclear Membrane-Associated Proteins in γ-Irradiation-Induced Senescent Cells. Cells 2020, 9, 999. [Google Scholar]
- Ketema, M.; Kreft, M.; Secades, P.; Janssen, H.; Sonnenberg, A. A Highlights from MBoC Selection: Nesprin-3 connects plectin and vimentin to the nuclear envelope of Sertoli cells but is not required for Sertoli cell function in spermatogenesis. Mol. Biol. Cell 2013, 24, 2454. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.; Quintremil, S.; Yi, J.; Vallee, R.B. Nesprin-2 recruitment of BicD2 to the nuclear envelope controls dynein/kinesin-mediated neuronal migration in vivo. Curr. Biol. 2020, 30, 3116–3129.e3114. [Google Scholar] [CrossRef]
- Holt, I.; Fuller, H.R.; Sewry, C.A.; Shirran, S.L.; Zhang, Q.; Shanahan, C.M.; Morris, G.E. Nesprin-1-alpha2 associates with kinesin at myotube outer nuclear membranes, but is restricted to neuromuscular junction nuclei in adult muscle. Sci. Rep. 2019, 9, 14202. [Google Scholar] [CrossRef] [Green Version]
- Porter, L.; Minaisah, R.-M.; Ahmed, S.; Ali, S.; Norton, R.; Zhang, Q.; Ferraro, E.; Molenaar, C.; Holt, M.; Cox, S. SUN1/2 are essential for RhoA/ROCK-regulated actomyosin activity in isolated vascular smooth muscle cells. Cells 2020, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Sakamoto, C.; Matsumori, H.; Katahira, J.; Yasuda, Y.; Yoshidome, K.; Tsujimoto, M.; Goldberg, I.G.; Matsuura, N.; Nakao, M. Loss of the integral nuclear envelope protein SUN1 induces alteration of nucleoli. Nucleus 2016, 7, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.; Lee, J.; Jeong, S.; Kang, S.-m.; Park, B.-J.; Ha, N.-C. Beta-strand-mediated dimeric formation of the Ig-like domains of human lamin A/C and B1. Biochem. Biophys. Res. Commun. 2021, 550, 191–196. [Google Scholar] [CrossRef]
- Parry, D.A.; Martin, C.-A.; Greene, P.; Marsh, J.A.; Blyth, M.; Cox, H.; Donnelly, D.; Greenhalgh, L.; Greville-Heygate, S.; Harrison, V. Heterozygous lamin B1 and lamin B2 variants cause primary microcephaly and define a novel laminopathy. Genet. Med. 2021, 23, 408–414. [Google Scholar] [CrossRef]
- Xie, W.; Chojnowski, A.; Boudier, T.; Lim, J.S.; Ahmed, S.; Ser, Z.; Stewart, C.; Burke, B. A-type lamins form distinct filamentous networks with differential nuclear pore complex associations. Curr. Biol. 2016, 26, 2651–2658. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-P.; Liu, J.-T.; Chen, Y.-X.; Wang, W.-B.; Han, Y.; Yao, Q.-P.; Qi, Y.-X. Suppressed nuclear envelope proteins activate autophagy of vascular smooth muscle cells during cyclic stretch application. Biochim. Biophys. Acta BBA Mol. Cell Res. 2021, 1868, 118855. [Google Scholar] [CrossRef]
- Shimi, T.; Kittisopikul, M.; Tran, J.; Goldman, A.E.; Adam, S.A.; Zheng, Y.; Jaqaman, K.; Goldman, R.D. Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol. Biol. Cell 2015, 26, 4075–4086. [Google Scholar] [CrossRef]
- Oldenburg, A.; Briand, N.; Sørensen, A.L.; Cahyani, I.; Shah, A.; Moskaug, J.Ø.; Collas, P. A lipodystrophy-causing lamin A mutant alters conformation and epigenetic regulation of the anti-adipogenic MIR335 locus. J. Cell Biol. 2017, 216, 2731–2743. [Google Scholar] [CrossRef] [Green Version]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410. [Google Scholar] [CrossRef]
- Serebryannyy, L.A.; Cruz, C.M.; De Lanerolle, P. A role for nuclear actin in HDAC 1 and 2 regulation. Sci. Rep. 2016, 6, 28460. [Google Scholar] [CrossRef]
- Philimonenko, V.V.; Zhao, J.; Iben, S.; Dingová, H.; Kyselá, K.; Kahle, M.; Zentgraf, H.; Hofmann, W.A.; de Lanerolle, P.; Hozák, P. Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 2004, 6, 1165–1172. [Google Scholar] [CrossRef]
- Hu, P.; Wu, S.; Hernandez, N. A role for β-actin in RNA polymerase III transcription. Genes Dev. 2004, 18, 3010–3015. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, M.; Moore, H.M.; Prajapati, B.; Dopie, J.; Meriläinen, L.; Honkanen, M.; Matos, R.C.; Poukkula, M.; Hietakangas, V.; Vartiainen, M.K. Nuclear actin is required for transcription during Drosophila oogenesis. iScience 2018, 9, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Fan, X.; Ding, M.; Li, R.; Shao, S.; Hou, Y.; Meng, S.; Tang, F.; Li, C.; Sun, Y. Nuclear actin regulates inducible transcription by enhancing RNA polymerase II clustering. Sci. Adv. 2020, 6, eaay6515. [Google Scholar] [CrossRef] [Green Version]
- Plessner, M.; Melak, M.; Chinchilla, P.; Baarlink, C.; Grosse, R. Nuclear F-actin formation and reorganization upon cell spreading. J. Biol. Chem. 2015, 290, 11209–11216. [Google Scholar] [CrossRef] [Green Version]
- Lattanzi, G.; Cenni, V.; Marmiroli, S.; Capanni, C.; Mattioli, E.; Merlini, L.; Squarzoni, S.; Maraldi, N.M. Association of emerin with nuclear and cytoplasmic actin is regulated in differentiating myoblasts. Biochem. Biophys. Res. Commun. 2003, 303, 764–770. [Google Scholar] [CrossRef]
- Lombardi, M.L.; Jaalouk, D.E.; Shanahan, C.M.; Burke, B.; Roux, K.J.; Lammerding, J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 2011, 286, 26743–26753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-K.; Louhghalam, A.; Lee, G.; Schafer, B.W.; Wirtz, D.; Kim, D.-H. Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun. 2017, 8, 2123. [Google Scholar] [CrossRef] [PubMed]
- Lamm, N.; Read, M.N.; Nobis, M.; Van Ly, D.; Page, S.G.; Masamsetti, V.P.; Timpson, P.; Biro, M.; Cesare, A.J. Nuclear F-actin counteracts nuclear deformation and promotes fork repair during replication stress. Nat. Cell Biol. 2020, 22, 1460–1470. [Google Scholar] [CrossRef]
- Cao, X.; Lin, Y.; Driscoll, T.P.; Franco-Barraza, J.; Cukierman, E.; Mauck, R.L.; Shenoy, V.B. A chemomechanical model of matrix and nuclear rigidity regulation of focal adhesion size. Biophys. J. 2015, 109, 1807–1817. [Google Scholar] [CrossRef] [Green Version]
- Chancellor, T.; Lee, J.; Thodeti, C.K.; Lele, T. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 2010, 99, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, L.M.; Smith, M.A.; Jensen, C.C.; Yoshigi, M.; Blankman, E.; Ullman, K.S.; Beckerle, M.C. Mechanical stress triggers nuclear remodeling and the formation of transmembrane actin nuclear lines with associated nuclear pore complexes. Mol. Biol. Cell 2020, 31, 1774–1787. [Google Scholar] [CrossRef]
- Ren, J.; Li, Y.; Hu, S.; Liu, Y.; Tsao, S.W.; Lau, D.; Luo, G.; Tsang, C.M.; Lam, R.H. Nondestructive quantification of single-cell nuclear and cytoplasmic mechanical properties based on large whole-cell deformation. Lab Chip 2020, 20, 4175–4185. [Google Scholar] [CrossRef]
