Multispectral Assessment of Sweet Pepper (Capsicum annuum L.) Fruit Quality Affected by Calcite Nanoparticles
Abstract
:1. Introduction
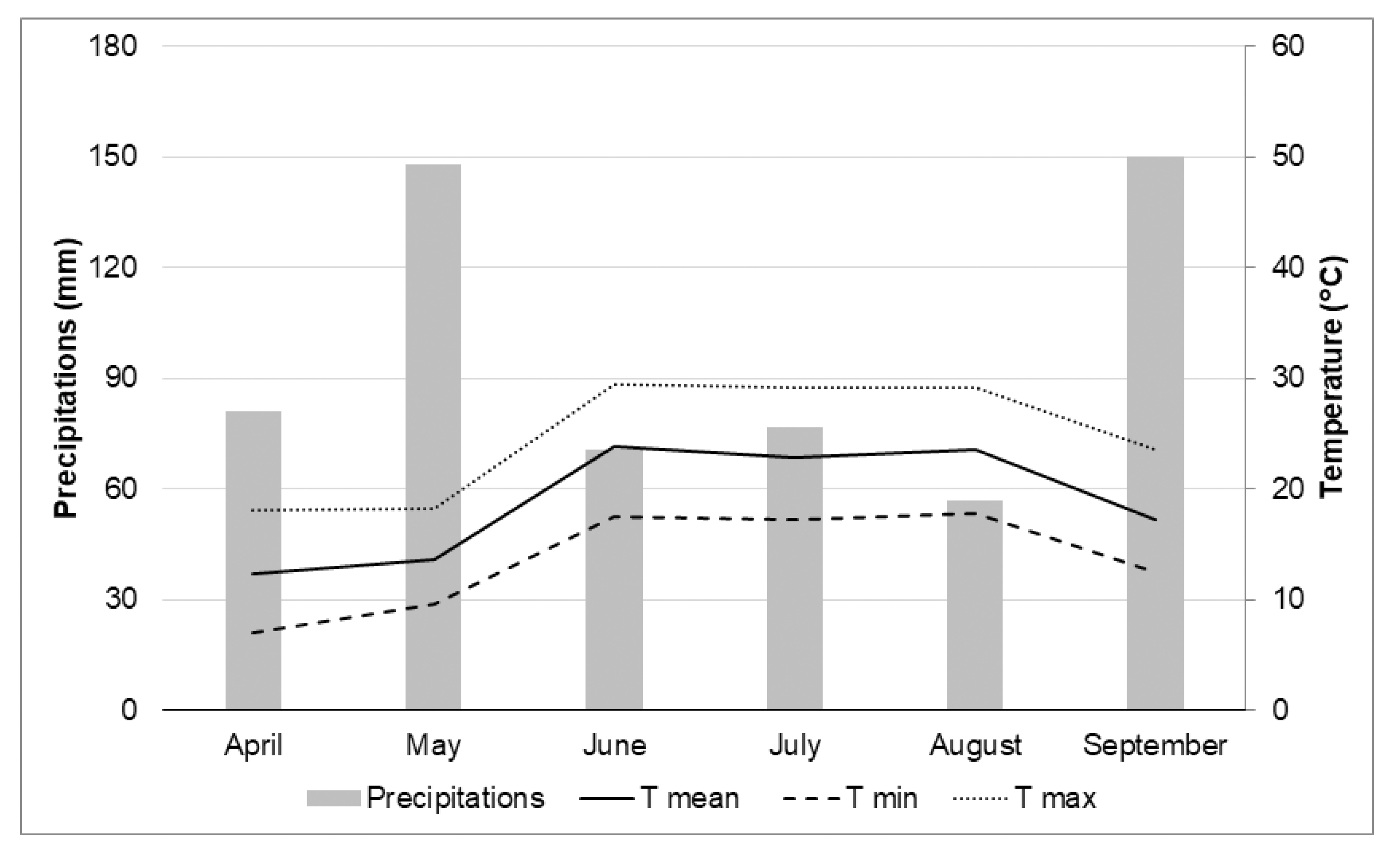
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Yield and Yield Components
2.3. Chemical Properties of the Fruit
2.4. Physical and Morphological Measurements of the Fruit
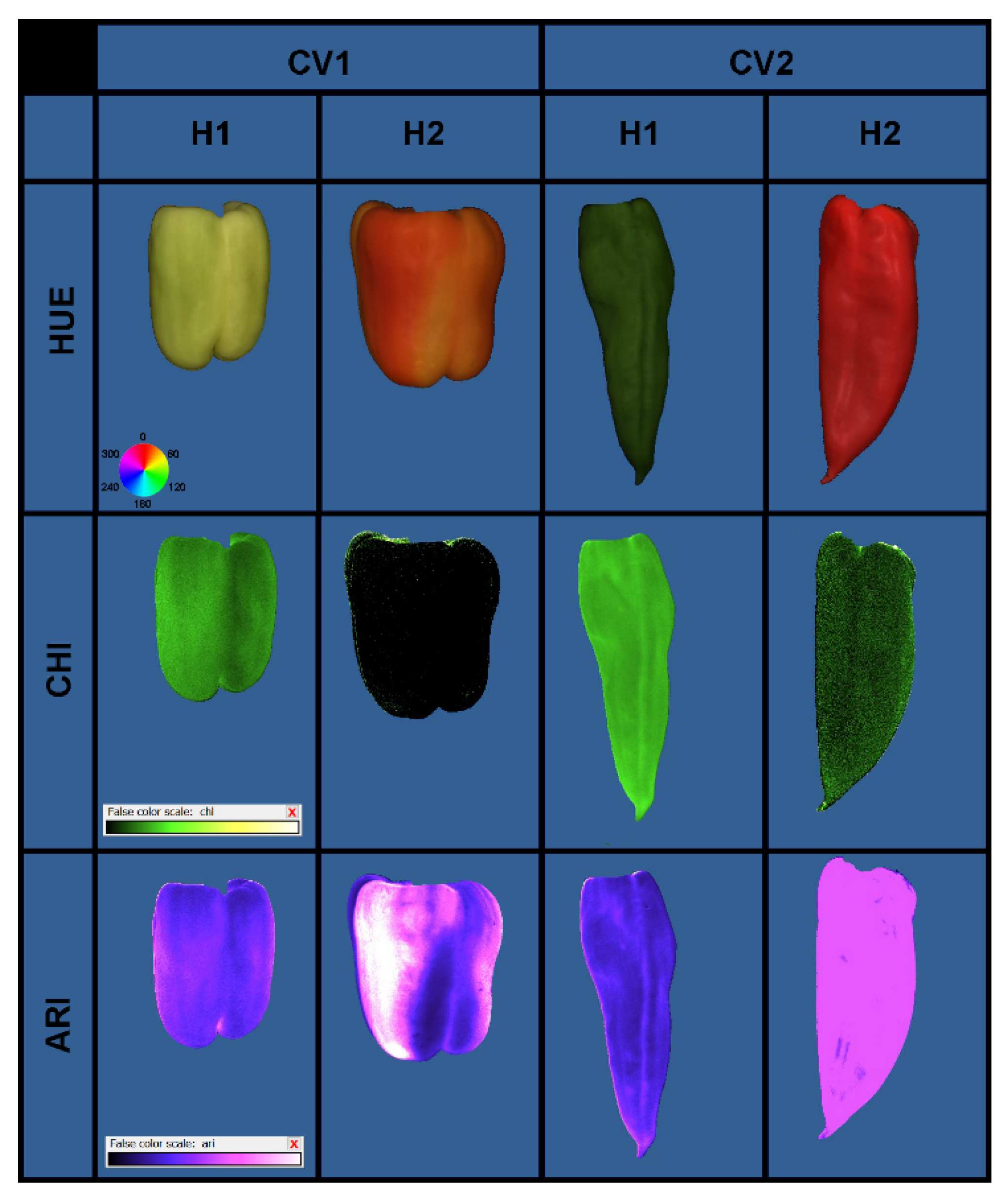
2.5. Multispectral Analysis of the Fruit
2.6. Statistical Data Analysis
3. Results
3.1. Yield
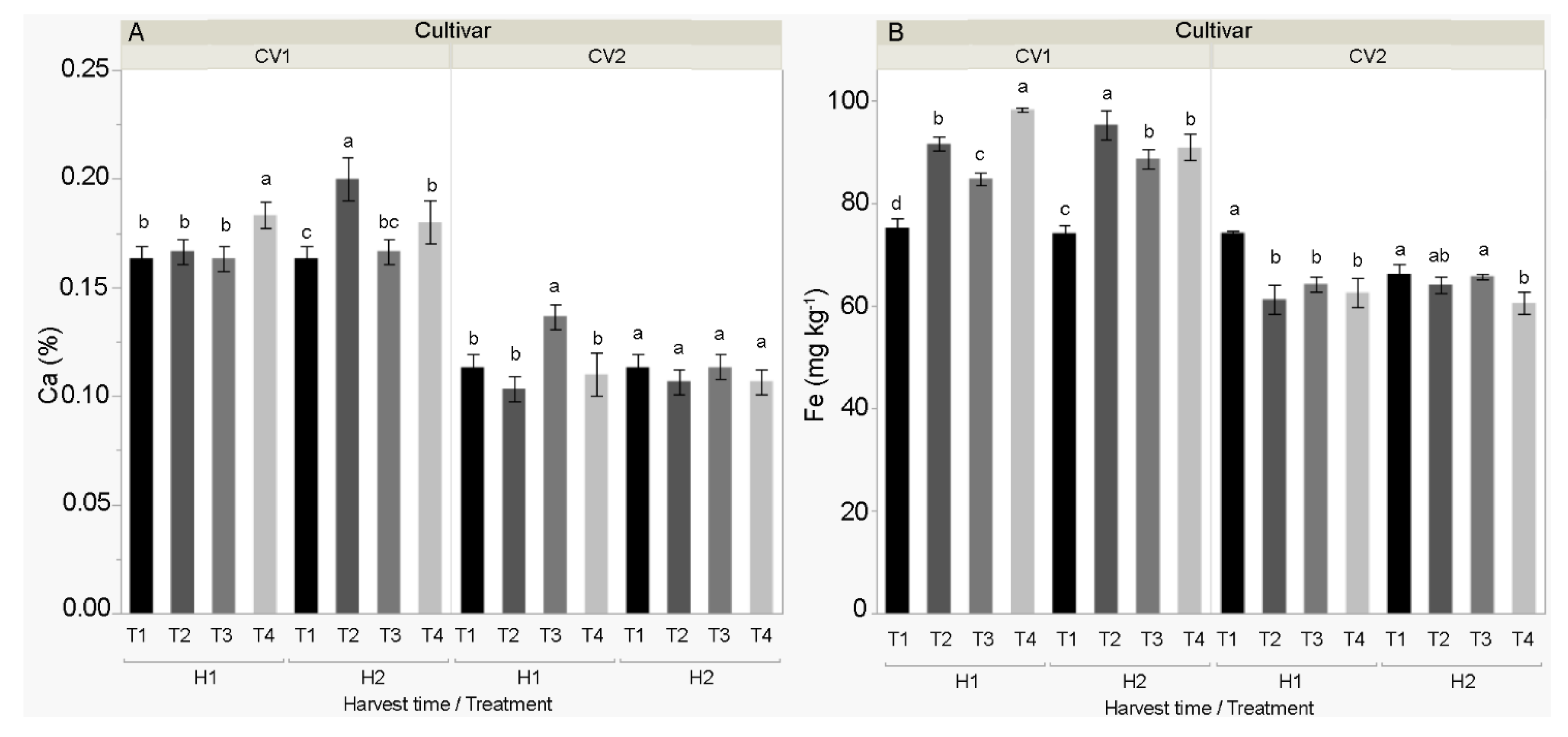
3.2. Chemical Properties of the Fruit
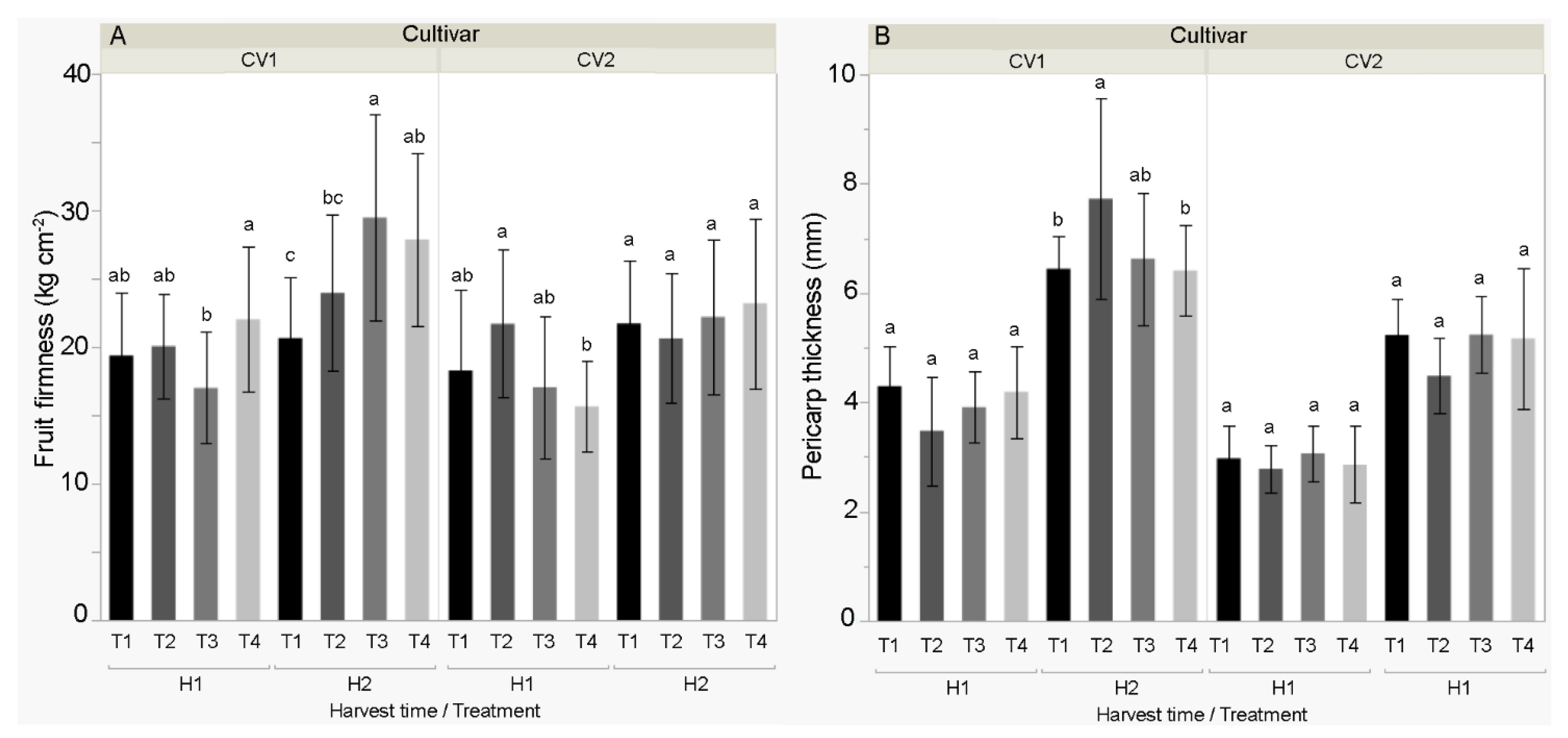
3.3. Physical and Morphological Properties of the Fruit
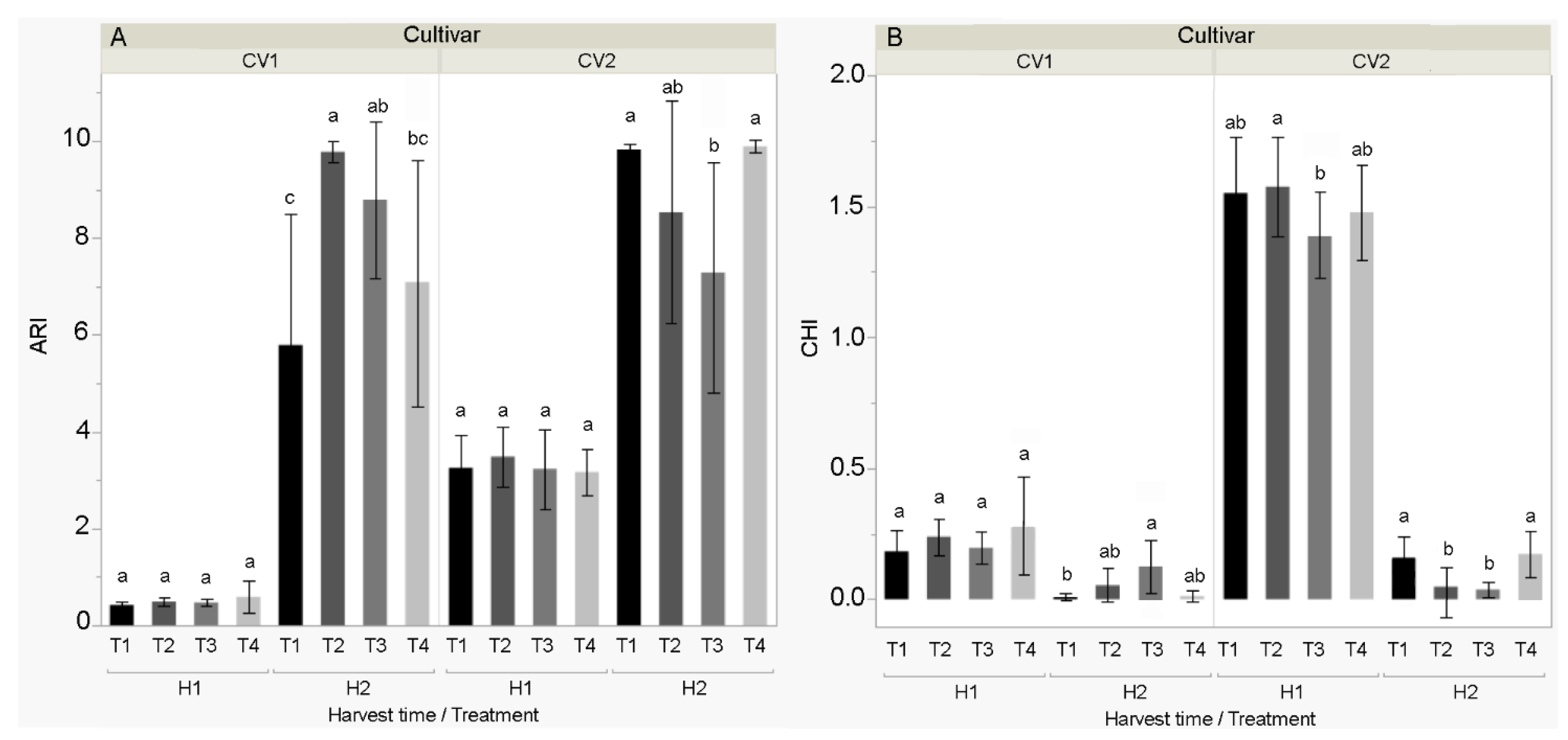
3.4. Multispectral Properties of the Fruit
4. Discussion
4.1. Yield
4.2. Chemical Properties of the Fruit
4.3. Physical and Morphological Properties of the Fruit
4.4. Multispectral Properties of the Fruit
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gao, Q.; Xiong, T.; Li, X.; Chen, W.; Zhu, X. Calcium and calcium sensors in fruit development and ripening. Sci. Hortic. (Amsterdam) 2019, 253, 412–421. [Google Scholar] [CrossRef]
- Gulbagca, F.; Burhan, H.; Elmusa, F.; Sen, F. Calcium Nutrition in Fruit Crops: Agronomic and Physiological Implications; Elsevier, Inc.: Philadelphia, PA, USA, 2019; ISBN 9780128187326. [Google Scholar]