- Guilluy, C.; Osborne, L.D.; Van Landeghem, L.; Sharek, L.; Superfine, R.; Garcia-Mata, R.; Burridge, K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 2014, 16, 376–381. [Google Scholar] [CrossRef]
- Buxboim, A.; Swift, J.; Irianto, J.; Spinler, K.R.; Dingal, P.D.P.; Athirasala, A.; Kao, Y.-R.C.; Cho, S.; Harada, T.; Shin, J.-W. Matrix elasticity regulates lamin-A, C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 2014, 24, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Tifft, K.E.; Bradbury, K.A.; Wilson, K.L. Tyrosine phosphorylation of nuclear-membrane protein emerin by Src, Abl and other kinases. J. Cell Sci. 2009, 122, 3780–3790. [Google Scholar] [CrossRef] [Green Version]
- Bera, M.; Kotamarthi, H.C.; Dutta, S.; Ray, A.; Ghosh, S.; Bhattacharyya, D.; Ainavarapu, S.R.K.; Sengupta, K. Characterization of Unfolding Mechanism of Human Lamin a Ig Fold by Single-Molecule Force Spectroscopy Implications in EDMD. Biochemistry 2014, 53, 7247–7258. [Google Scholar] [CrossRef]
- Yao, M.; Qiu, W.; Liu, R.; Efremov, A.; Cong, P.; Seddiki, R.; Payre, M.; Lim, C.; Ladoux, B.; Mege, R.-M.; et al. Force-dependent conformational switch of α-catenin controls vinculin binding. Nat. Commun. 2014, 5, 4525. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.J.; Fischer, M.; Jabre, S.; Moog, S.; Mamchaoui, K.; Butler-Browne, G.; Coirault, C. Lamin mutations cause increased YAP nuclear entry in muscle stem cells. Cells 2020, 9, 816. [Google Scholar] [CrossRef] [Green Version]
- Stephens, A.D.; Banigan, E.J.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell 2017, 28, 1984–1996. [Google Scholar] [CrossRef]
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; Arcos, J.M.G. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 2020, 181, 800–817.e822. [Google Scholar] [CrossRef]
- Quang Le, H.; Ghatak, S.; Chloé Yeung, C.-Y.; Tellkamp, F.; Günschmann, C.; Dieterich, C.; Yeroslaviz, A.; Habermann, B.; Pombo, A.; Niessen, C.M.; et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 2016, 18, 864–875. [Google Scholar]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef] [Green Version]
- Gadban, N.; Weinberg, E.; Zoabi, A.; Ashkenazi, M.; Yaffe, A.; Binderman, I. Strain reduction of human gingival fibroblasts induces the ATP pathway. J. Interdiscipl. Med. Dent. Sci. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Denes, B.J.; Bolton, C.; Illsley, C.; Kok, W.; Walker, J.; Poetsch, A.; Tredwin, C.; Kiliaridis, S.; Hu, B. Notch coordinates periodontal ligament maturation through regulating Lamin, A. J. Dent. Res. 2019, 98, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.P.; Lv, W.; Rhoades, J.H.; Poleshko, A.; Abbey, D.; Caporizzo, M.A.; Linares-Saldana, R.; Heffler, J.G.; Sayed, N.; Thomas, D. Pathogenic LMNA variants disrupt cardiac lamina-chromatin interactions and de-repress alternative fate genes. Cell Stem Cell 2021, 28, 938–954.e9. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Mishima, H.; Barc, J.; Takahashi, M.P.; Hirono, K.; Terada, S.; Kowase, S.; Sato, T.; Mukai, Y.; Yui, Y. Cardiac Emerinopathy: A Nonsyndromic Nuclear Envelopathy With Increased Risk of Thromboembolic Stroke Due to Progressive Atrial Standstill and Left Ventricular Noncompaction. Circ. Arrhythmia Electrophysiol. 2020, 13, e008712. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Goodsell, K.; Grogan, M.; Ackerman, M.J. LMNA-mediated arrhythmogenic right ventricular cardiomyopathy and charcot-marie-tooth type 2B1: A patient-discovered unifying diagnosis. J. Cardiovasc. Electrophysiol. 2016, 27, 868–871. [Google Scholar] [CrossRef]
- Huang, J.; Wan, Q.; Zou, Y.; Wang, L.; Pan, Y. Familial dilated cardiomyopathy caused by a novel variant in the Lamin A/C gene: A case report. BMC Cardiovasc. Disord. 2020, 20, 423. [Google Scholar] [CrossRef] [PubMed]
- Erdos, M.R.; Cabral, W.A.; Tavarez, U.L.; Cao, K.; Gvozdenovic-Jeremic, J.; Narisu, N.; Zerfas, P.M.; Crumley, S.; Boku, Y.; Hanson, G. A targeted antisense therapeutic approach for Hutchinson–Gilford progeria syndrome. Nat. Med. 2021, 27, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Peric-Hupkes, D.; Meuleman, W.; Pagie, L.; Bruggeman, S.W.; Solovei, I.; Brugman, W.; Gräf, S.; Flicek, P.; Kerkhoven, R.M.; van Lohuizen, M. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 2010, 38, 603–613. [Google Scholar] [CrossRef]
- Killaars, A.R.; Walker, C.J.; Anseth, K.S. Nuclear mechanosensing controls MSC osteogenic potential through HDAC epigenetic remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 21258–21266. [Google Scholar] [CrossRef] [PubMed]
- Mozzetta, C.; Tedesco, F.S. Challenging the “chromatin hypothesis” of cardiac laminopathies with LMNA mutant iPS cells. J. Cell Biol. 2019, 218, 2826–2828. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.; Buxboim, A.; Harada, T.; Dingal, P.; Pinter, J.; Pajerowski, J.; Spinler, K.; Shin, J.; Manorama, T.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef] [Green Version]
- Torvaldson, E.; Kochin, V.; Eriksson, J.E. Phosphorylation of lamins determine their structural properties and signaling functions. Nucleus 2015, 6, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K. Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev. Cell 2019, 49, 920–935.e925. [Google Scholar] [CrossRef]
- VanGompel, M.J.; Nguyen, K.C.; Hall, D.H.; Dauer, W.T.; Rose, L.S. A novel function for the Caenorhabditis elegans torsin OOC-5 in nucleoporin localization and nuclear import. Mol. Biol. Cell 2015, 26, 1752–1763. [Google Scholar] [CrossRef]
- Li, J.; Levin, D.S.; Kim, A.J.; Pappas, S.S.; Dauer, W.T. TorsinA restoration in a mouse model identifies a critical therapeutic window for DYT1 dystonia. J. Clin. Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.K.; Ly, C.; Kim, P.H.; Saunders, C.A.; Fong, L.G.; Young, S.G.; Luxton, G.; Rowat, A.C. DYT1 dystonia patient-derived fibroblasts have increased deformability and susceptibility to damage by mechanical forces. Front. Cell Dev. Biol. 2019, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Huelgas-Morales, G.; Sanders, M.; Mekonnen, G.; Tsukamoto, T.; Greenstein, D. Decreased mechanotransduction prevents nuclear collapse in a Caenorhabditis elegans laminopathy. Proc. Natl. Acad. Sci. USA 2020, 117, 31301–31308. [Google Scholar] [CrossRef]
- Maloney, W. The integral role of the dentist in treating individuals with Hutchinson-Gilford progeria syndrome. DENTISTRY 2010, 1, WMC00446. [Google Scholar]
- Reichert, C.; Gölz, L.; Götz, W.; Wolf, M.; Deschner, J.; Jäger, A. Dental and craniofacial characteristics in a patient with Hutchinson–Gilford progeria syndrome. J. Orofac. Orthop. Fortschr. Kieferorthopädie 2014, 75, 251–263. [Google Scholar] [CrossRef]
- Bengtsson, V.W.; Persson, G.R.; Berglund, J.S.; Renvert, S. Periodontitis related to cardiovascular events and mortality: A long-time longitudinal study. Clin. Oral Investig. 2021, 25, 4085–4095. [Google Scholar] [CrossRef]
- Almeida-Santos, A.; Martins-Mendes, D.; Gayà-Vidal, M.; Pérez-Pardal, L.; Beja-Pereira, A. Characterization of the oral microbiome of medicated type-2 diabetes patients. Front. Microbiol. 2021, 12, 56. [Google Scholar] [CrossRef]
- Feng, Y.-K.; Wu, Q.-L.; Peng, Y.-W.; Liang, F.-Y.; You, H.-J.; Feng, Y.-W.; Li, G.; Li, X.-J.; Liu, S.-H.; Li, Y.-C.; et al. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J. Neuroinflammation 2020, 17, 347. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.E.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontology 2000 2015, 69, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.M.; Ling, M.R.; Insall, R.; Kalna, G.; Spengler, J.; Grant, M.M.; Chapple, I.L. Impaired neutrophil directional chemotactic accuracy in chronic periodontitis patients. J. Clin. Periodontol. 2015, 42, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Mortensen, M.S.; Schjørring, S.; Trivedi, U.; Vestergaard, G.; Stokholm, J.; Bisgaard, H.; Krogfelt, K.A.; Sørensen, S.J. Amplicon sequencing provides more accurate microbiome information in healthy children compared to culturing. Commun. Biol. 2019, 2, 291. [Google Scholar] [CrossRef] [Green Version]
- Arweiler, N.B.; Auschill, T.M.; Heumann, C.; Hellwig, E.; Al-Ahmad, A. Influence of Probiotics on the Salivary Microflora Oral Streptococci and Their Integration into Oral Biofilm. Antibiotics 2020, 9, 803. [Google Scholar] [CrossRef]
- Moore, W.; Moore, L.V. The bacteria of periodontal diseases. Periodontology 2000 1994, 5, 66–77. [Google Scholar] [CrossRef]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef] [Green Version]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Hong, B.-Y.; Araujo, M.V.F.; Strausbaugh, L.D.; Terzi, E.; Ioannidou, E.; Diaz, P.I. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS ONE 2015, 10, e0127077. [Google Scholar] [CrossRef] [Green Version]
- Souto, G.R.; Queiroz-Junior, C.M.; de Abreu, M.H.N.G.; Costa, F.O.; Mesquita, R.A. Pro-inflammatory, Th1, Th2, Th17 cytokines and dendritic cells: A cross-sectional study in chronic periodontitis. PLoS ONE 2014, 9, e91636. [Google Scholar] [CrossRef]
- Özkavaf, A.; Aras, H.; Huri, C.B.; Mottaghian-Dini, F.; Tözüm, T.F.; Etikan, I.; Yamalik, N.; Çaglayan, F. Relationship between the quantity of gingival crevicular fluid and clinical periodontal status. J. Oral Sci. 2000, 42, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Sandholm, L. Proteases and their inhibitors in chronic inflammatory periodontal disease. J. Clin. Periodontol. 1986, 13, 19–26. [Google Scholar] [CrossRef]
- Laugisch, O.; Schacht, M.; Guentsch, A.; Kantyka, T.; Sroka, A.; Stennicke, H.; Pfister, W.; Sculean, A.; Potempa, J.; Eick, S. Periodontal pathogens affect the level of protease inhibitors in gingival crevicular fluid. Mol. Oral Microbiol. 2012, 27, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’Amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciù, M. Porphyromonas gingivalis, periodontal and systemic implications: A systematic review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Nunes, J.M.; Fillis, T.; Page, M.J.; Venter, C.; Lancry, O.; Kell, D.B.; Windberger, U.; Pretorius, E. Gingipain R1 and lipopolysaccharide from Porphyromonas gingivalis have major effects on blood clot morphology and mechanics. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Katz, J.; Yang, Q.-B.; Zhang, P.; Potempa, J.; Travis, J.; Michalek, S.M.; Balkovetz, D.F. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect. Immun. 2002, 70, 2512–2518. [Google Scholar] [CrossRef] [Green Version]
- Feghali, K.; Grenier, D. Priming effect of fibronectin fragments on the macrophage inflammatory response: Potential contribution to periodontitis. Inflammation 2012, 35, 1696–1705. [Google Scholar] [CrossRef]
- Lindemann, W.R.; Mijalis, A.J.; Alonso, J.L.; Borbat, P.P.; Freed, J.H.; Arnaout, M.A.; Pentelute, B.L.; Ortony, J.H. Conformational dynamics in extended RGD-containing peptides. Biomacromolecules 2020, 21, 2786–2794. [Google Scholar] [CrossRef]
- Sela, M.N.; Babitski, E.; Steinberg, D.; Kohavi, D.; Rosen, G. Degradation of collagen-guided tissue regeneration membranes by proteolytic enzymes of Porphyromonas gingivalis and its inhibition by antibacterial agents. Clin. Oral Implant. Res. 2009, 20, 496–502. [Google Scholar] [CrossRef]
- Mo, W.; Luo, H.; Wu, J.; Xu, N.; Zhang, F.; Qiu, Q.; Zhu, W.; Liang, M. Gingipains promote RANKL-induced osteoclastogenesis through the enhancement of integrin β3 in RAW264. 7 cells. J. Mol. Histol. 2020, 51, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Hu, C.-C.; Wu, Y.-Y.; Ueng, S.W.; Chang, C.-H.; Chen, M.-F. Ibudilast Mitigates Delayed Bone Healing Caused by Lipopolysaccharide by Altering Osteoblast and Osteoclast Activity. Int. J. Mol. Sci. 2021, 22, 1169. [Google Scholar] [CrossRef] [PubMed]
- Okahashi, N.; Inaba, H.; Nakagawa, I.; Yamamura, T.; Kuboniwa, M.; Nakayama, K.; Hamada, S.; Amano, A. Porphyromonas gingivalis induces receptor activator of NF-κB ligand expression in osteoblasts through the activator protein 1 pathway. Infect. Immun. 2004, 72, 1706–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, T.; Miyamoto, Y.; Yoshimura, K.; Yamada, A.; Takami, M.; Suzawa, T.; Hoshino, M.; Imamura, T.; Akiyama, C.; Yasuhara, R. Porphyromonas gingivalis-derived lysine gingipain enhances osteoclast differentiation induced by tumor necrosis factor-α and interleukin-1β but suppresses that by interleukin-17A: Importance of proteolytic degradation of osteoprotegerin by lysine gingipain. J. Biol. Chem. 2014, 289, 15621–15630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamashunas, A.C.; Katiyar, A.; Zhang, Q.; Purkayastha, P.; Singh, P.K.; Chukkapalli, S.S.; Lele, T.P. Osteoprotegerin is sensitive to actomyosin tension in human periodontal ligament fibroblasts. J. Cell. Physiol. 2021, 236, 5715–5724. [Google Scholar] [CrossRef]
- Bergsma, A.; Ganguly, S.S.; Wiegand, M.E.; Dick, D.; Williams, B.O.; Miranti, C.K. Regulation of cytoskeleton and adhesion signaling in osteoclasts by tetraspanin CD82. Bone Rep. 2019, 10, 100196. [Google Scholar] [CrossRef]