- Brunetto, G.; De Melo, G.W.B.; Toselli, M.; Quartieri, M.; Tagliavini, M. the Role of Mineral Nutrition on Yields and Fruit Quality in Grapevine, Pear and Apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Singh, U.M.; Manohar, M.; Gaur, V.S. Calcium transport from source to sink: Understanding the mechanism(s) of acquisition, translocation, and accumulation for crop biofortification. Acta Physiol. Plant. 2015, 37, 1722. [Google Scholar] [CrossRef]
- Fageria, N.K.; Filho, M.P.B.; Moreira, A.; Guimarães, C.M. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Sabir, A.; Yazar, K.; Sabir, F.; Kara, Z.; Yazici, M.A.; Goksu, N. Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertilizer pulverizations. Sci. Hortic. (Amsterdam) 2014, 175, 1–8. [Google Scholar] [CrossRef]
- Gajc-Wolska, J.; Mazur, K.; Niedzińska, M.; Kowalczyk, K.; Zołnierczyk, P. The influence of foliar fertilizers on the quality and yield of sweet pepper (Capsicum annuum L.). Folia Hortic. 2018, 30, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants 2021, 10, 2. [Google Scholar] [CrossRef]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Avila-Juarez, L.; Torres-Pacheco, I. Nanoparticles as potential antivirals in agriculture. Agriculture 2020, 10, 444. [Google Scholar] [CrossRef]
- El-Zawily, A.I.; El-Sawy, M.B.; EL-Semellawy, E.M.H.; Abd-EL-Ghaffar, R.S.A. Effect of Magnetization and Nano Potassium Particles on Growth, Yield and Fruit Quality of Cucumber Under Plastic House Conditions. J. Product. Dev. 2018, 23, 627–652. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, S.M.; Karimi, M.; Teixeira da Silva, J.A. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 2020, 100, 25–31. [Google Scholar] [CrossRef]
- Prifti, D.; Maçi, A. Effect of Herbagreen Nano-Particles on Biochemical and Technological Parameters of Cereals (Wheat and Corn). Eur. Sci. J. ESJ 2017, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Herceg, Z.; Batur, V.; Režek Jambrak, A.; Vukušić, T.; Gmajnički, I.; Špoljarić, I. The Effect of Tribomechanical Micronization and Activation on Rheological, Thermophysical, and Some Physical Properties of Tapioca Starch. Int. J. Carbohydr. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chithrani, K.; Premalal, G.G.C.; Wickramasinghe, J.P. Effect of ’Calcite Foliar Fertilizer’ on Growth, Yield and Nutritional Composition of Sorghum (Sorghum bicolor L. Moench) Cultivated under Field Condition as a Fodder for Dairy Cattle. In Proceedings of the 14th Agricultural Research Symposium, Kuliyapitiya, Sri Lanka, 25–26 June 2015; pp. 302–306. [Google Scholar]
- Hua, K.H.; Wang, H.C.; Chung, R.S.; Hsu, J.C. Calcium carbonate nanoparticles can enhance plant nutrition and insect pest tolerance. J. Pestic. Sci. 2015, 40, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Rana, V.S.; Pawar, R.; Lakra, J.; Racchapannavar, V.K. Nanofertilizers for sustainable fruit production: A review. Environ. Chem. Lett. 2020, 19, 1693–1714. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, S.N.; Matsuoka, T. Non-Destructive Techniques for Quality Evaluation of Intact Fruits and Vegetables. Food Sci. Technol. Res. 2000, 6, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Tripodi, P.; Massa, D.; Venezia, A.; Cardi, T. Sensing Technologies for Precision Phenotyping in Vegetable Crops: Current Status and Future Challenges. Agronomy 2018, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Lazarević, B.; Šatović, Z.; Nimac, A.; Vidak, M.; Gunjača, J.; Politeo, O.; Carović-Stanko, K. Application of Phenotyping Methods in Detection of Drought and Salinity Stress in Basil (Ocimum basilicum L.). Front. Plant Sci. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic plant biostimulants and fruit quality—A review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Bejo, S.K.; Shamsudin, R. Rapid and nondestructive techniques for internal and external quality evaluation of watermelons: A review. Sci. Hortic. (Amsterdam) 2017, 225, 689–699. [Google Scholar] [CrossRef]
- Yildiz, F.; Özdemir, A.T.; Uluişik, S. Evaluation Performance of Ultrasonic Testing on Fruit Quality Determination. J. Food Qual. 2019, 6810865, 7. [Google Scholar] [CrossRef]
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Lee, S.Y.; Baek, D.Y.; Park, S.Y.; Lee, S.G.; Ryu, T.H.; Lee, S.K.; Kang, H.J.; Kwon, O.H.; Kil, M.; et al. A comparison of the nutrient composition and statistical profile in red pepper fruits (Capsicums annuum L.) based on genetic and environmental factors. Appl. Biol. Chem. 2019, 62, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, C.V.; de Mendonça, V.Z.; Mendes de Sousa Gouveia, A.; Gonçalves Carpanetti, M.; Barbosa Tavares, A.E.; de Brito Lima Lanna, N.; Evangelista, R.M.; Inácio Cardoso, A.I. Physicochemical and biochemical traits of sweet pepper hybrids as a function of harvest times. Food Chem. 2018, 257, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Żurawik, A.; Jadczak, D.; Panayotov, N.; Żurawik, P. Macro-and micronutrient content in selected cultivars of Capsicum annuum L. depending on fruit coloration. Plant Soil Environ. 2020, 69, 155–161. [Google Scholar] [CrossRef]
- Buczkowska, H.; Michałojć, Z.; Konopińska, J.; Kowalik, P. Content of macro- and microelements in sweet pepper fruits depending on foliar feeding with calcium. J. Elem. 2015, 20, 261–272. [Google Scholar] [CrossRef]
- Ozbay, N. Studies on Seed Priming in Pepper (Capsicum annuum L.). In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 209–239. ISBN 9789811300325. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- AOAC Officinal. Method of Analysis of AOAC International; AOAC Officinal: Gaithersburg, MD, USA, 2015. [Google Scholar]