- Hočevar, K.; Vizovišek, M.; Wong, A.; Kozieł, J.; Fonović, M.; Potempa, B.; Lamont, R.J.; Potempa, J.; Turk, B. Proteolysis of Gingival Keratinocyte Cell Surface Proteins by Gingipains Secreted from Porphyromonas gingivalis–Proteomic Insights Into Mechanisms Behind Tissue Damage in the Diseased Gingiva. Front. Microbiol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Ruggiero, S.; Cosgarea, R.; Potempa, J.; Potempa, B.; Eick, S.; Chiquet, M. Cleavage of extracellular matrix in periodontitis: Gingipains differentially affect cell adhesion activities of fibronectin and tenascin-C. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2013, 1832, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.; Zhang, F.; Wu, J.; Xu, N.; Liang, M. Gingipains disrupt F-actin and cause osteoblast apoptosis via integrin β1. J. Periodontal Res. 2018, 53, 762–776. [Google Scholar] [CrossRef]
- Aliko, A.; Kamińska, M.; Bergum, B.; Gawron, K.; Benedyk, M.; Lamont, R.J.; Malicki, S.; Delaleu, N.; Potempa, J.; Mydel, P. Impact of Porphyromonas gingivalis peptidylarginine deiminase on bacterial biofilm formation, epithelial cell invasion, and epithelial cell transcriptional landscape. Sci. Rep. 2018, 8, 14144. [Google Scholar] [CrossRef] [Green Version]
- Kinane, J.A.; Benakanakere, M.R.; Zhao, J.; Hosur, K.B.; Kinane, D.F. Porphyromonas gingivalis influences actin degradation within epithelial cells during invasion and apoptosis. Cell. Microbiol. 2012, 14, 1085–1096. [Google Scholar] [CrossRef]
- Sheets, S.M.; Potempa, J.; Travis, J.; Fletcher, H.M.; Casiano, C.A. Gingipains from Porphyromonas gingivalis W83 synergistically disrupt endothelial cell adhesion and can induce caspase-independent apoptosis. Infect. Immun. 2006, 74, 5667–5678. [Google Scholar] [CrossRef] [Green Version]
- Bugueno, I.M.; Batool, F.; Keller, L.; Kuchler-Bopp, S.; Benkirane-Jessel, N.; Huck, O. Porphyromonas gingivalis bypasses epithelial barrier and modulates fibroblastic inflammatory response in an in vitro 3D spheroid model. Sci. Rep. 2018, 8, 14914. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Sasaki, N.; Yamaga, S.; Kuboniwa, M.; Matsusaki, M.; Amano, A. Porphyromonas gingivalis induces penetration of lipopolysaccharide and peptidoglycan through the gingival epithelium via degradation of junctional adhesion molecule 1. PLoS Pathog. 2019, 15, e1008124. [Google Scholar] [CrossRef]
- Eick, S.; Gadzo, N.; Tacchi, M.; Sculean, A.; Potempa, J.; Stavropoulos, A. Gingipains impair attachment of epithelial cell to dental titanium abutment surfaces. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2019, 107, 2549–2556. [Google Scholar] [CrossRef]
- Verrelli, D.I.; Albijanic, B. A comparison of methods for measuring the induction time for bubble–particle attachment. Miner. Eng. 2015, 80, 8–13. [Google Scholar] [CrossRef]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of pro MMP 9 and its activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Abdulkareem, A.; Shelton, R.; Landini, G.; Cooper, P.; Milward, M. Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial-mesenchymal transition. J. Periodontal Res. 2018, 53, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huck, O.; Mulhall, H.; Rubin, G.; Kizelnik, Z.; Iyer, R.; Perpich, J.D.; Haque, N.; Cani, P.D.; de Vos, W.M.; Amar, S. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J. Clin. Periodontol. 2020, 47, 202–212. [Google Scholar] [CrossRef]
- Bhalla, U.S.; Iyengar, R. Emergent properties of networks of biological signaling pathways. Science 1999, 283, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Antmen, E.; Demirci, U.; Hasirci, V. Micropatterned Surfaces Expose the Coupling between Actin Cytoskeleton-Lamin/Nesprin and Nuclear Deformability of Breast Cancer Cells with Different Malignancies. Adv. Biol. 2021, 5, 2000048. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, S.-H.; Kim, S.-H.; Lim, J.C.; Kim, S.H. Synthesis and characterization of the biodegradable and elastic terpolymer poly (glycolide-co-L-lactide-co-ϵ-caprolactone) for mechano-active tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Hörner, M.; Raute, K.; Hummel, B.; Madl, J.; Creusen, G.; Thomas, O.S.; Christen, E.H.; Hotz, N.; Gübeli, R.J.; Engesser, R. Phytochrome-based extracellular matrix with reversibly tunable mechanical properties. Adv. Mater. 2019, 31, 1806727. [Google Scholar] [CrossRef] [PubMed]
- Kortsmit, J.; Davies, N.H.; Miller, R.; Macadangdang, J.R.; Zilla, P.; Franz, T. The effect of hydrogel injection on cardiac function and myocardial mechanics in a computational post-infarction model. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 1185–1195. [Google Scholar] [CrossRef]
- Wu, B.; Fu, Y.; Shi, H.; Yan, B.; Lu, R.; Ma, S.; Markert, B. Tensile testing of the mechanical behavior of the human periodontal ligament. Biomed. Eng. Online 2018, 17, 172. [Google Scholar] [CrossRef] [Green Version]
- Uhlir, R.; Mayo, V.; Lin, P.H.; Chen, S.; Lee, Y.-T.; Hershey, G.; Lin, F.-C.; Ko, C.-C. Biomechanical characterization of the periodontal ligament: Orthodontic tooth movement. Angle Orthod. 2017, 87, 183–192. [Google Scholar] [CrossRef]
- Pfisterer, K.; Lumicisi, B.; Parsons, M. Imaging of Human Cancer Cells in 3D Collagen Matrices. Bio Protoc. 2021, 11, e3889. [Google Scholar] [CrossRef]
- Maeda, E.; Tsutsumi, T.; Kitamura, N.; Kurokawa, T.; Gong, J.P.; Yasuda, K.; Ohashi, T. Significant increase in Young’s modulus of ATDC5 cells during chondrogenic differentiation induced by PAMPS/PDMAAm double-network gel: Comparison with induction by insulin. J. Biomech. 2014, 47, 3408–3414. [Google Scholar] [CrossRef]
- Ettelt, V.; Belitsky, A.; Lehnert, M.; Loidl-Stahlhofen, A.; Epple, M.; Veith, M. Enhanced selective cellular proliferation by multi-biofunctionalization of medical implant surfaces with heterodimeric BMP-2/6, fibronectin, and FGF-2. J. Biomed. Mater. Res. Part. A 2018, 106, 2910–2922. [Google Scholar] [CrossRef]
- Doyle, A.D.; Carvajal, N.; Jin, A.; Matsumoto, K.; Yamada, K.M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 2015, 6, 8720. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Shi, C.; Li, J.; Xu, S.; Han, Y.; Li, J.; Zhang, L. Yes-associated protein promotes cell migration via activating Wiskott-Aldrich syndrome protein family member 1 in oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 290–298. [Google Scholar] [CrossRef]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, S.; Babensee, J.E. Controlled Delivery of Immunomodulators from a Biomaterial Scaffold Niche to Induce a Tolerogenic Phenotype in Human Dendritic Cells. ACS Biomater. Sci. Eng. 2020, 6, 4062–4076. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.; Koçtürk, S.; Bilici, G.; Havitcioglu, H. Development of biological meniscus scaffold: Decellularization method and recellularization with meniscal cell population derived from mesenchymal stem cells. J. Biomater. Appl. 2021, 0885328220981189. [Google Scholar] [CrossRef]
- Amler, A.-K.; Thomas, A.; Tüzüner, S.; Lam, T.; Geiger, M.-A.; Kreuder, A.-E.; Palmer, C.; Nahles, S.; Lauster, R.; Kloke, L. 3D bioprinting of tissue-specific osteoblasts and endothelial cells to model the human jawbone. Sci. Rep. 2021, 11, 4876. [Google Scholar] [CrossRef]
- Zeng, W.-Y.; Ning, Y.; Huang, X. Advanced technologies in periodontal tissue regeneration based on stem cells: Current status and future perspectives. J. Dent. Sci. 2020, 16, 501–507. [Google Scholar] [CrossRef]
- Lepsky, V.R.; Natan, S.; Tchaicheeyan, O.; Kolel, A.; Zussman, M.; Zilberman, M.; Lesman, A. FITC-Dextran Release from Cell-Embedded Fibrin Hydrogels. Biomolecules 2021, 11, 337. [Google Scholar] [CrossRef]
- Selig, M.; Lauer, J.C.; Hart, M.L.; Rolauffs, B. Mechanotransduction and Stiffness-Sensing: Mechanisms and Opportunities to Control Multiple Molecular Aspects of Cell Phenotype as a Design Cornerstone of Cell-Instructive Biomaterials for Articular Cartilage Repair. Int. J. Mol. Sci. 2020, 21, 5399. [Google Scholar] [CrossRef]
- Ayuningtyas, F.D.; Kim, M.-H.; Kino-Oka, M. Muscle lineage switching by migratory behaviour-driven epigenetic modifications of human mesenchymal stem cells on a dendrimer-immobilized surface. Acta Biomater. 2020, 106, 170–180. [Google Scholar] [CrossRef]
- Merrett, K.; Wan, F.; Lee, C.-J.; Harden, J.L. Enhanced Collagen-like Protein for Facile Biomaterial Fabrication. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Guo, J.; Kim, Y.; Xie, V.; Smith, B.; Watson, E.; Lam, J.; Pearce, H.; Engel, P.; Mikos, A. Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering. Sci. Adv. 2019, 5, eaaw7396. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.L.; Li, A.; Kim, Y.S.; Xie, V.Y.; Smith, B.T.; Watson, E.; Bao, G.; Mikos, A.G. Click functionalized, tissue-specific hydrogels for osteochondral tissue engineering. J. Biomed. Mater. Res. Part. A 2020, 108, 684–693. [Google Scholar] [CrossRef]
- Yuan, Y.; Loh, Y.-h.E.; Han, X.; Feng, J.; Ho, T.-V.; He, J.; Jing, J.; Groff, K.; Wu, A.; Chai, Y. Spatiotemporal cellular movement and fate decisions during first pharyngeal arch morphogenesis. Sci. Adv. 2020, 6, eabb0119. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [Green Version]
- Hernández Martinez, V.M.; Garcia Benavides, L.; Totsuka Sutto, S.E.; Cardona Muñoz, E.G.; Campos Bayardo, T.I.; Pascoe Gonzalez, S. Effectiveness of degradable and non-degradable implants to close large septal perforations in an experimental model. J. Plast. Surg. Hand Surg. 2016, 50, 222–226. [Google Scholar] [CrossRef]
- Colaris, M.J.; de Boer, M.; van der Hulst, R.R.; Tervaert, J.W.C. Two hundreds cases of ASIA syndrome following silicone implants: A comparative study of 30 years and a review of current literature. Immunol. Res. 2017, 65, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hong, J.Y.; Jeon, W.-J.; Lee, J.; Ha, I.-H. Evaluation of the effects of differences in silicone hardness on rat model of lumbar spinal stenosis. PLoS ONE 2021, 16, e0251464. [Google Scholar] [CrossRef]
- Sharma, S.; Mandhani, A.; Bose, S.; Basu, B. Dynamically crosslinked polydimethylsiloxane-based polyurethanes with contact-killing antimicrobial properties as implantable alloplasts for urological reconstruction. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Valipour, F.; Valipour, F.; Rahbarghazi, R.; Navali, A.M.; Rashidi, M.R.; Davaran, S. Novel hybrid polyester-polyacrylate hydrogels enriched with platelet-derived growth factor for chondrogenic differentiation of adipose-derived mesenchymal stem cells in vitro. J. Biol. Eng. 2021, 15, 6. [Google Scholar] [CrossRef]
- Khandaker, M.; Kotturi, H.; Progri, H.; Tummala, S.; Nikfarjam, S.; Rao, P.; Hosna, A.U.; Arasu, D.T.; Williams, W.; Haleem, A. In vitro and in vivo effect of polycaprolactone nanofiber coating on polyethylene glycol diacrylate scaffolds for intervertebral disc repair. Biomed. Mater. 2021. [Google Scholar] [CrossRef]
- Milojević, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradišnik, L.; Zidarič, T.; Stana Kleinschek, K.; Maver, U.; Maver, T. Hybrid 3D Printing of Advanced Hydrogel-Based Wound Dressings with Tailorable Properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Eguiluz, A.; Vazquez, E.; Guerrero, J.D.; Gonzalez, Z.; Sanchez-Herencia, A.J.; Ferrari, B. Controlled SrR Delivery by the Incorporation of Mg Particles on Biodegradable PLA-Based Composites. Polymers 2021, 13, 1061. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zhang, H.; Tang, Z.; Cui, X.; Li, X.; Liang, J.; Wang, Q.; Fan, Y.; Zhang, X. Solubilized Cartilage ECM Facilitates the Recruitment and Chondrogenesis of Endogenous BMSCs in Collagen Scaffolds for Enhancing Microfracture Treatment. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef]
- Rahoui, N.; Jiang, B.; Taloub, N.; Huang, Y.D. Spatio-temporal control strategy of drug delivery systems based nano structures. J. Control. Release 2017, 255, 176–201. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2021. [Google Scholar] [CrossRef]
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.-T.V.; Thomas, S.; Huang, Y.-C.; Kong, Z.-L. Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci. Rep. 2020, 10, 18037. [Google Scholar]
- Asparuhova, M.B.; Stähli, A.; Guldener, K.; Sculean, A. A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 4051. [Google Scholar] [CrossRef]
- Chen, H.; Dai, Y.; Cui, J.; Yin, X.; Feng, W. Carbon Monoxide Releasing Molecule-3 Enhances Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Carbon Monoxide Release. Drug Des. Dev. Ther. 2021, 15, 1691. [Google Scholar] [CrossRef]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 216. [Google Scholar] [CrossRef]
- Synytsya, A.; Poučková, P.; Zadinová, M.; Troshchynska, Y.; Štětina, J.; Synytsya, A.; Saloň, I.; Král, V. Hydrogels based on low-methoxyl amidated citrus pectin and flaxseed gum formulated with tripeptide glycyl-l-histidyl-l-lysine improve the healing of experimental cutting wounds in rats. Int. J. Biol. Macromol. 2020, 165, 3156–3168. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, J.; Zhao, L.; Deng, J.; Li, Q. A simvastatin-releasing scaffold with periodontal ligament stem cell sheets for periodontal regeneration. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800019900094. [Google Scholar] [CrossRef]