- Croatian Standards Institute HRN ISO 11277:2011. Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation (ISO 11277:2009). 2011. Available online: http://31.45.242.218/HZN/Todb.nsf/wFrameset2?OpenFrameSet&Frame=Down&Src=%2FHZN%2FTodb.nsf%2Fcd07510acb630f47c1256d2c006ec863%2Fb23c712f88d6b685c1257809005388c0%3FOpenDocument%26AutoFramed (accessed on 14 October 2020).
- Croatian Standards Institute HRN ISO 10694:2004. Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis) (ISO 10694:1995). 2004. Available online: http://31.45.242.218/HZN/Todb.nsf/wFrameset2?OpenFrameSet&Frame=Down&Src=%2FHZN%2FTodb.nsf%2Fcd07510acb630f47c1256d2c006ec863%2Fc1256c8f003565d5c1256d29003db0c4%3FOpenDocument%26AutoFramed (accessed on 14 October 2020).
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchung über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustanden der Böden. II, Chemische Extraktionsmethoden zur Phosphor und Kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- EEC Commission Concerning the Establishment of Common Analytical Methods in the Sector of Wine. Regulation No. 2676/90. Off. J. Eur. Commun. 1990, L272, 1–192. [Google Scholar]
- Croatian Standards Institute HRN ISO 11261:2004. Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method (HRN ISO 11261:1995). 2004. Available online: http://31.45.242.218/HZN/Todb.nsf/wFrameset2?OpenFrameSet&Frame=Down&Src=%2FHZN%2FTodb.nsf%2F66011c0bda2bd4dfc1256cf300764c2d%2Fc1256c8f003565d5c1256d29003da9b9%3FOpenDocument%26AutoFramed (accessed on 14 October 2020).