- Panzeri, S.; Arosio, D.; Gazzola, S.; Belvisi, L.; Civera, M.; Potenza, D.; Vasile, F.; Kemker, I.; Ertl, T.; Sewald, N. Cyclic RGD and isoDGR Integrin Ligands Containing cis-2-amino-1-cyclopentanecarboxylic (cis-β-ACPC) Scaffolds. Molecules 2020, 25, 5966. [Google Scholar] [CrossRef] [PubMed]
- Almonte-Becerril, M.; Gimeno-LLuch, I.; Villarroya, O.; Benito-Jardón, M.; Kouri, J.B.; Costell, M. Genetic abrogation of the fibronectin-α5β1 integrin interaction in articular cartilage aggravates osteoarthritis in mice. PLoS ONE 2018, 13, e0198559. [Google Scholar] [CrossRef] [PubMed]
- Massam-Wu, T.; Chiu, M.; Choudhury, R.; Chaudhry, S.S.; Baldwin, A.K.; McGovern, A.; Baldock, C.; Shuttleworth, C.A.; Kielty, C.M. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGFβ. J. Cell Sci. 2010, 123, 3006–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorolsuren, Z.; Lang, O.; Pallinger, E.; Foldes, A.; Szabolcs, G.G.; Varga, G.; Mezo, G.; Vag, J.; Kohidai, L. Functional and cell surface characteristics of periodontal ligament cells (PDLCs) on RGD-synthetic polypeptide conjugate coatings. J. Periodontal Res. 2020, 55, 713–723. [Google Scholar] [CrossRef]
- Matsugami, D.; Murakami, T.; Yoshida, W.; Imamura, K.; Bizenjima, T.; Seshima, F.; Saito, A. Treatment with functionalized designer self-assembling peptide hydrogels promotes healing of experimental periodontal defects. J. Periodontal Res. 2021, 56, 162–172. [Google Scholar] [CrossRef]
- Etemadi Sh, M.; Hsieh, N.-C.; Movahed Mohammadi, S.S.; Momeni, S.; Razavi, S.M.; Alizargar, J. Histological and Radiological Evaluation of Low-Intensity Pulsed Ultrasound Versus Whole Body Vibration on Healing of Mandibular Bone Defects in Rats. Medicina 2020, 56, 457. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Tan, M.; Feng, G.; Kuang, Y.; Li, J.; Song, J. Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res. Ther. 2020, 11, 215. [Google Scholar]
- Shobara, K.; Ogawa, T.; Shibamoto, A.; Miyashita, M.; Ito, A.; Sitalaksmi, R.M. Osteogenic effect of low-intensity pulsed ultrasound and whole-body vibration on peri-implant bone. An experimental in vivo study. Clin. Oral Implant. Res. 2021, 32, 641–650. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Wu, L.; Li, S. Effects of collimated and focused low-intensity pulsed ultrasound stimulation on the mandible repair in rabbits. Ann. Transl. Med. 2020, 8, 98. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujita, N.; Tsuji-Tamura, K.; Kitagawa, Y.; Fujisawa, T.; Tamura, M.; Sato, M. Osteocytes as main responders to low-intensity pulsed ultrasound treatment during fracture healing. Sci. Rep. 2021, 11, 10298. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Y.; Li, J.; Zhao, C.; Song, J. Low-intensity pulsed ultrasound promotes alveolar bone regeneration in a periodontal injury model. Ultrasonics 2018, 90, 166–172. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhu, M.; Ying, S.; Li, L.; Chen, D.; Li, J.; Song, J. Low-intensity pulsed ultrasound promotes the formation of periodontal ligament stem cell sheets and ectopic periodontal tissue regeneration. J. Biomed. Mater. Res. Part. A 2021, 109, 1101–1112. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Feng, G.; He, Y.; Li, J.; Song, J. Low-intensity pulsed ultrasound activates autophagy in periodontal ligament cells in the presence or absence of lipopolysaccharide. Arch. Oral Biol. 2020, 117, 104769. [Google Scholar] [CrossRef]
- Ying, S.; Tan, M.; Feng, G.; Kuang, Y.; Chen, D.; Li, J.; Song, J. Low-intensity Pulsed Ultrasound regulates alveolar bone homeostasis in experimental Periodontitis by diminishing Oxidative Stress. Theranostics 2020, 10, 9789. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, M.; Li, J.; Hu, B.; Jiang, D.; Huang, H.; Song, J. LIPUS inhibited the expression of inflammatory factors and promoted the osteogenic differentiation capacity of hPDLCs by inhibiting the NF-κB signaling pathway. J. Periodontal Res. 2020, 55, 125–140. [Google Scholar] [CrossRef]
- Alshihah, N.; Alhadlaq, A.; El-Bialy, T.; Aldahmash, A.; Bello, I. The effect of low intensity pulsed ultrasound on dentoalveolar structures during orthodontic force application in diabetic ex-vivo model. Arch. Oral Biol. 2020, 119, 104883. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Y.; Xiong, Y.; Wang, B.; Guo, Y.; Gong, P.; Zhang, L. Low-intensity pulsed ultrasound improves osseointegration of dental implant in mice by inducing local neuronal production of αCGRP. Arch. Oral Biol. 2020, 115, 104736. [Google Scholar] [CrossRef]
- Callhoff, J.; Dietrich, T.; Chubrieva, M.; Klotsche, J.; Zink, A. A patient-reported questionnaire developed in a German early arthritis cohort to assess periodontitis in patients with rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 197. [Google Scholar] [CrossRef] [Green Version]
- Strassburg, S.; Caduc, M.; Stark, G.B.; Jedrusik, N.; Tomakidi, P.; Steinberg, T.; Simunovic, F.; Finkenzeller, G. In vivo evaluation of an electrospun gelatin nonwoven mat for regeneration of epithelial tissues. J. Biomed. Mater. Res. Part A 2019, 107, 1605–1614. [Google Scholar] [CrossRef]
- Schulz, S.; Angarano, M.; Fabritius, M.; Mülhaupt, R.; Dard, M.; Obrecht, M.; Tomakidi, P.; Steinberg, T. Nonwoven-based gelatin/polycaprolactone membrane proves suitability in a preclinical assessment for treatment of soft tissue defects. Tissue Eng. Part. A 2014, 20, 1935–1947. [Google Scholar] [CrossRef] [Green Version]
- Jedrusik, N.; Steinberg, T.; Husari, A.; Volk, L.; Wang, X.; Finkenzeller, G.; Strassburg, S.; Tomakidi, P. Gelatin nonwovens-based epithelial morphogenesis involves a signaling axis comprising EGF-receptor, MAP kinases ERK 1/2, and β1 integrin. J. Biomed. Mater. Res. Part. A 2019, 107, 663–677. [Google Scholar] [CrossRef]
- Jedrusik, N.; Meyen, C.; Finkenzeller, G.; Stark, G.B.; Meskath, S.; Schulz, S.D.; Steinberg, T.; Eberwein, P.; Strassburg, S.; Tomakidi, P. Nanofibered gelatin-based nonwoven elasticity promotes epithelial Histogenesis. Adv. Healthc. Mater. 2018, 7, 1700895. [Google Scholar] [CrossRef]
- Dunnwald, M.; Tomanek-Chalkley, A.; Alexandrunas, D.; Fishbaugh, J.; Bickenbach, J. Isolating a pure population of epidermal stem cells for use in tissue engineering. Exp. Dermatol. 2001, 10, 45–54. [Google Scholar] [CrossRef]
- Franzè, E.; Monteleone, I.; Laudisi, F.; Rizzo, A.; Dinallo, V.; Di Fusco, D.; Colantoni, A.; Ortenzi, A.; Giuffrida, P.; Di Carlo, S. Cadherin-11 is a Regulator of intestinal fibrosis. J. Crohn’s Colitis 2020, 14, 406–417. [Google Scholar] [CrossRef]