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SAS® 9.3 Procedures Guide: Statistical Procedures; SAS Institute, Inc: Cary, NC, USA, 2011. [Google Scholar]
- Lee, C.H.; Lee, D.W.; Choi, S.Y. Effects of Fruit Drop and Polyembryony of Ardisia pusilla as Influenced by Calcite, Promalin, and 2,4-DP. Hortic. Environ. Biotechnol. 2006, 47, 93–99. [Google Scholar]
- Tantawy, A.S.M.; Salama, Y.A.; Abdel-Mawgoud, A.; Ghoname, A. Comparison of Chelated Calcium with Nano Calcium on Alleviation of Salinity Negative Effects on Tomato Plants. Middle East J. Agric. Res. 2014, 3, 912–916. [Google Scholar]
- Zaman, L.; Shafqat, W.; Sharief, N.; Raza, K.; ud Din, S.; Ahsan Qureshi, M.; Ahmad, S.; Jiskani, M.J. Effect of Foliar Spray of Calcium Carbonate and Zinc Sulphate on Fruit Quality of Kinnow Mandarin. J. Glob. Innov. Agric. Soc. Sci. 2020, 8, 5–9. [Google Scholar] [CrossRef]
- Sajyan, T.K.; Alturki, S.M.; Sassine, Y.N. Nano-fertilizers and their impact on vegetables: Contribution of Nano-chelate Super Plus ZFM and Lithovit®-standard to improve salt-tolerance of pepper. Ann. Agric. Sci. 2020, 65, 200–208. [Google Scholar] [CrossRef]
- Rubio, C.; Hardisson, A.; Martín, R.E.; Báez, A.; Martín, M.M.; Álvarez, R. Mineral composition of the red and green pepper (Capsicum annuum) from Tenerife Island. Eur. Food Res. Technol. 2002, 214, 501–504. [Google Scholar] [CrossRef]
- López, A.; Fenoll, J.; Hellín, P.; Flores, P. Physical characteristics and mineral composition of two pepper cultivars under organic, conventional and soilless cultivation. Sci. Hortic. (Amsterdam) 2013, 150, 259–266. [Google Scholar] [CrossRef]
- Emmanuel-Ikpeme, C. Comparative Evaluation of the Nutritional, Phytochemical and Microbiological Quality of Three Pepper Varieties. J. Food Nutr. Sci. 2014, 2, 74. [Google Scholar] [CrossRef]
- Jadczak, D.; Grzeszczuk, M.; Kosecka, D. Quality characteristics and content of mineral compounds in fruit of some cultivars of sweet pepper (Capsicum annuum L.). J. Elem. 2010, 15, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Guilherme, R.; Reboredo, F.; Guerra, M.; Ressurreição, S.; Alvarenga, N. Elemental composition and some nutritional parameters of sweet pepper from organic and conventional agriculture. Plants 2020, 9, 863. [Google Scholar] [CrossRef]
- Österås, A.H.; Greger, M. Interactions between calcium and copper or cadmium in Norway spruce. Biol. Plant. 2006, 50, 647–652. [Google Scholar] [CrossRef]
- Azeez, L.; Adejumo, A.L.; Simiat, O.M.; Lateef, A. Influence of calcium nanoparticles (CaNPs) on nutritional qualities, radical scavenging attributes of Moringa oleifera and risk assessments on human health. J. Food Meas. Charact. 2020, 14, 2185–2195. [Google Scholar] [CrossRef]
- Patanè, C.; Pellegrino, A.; DI Silvestro, I. Effects of calcium carbonate application on physiology, yield and quality of field-grown tomatoes in a semi-arid Mediterranean climate. Crop Pasture Sci. 2018, 69, 411–418. [Google Scholar] [CrossRef]
- Maya-Meraz, I.O.; Pérez-Leal, R.; Ornelas-Paz, J.J.; Jacobo-Cuéllar, J.L.; Rodríguez-Roque, M.J.; Yañez-Muñoz, R.M.; Cabello-Pasini, A. Effect of calcium carbonate residues from cement industries on the phenolic composition and yield of Shiraz grapes. S. Afr. J. Enol. Vitic. 2020, 41, 33–43. [Google Scholar] [CrossRef]
- Kowalik, P.; Lipa, T.; Michałojć, Z.; Chwil, M. Ultrastructure of cells and microanalysis in malus domestica borkh. “Szampion” fruit in relation to varied calcium foliar feeding. Molecules 2020, 25, 4622. [Google Scholar] [CrossRef]
- Stow, J.R.; Dover, C.J.; Genge, P.M. Control of ethylene biosynthesis and softening in ‘Cox’s Orange Pippin’ apples during low-ethylene, low-oxygen storage. Postharvest Biol. Technol. 2000, 18, 215–225. [Google Scholar] [CrossRef]
- Róth, E.; Kovács, E.; Hertog, M.L.A.T.M.; Vanstreels, E.; Nicolaï, B. Relationship between physical and biochemical parameters in apple softening. Acta Hortic. 2005, 682, 573–578. [Google Scholar] [CrossRef]
- Aini, F.; Bayat, L.; Hosseinkhani, S. Influence of preharvest nano calcium applications on postharvest of sweet pepper (Capsicum annum). Nusant. Biosci. 2016, 8, 215–220. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, E.; Righetti, T.L.; Raese, J.T. Ranking tissue mineral analysis to identify mineral limitations on quality in fruit. J. Am. Soc. Hortic. Sci. 1988, 113, 382–389. [Google Scholar]
- Benavides, A.; Recasens, I.; Casero, T.; Soria, Y.; Puy, J. Multivariate Analysis of Quality and Mineral Parameters on Golden Smoothee Apples Treated Before Harvest with Calcium and Stored in Controlled Atmosphere. Food Sci. Technol. Int. 2002, 8, 139–146. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D. Effect of foliar Zn, form and timing of Ca sprays on fruit Ca concentration in new apple cultivars. Acta Hortic. 2002, 594, 435–443. [Google Scholar] [CrossRef]
- Raese, J.T.; Drake, S.R.; Staiff, D.C. Influence of different calcium materials and spray timing on mineral composition, yield, fruit quality, and control of fruit disorders of ‘anjou’ pears. J. Plant Nutr. 1995, 18, 823–838. [Google Scholar] [CrossRef]
- Smith, E.D. The effect of foliar calcium treatments on fruit weight and firmness of rabbiteye blueberry (Vaccinium virgatum Aiton). J. Am. Pomol. Soc. 2016, 70, 74–81. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; ISBN 978-0-12-473542-2. [Google Scholar]
- Tao, X.; Li, T.L.; Qi, M.F. Analysis of Calcium Content, Hormones, and Degrading Enzymes in Tomato Pedicel Explants During Calcium-Inhibited Abscission. Agric. Sci. China 2009, 8, 556–563. [Google Scholar] [CrossRef]
- Oren-Shamir, M. Does anthocyanin degradation play a significant role in determining pigment concentration in plants? Plant Sci. 2009, 177, 310–316. [Google Scholar] [CrossRef]


| pHH2O a | pHKCl a | % | mg 100 g−1 | % | ||||
|---|---|---|---|---|---|---|---|---|
| Corg b | N c | P2O5 d | K2O d | Sand e | Silt e | Clay e | ||
| 6.19 | 5.01 | 0.85 | 0.08 | 12.9 | 18.8 | 16.8 | 64.9 | 18.3 |
| Abbreviation | Treatment (Product) | Product Calcite Content | Product Calcium Content | Applied Recommended Concentration (g L−1) |
|---|---|---|---|---|
| T1 | Control | − | Water only | |
| T2 | Eco Green | 50.80% CaCO3 | 20.32% Ca | 3 |
| T3 | Eco Green | 5 | ||
| T4 | Zeogreen+P | 69.80% CaCO3 | 27.95% Ca | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidak, M.; Lazarević, B.; Petek, M.; Gunjača, J.; Šatović, Z.; Budor, I.; Carović-Stanko, K. Multispectral Assessment of Sweet Pepper (Capsicum annuum L.) Fruit Quality Affected by Calcite Nanoparticles. Biomolecules 2021, 11, 832. https://doi.org/10.3390/biom11060832
Vidak M, Lazarević B, Petek M, Gunjača J, Šatović Z, Budor I, Carović-Stanko K. Multispectral Assessment of Sweet Pepper (Capsicum annuum L.) Fruit Quality Affected by Calcite Nanoparticles. Biomolecules. 2021; 11(6):832. https://doi.org/10.3390/biom11060832
Chicago/Turabian StyleVidak, Monika, Boris Lazarević, Marko Petek, Jerko Gunjača, Zlatko Šatović, Ivica Budor, and Klaudija Carović-Stanko. 2021. "Multispectral Assessment of Sweet Pepper (Capsicum annuum L.) Fruit Quality Affected by Calcite Nanoparticles" Biomolecules 11, no. 6: 832. https://doi.org/10.3390/biom11060832