- Niu, C.; Yang, P.; Yao, B. Engineering Protease-Resistant and Highly Active Phytases. In Inositol Phosphates; Springer: New York, NY, USA, 2020; pp. 155–162. [Google Scholar]
- Frank, C.F.; Hostetter, M.K. Cleavage of E-cadherin: A mechanism for disruption of the intestinal epithelial barrier by Candida albicans. Transl. Res. 2007, 149, 211–222. [Google Scholar] [CrossRef]
- Oh, C.; Kim, H.J.; Kim, H.-M. Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-α. J. Periodontal Implant. Sci. 2019, 49, 270. [Google Scholar] [CrossRef]
- Jin, C.; Lee, G.; Oh, C.; Kim, H.J.; Kim, H.-M. Substrate roughness induces the development of defective E-cadherin junctions in human gingival keratinocytes. J. Periodontal Implant. Sci. 2017, 47, 116. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, M.C.; Lieb, S.; Renkawitz-Pohl, R.; Bogdan, S. Filopodia-based contact stimulation of cell migration drives tissue morphogenesis. Nat. Commun. 2021, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Dyberg, C.; Fransson, S.; Andonova, T.; Sveinbjörnsson, B.; Lännerholm-Palm, J.; Olsen, T.K.; Forsberg, D.; Herlenius, E.; Martinsson, T.; Brodin, B. Rho-associated kinase is a therapeutic target in neuroblastoma. Proc. Natl. Acad. Sci. USA 2017, 114, E6603–E6612. [Google Scholar] [CrossRef] [Green Version]
- Macks, C.; Jeong, D.; Lee, J.S. Local delivery of RhoA siRNA by PgP nanocarrier reduces inflammatory response and improves neuronal cell survival in a rat TBI model. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102343. [Google Scholar] [CrossRef]
- Fogli, S.; Stefanelli, F.; Battolla, B.; Bianchi, F.; Breschi, M.C.; Mattii, L. Salbutamol inhibits RhoA activation in normal but not in desensitized bronchial smooth muscle cells. J. Pharm. Pharmacol. 2015, 67, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Sato-Ohira, S.; Tanihara, H.; Inoue, T. RhoA activation decreases phagocytosis of trabecular meshwork cells. Curr. Eye Res. 2020, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.-i.; Yamamoto, G.; Asakura, M. Gelatin Methacryloyl–Riboflavin (GelMA–RF) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, J.Y.; Ballance, W.C.; Sutton, B.P.; Shin, D.H.; Jang, K.H.; Shin, M.; Kong, H.; Kim, J.W. Fabrication of cell penetrating peptide-conjugated bacterial cellulose nanofibrils with remarkable skin adhesion and water retention performance. Int. J. Pharm. 2021, 600, 120476. [Google Scholar] [CrossRef] [PubMed]
- Tone, Y.; Mamchaoui, K.; Tsoumpra, M.K.; Hashimoto, Y.; Terada, R.; Maruyama, R.; Gait, M.J.; Arzumanov, A.A.; McClorey, G.; Imamura, M. Immortalized Canine Dystrophic Myoblast Cell Lines for Development of Peptide-Conjugated Splice-Switching Oligonucleotides. Nucleic Acid Ther. 2021, 31, 172–181. [Google Scholar] [CrossRef]
- Unkart, J.T.; Chen, S.L.; Wapnir, I.L.; González, J.E.; Harootunian, A.; Wallace, A.M. Intraoperative tumor detection using a ratiometric activatable fluorescent peptide: A first-in-human phase 1 study. Ann. Surg. Oncol. 2017, 24, 3167–3173. [Google Scholar] [CrossRef]
- Lulla, R.R.; Goldman, S.; Yamada, T.; Beattie, C.W.; Bressler, L.; Pacini, M.; Pollack, I.F.; Fisher, P.G.; Packer, R.J.; Dunkel, I.J. Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A Pediatric Brain Tumor Consortium Study. Neuro Oncol. 2016, 18, 1319–1325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Dai, S.; Chen, Y.; Wang, H.; Chen, T.; Shu, Q.; Chen, S.; Shou, L.; Cai, X. Aqueous extract of Taxus chinensis var. mairei regulates the Hippo-YAP pathway and promotes apoptosis of non-small cell lung cancer via ATF3 in vivo and in vitro. Biomed. Pharmacother. 2021, 138, 111506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Feng, X.; Tian, H.; Fu, X.; Gu, W.; Wen, Y. Metformin inhibits mTOR and c-Myc by decreasing YAP protein expression in OSCC cells. Oncol. Rep. 2021, 45, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.H.; Lai, T.Y.; Takahashi, K.; Wong, T.Y.; Chen, L.-J.; Ruamviboonsuk, P.; Tan, C.S.; Lee, W.K.; Cheung, C.M.G.; Ngah, N.F. Comparison of ranibizumab with or without Verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: The EVEREST II Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 935–942. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Feng, W.; Yu, Y.; Jeong, K.; Guo, W.; Lu, Y.; Mills, G.B. Verteporfin inhibits YAP function through up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am. J. Cancer Res. 2016, 6, 27–37. [Google Scholar] [PubMed]
- Zhou, L.; Wang, Q.; Zhang, H.; Li, Y.; Xie, S.; Xu, M. YAP Inhibition by Nuciferine via AMPK-Mediated Downregulation of HMGCR Sensitizes Pancreatic Cancer Cells to Gemcitabine. Biomolecules 2019, 9, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassino, T.R.; Drowley, L.; Okada, M.; Beckman, S.A.; Keller, B.; Tobita, K.; LeDuc, P.R.; Huard, J. Mechanical loading of stem cells for improvement of transplantation outcome in a model of acute myocardial infarction: The role of loading history. Tissue Eng. Part A 2012, 18, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götschi, T.; Schulz, N.; Snedeker, J.G.; Hanimann, J.; Franchi, M.V.; Spörri, J. Three-Dimensional Mapping of Shear Wave Velocity in Human Tendon: A Proof of Concept Study. Sensors 2021, 21, 1655. [Google Scholar] [CrossRef]
- Wang, Z.; Weng, Y.; Ishihara, Y.; Odagaki, N.; Hlaing, E.E.H.; Izawa, T.; Okamura, H.; Kamioka, H. Loading history changes the morphology and compressive force-induced expression of receptor activator of nuclear factor kappa B ligand/osteoprotegerin in MLO-Y4 osteocytes. PeerJ 2020, 8, e10244. [Google Scholar] [CrossRef]
- Bidan, C.M.; Fratzl, M.; Coullomb, A.; Moreau, P.; Lombard, A.H.; Wang, I.; Balland, M.; Boudou, T.; Dempsey, N.M.; Devillers, T. Magneto-active substrates for local mechanical stimulation of living cells. Sci. Rep. 2018, 8, 1464. [Google Scholar] [CrossRef]
- Kojima, T.; Husari, A.; Dieterle, M.P.; Fontaine, S.; Prucker, O.; Tomakidi, P.; Rühe, J. PnBA/PDMAA-Based Iron-Loaded Micropillars Allow for Discrete Cell Adhesion and Analysis of Actuation-Related Molecular Responses. Adv. Mater. Interfaces 2020, 7, 1901806. [Google Scholar] [CrossRef]
- Chatelet, M.; Afota, F.; Savoldelli, C. Review of bone graft and implant survival rate: A comparison between autogenous bone block versus guided bone regeneration. J. Stomatol. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef]
- Yu, D.; Huang, C.; Jiang, C.; Zhu, H. Features of a simvastatin-loaded multi-layered co-electrospun barrier membrane for guided bone regeneration. Exp. Ther. Med. 2021, 22, 713. [Google Scholar] [CrossRef]
- Porrelli, D.; Mardirossian, M.; Musciacchio, L.; Pacor, M.; Berton, F.; Crosera, M.; Turco, G. Antibacterial Electrospun Polycaprolactone Membranes Coated with Polysaccharides and Silver Nanoparticles for Guided Bone and Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 17255–17267. [Google Scholar] [CrossRef]
- De Souza Balbinot, G.; da Cunha Bahlis, E.A.; Visioli, F.; Leitune, V.C.B.; Soares, R.M.D.; Collares, F.M. Polybutylene-adipate-terephthalate and niobium-containing bioactive glasses composites: Development of barrier membranes with adjusted properties for guided bone regeneration. Mater. Sci. Eng. C 2021, 125, 112115. [Google Scholar] [CrossRef]
- Küçüktürkmen, B.; Öz, U.C.; Toptaş, M.; Devrim, B.; Saka, O.M.; Bilgili, H.; Deveci, M.S.; Ünsal, E.; Bozkır, A. Development of Zoledronic Acid Containing Biomaterials for Enhanced Guided Bone Regeneration. J. Pharm. Sci. 2021. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Osorio, R.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C. Doxycycline-doped membranes induced osteogenic gene expression on osteoblastic cells. J. Dent. 2021, 109, 103676. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C.; Osorio, R. Doxycycline-Doped Polymeric Membranes Induced Growth, Differentiation and Expression of Antigenic Phenotype Markers of Osteoblasts. Polymers 2021, 13, 1063. [Google Scholar] [CrossRef]
- Naenni, N.; Stucki, L.; Huesler, J.; Schneider, D.; Hämmerle, C.H.; Jung, R.E.; Thoma, D.S. Implants sites with concomitant bone regeneration using a resorbable or non-resorbable membrane result in stable marginal bone levels and similar profilometric outcomes over 5 years. Clin. Oral Implant. Res. 2021. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.-F.; Wan, Q.-Q.; Shen, M.-J.; Ma, Y.-X.; Gu, J.-T.; Gao, P.; Tang, X.-Y.; Yu, F.; Chen, J.-H. Matrix stiffening by self-mineralizable guided bone regeneration. Acta Biomater. 2021, 125, 112–125. [Google Scholar] [CrossRef]
- Barajaa, M.A.; Nair, L.S.; Laurencin, C.T. Bioinspired scaffold designs for regenerating musculoskeletal tissue interfaces. Regen. Eng. Transl. Med. 2020, 6, 451–483. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, Y.; Yao, B.; Li, Z.; Song, W.; Wang, Y.; Duan, X.; Yuan, X.; Fu, X. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021, 9, 397. [Google Scholar]
- De Medeiros, G.; Kromm, D.; Balazs, B.; Norlin, N.; Günther, S.; Izquierdo, E.; Ronchi, P.; Komoto, S.; Krzic, U.; Schwab, Y. Cell and tissue manipulation with ultrashort infrared laser pulses in light-sheet microscopy. Sci. Rep. 2020, 10, 1942. [Google Scholar]
- Ma, Y.; Lin, M.; Huang, G.; Li, Y.; Wang, S.; Bai, G.; Lu, T.J.; Xu, F. 3D spatiotemporal mechanical microenvironment: A hydrogel-based platform for guiding stem cell fate. Adv. Mater. 2018, 30, 1705911. [Google Scholar] [CrossRef]
- Richter, B.; Hahn, V.; Bertels, S.; Claus, T.K.; Wegener, M.; Delaittre, G.; Barner-Kowollik, C.; Bastmeyer, M. Guiding cell attachment in 3D microscaffolds selectively functionalized with two distinct adhesion proteins. Adv. Mater. 2017, 29, 1604342. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The horizon of materiobiology: A perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- Kang, E.-S.; Kim, D.-S.; Suhito, I.R.; Lee, W.; Song, I.; Kim, T.-H. Two-dimensional material-based bionano platforms to control mesenchymal stem cell differentiation. Biomater. Res. 2018, 22, 10. [Google Scholar] [CrossRef]
- Suhito, I.R.; Han, Y.; Kim, D.-S.; Son, H.; Kim, T.-H. Effects of two-dimensional materials on human mesenchymal stem cell behaviors. Biochem. Biophys. Res. Commun. 2017, 493, 578–584. [Google Scholar] [CrossRef]
- Song, J.; Michas, C.; Chen, C.S.; White, A.E.; Grinstaff, M.W. Controlled Cell Alignment Using Two-Photon Direct Laser Writing-Patterned Hydrogels in 2D and 3D. Macromol. Biosci. 2021, 21, 2100051. [Google Scholar] [CrossRef]
- Cheng, D.; Jayne, R.K.; Tamborini, A.; Eyckmans, J.; White, A.E.; Chen, C.S. Studies of 3D directed cell migration enabled by direct laser writing of curved wave topography. Biofabrication 2019, 11, 021001. [Google Scholar] [CrossRef]
- Dietrich, M.; Le Roy, H.; Brückner, D.B.; Engelke, H.; Zantl, R.; Rädler, J.O.; Broedersz, C.P. Guiding 3D cell migration in deformed synthetic hydrogel microstructures. Soft Matter 2018, 14, 2816–2826. [Google Scholar] [CrossRef] [Green Version]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing of living cells. Biotechnol. Bioeng. 2000, 67, 312–318. [Google Scholar] [CrossRef]
- KehrIbar, D.; Özgen, M.; YolbaŞ, S.; Yildirim, A.; Önalan, E.; ÇİftÇİ, O.; Özercan, İ.; Koca, S. The inhibition of Src kinase suppresses the production of matrix metalloproteinases in from synovial fibroblasts and inhibits MAPK and STATs pathways. Turk. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Darwish, I.A.; Khalil, N.Y.; Alsaif, N.A.; Herqash, R.N.; Sayed, A.Y.; Abdel-Rahman, H.M. Charge-Transfer Complex of Linifanib with 2, 3-dichloro-3, 5-dicyano-1, 4-benzoquinone: Synthesis, Spectroscopic Characterization, Computational Molecular Modelling and Application in the Development of Novel 96-microwell Spectrophotometric Assay. Drug Des. Dev. Ther. 2021, 15, 1167. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef] [PubMed]
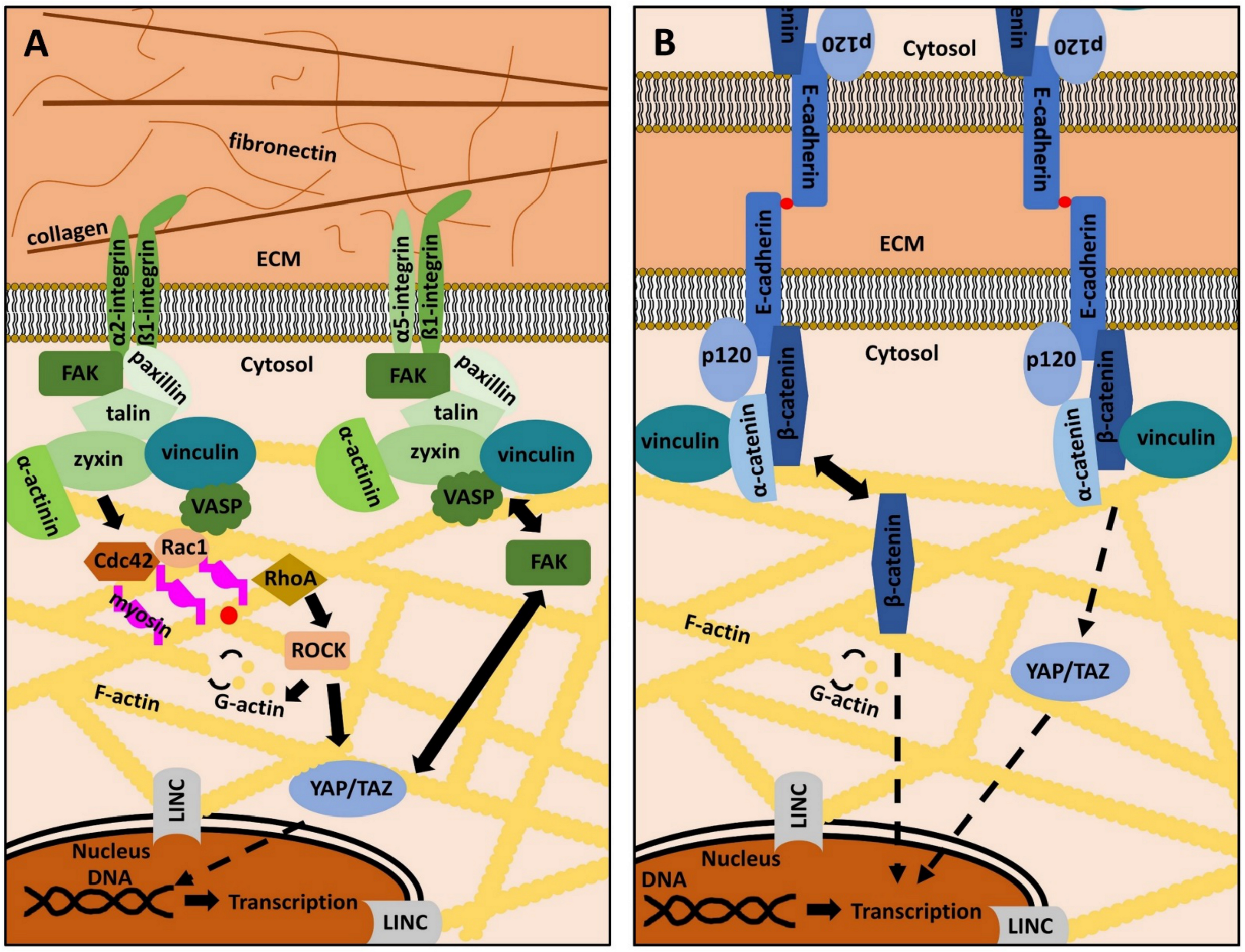

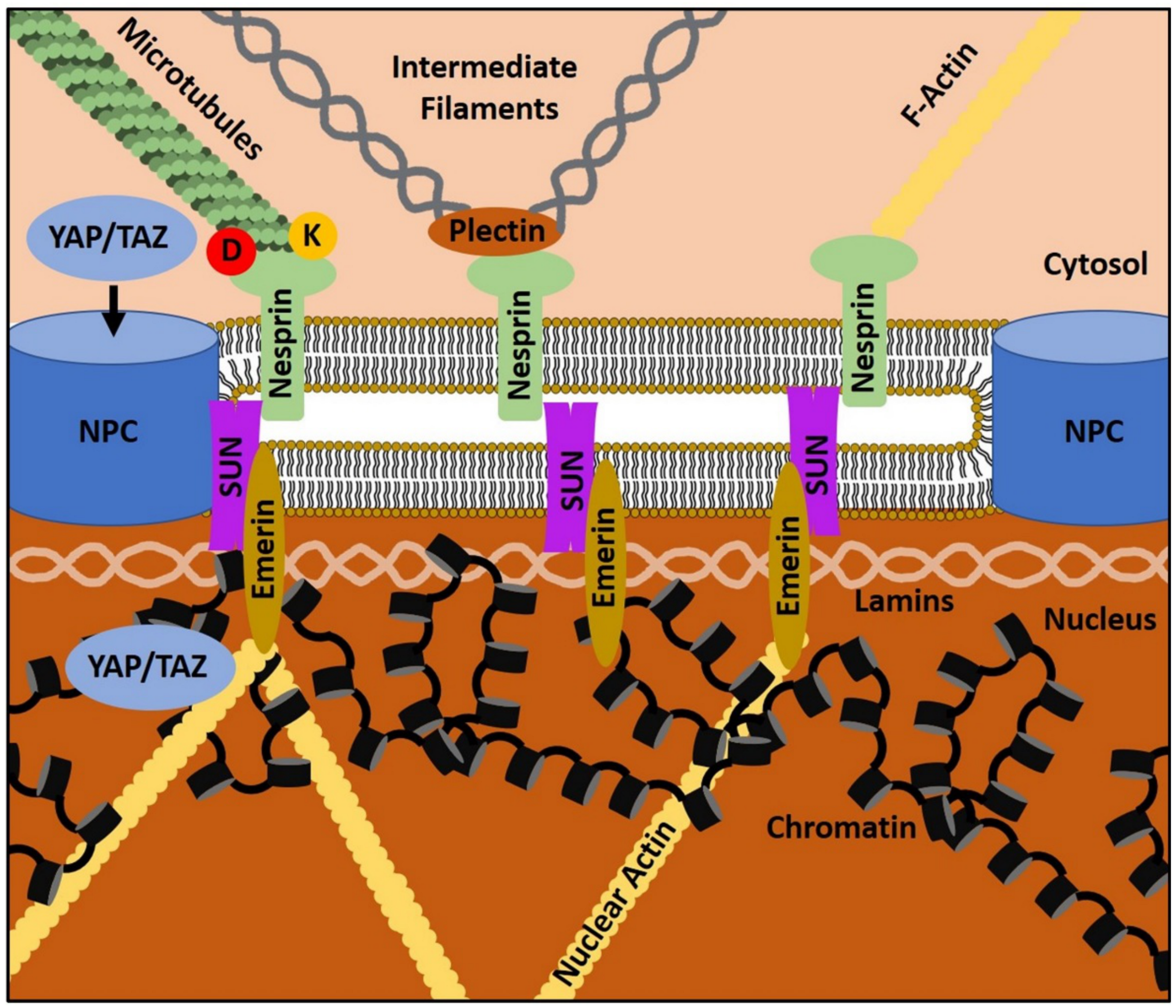

| Integrin Heterodimers | α1β1 | α2β1 | α3β1 | α5β1 | αVβ1 | αVβ3 | αVβ5 |
|---|---|---|---|---|---|---|---|
| Ligand(s) | Different collagens (e.g. type I) | Different collagens (e.g. type I) | laminins | fibronectin | fibronectin | fibronectin | vitronectin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieterle, M.P.; Husari, A.; Steinberg, T.; Wang, X.; Ramminger, I.; Tomakidi, P. From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues. Biomolecules 2021, 11, 824. https://doi.org/10.3390/biom11060824
Dieterle MP, Husari A, Steinberg T, Wang X, Ramminger I, Tomakidi P. From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues. Biomolecules. 2021; 11(6):824. https://doi.org/10.3390/biom11060824
Chicago/Turabian StyleDieterle, Martin Philipp, Ayman Husari, Thorsten Steinberg, Xiaoling Wang, Imke Ramminger, and Pascal Tomakidi. 2021. "From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues" Biomolecules 11, no. 6: 824. https://doi.org/10.3390/biom11060824
APA StyleDieterle, M. P., Husari, A., Steinberg, T., Wang, X., Ramminger, I., & Tomakidi, P. (2021). From the Matrix to the Nucleus and Back: Mechanobiology in the Light of Health, Pathologies, and Regeneration of Oral Periodontal Tissues. Biomolecules, 11(6), 824. https://doi.org/10.3390/biom11060824







