Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants
Abstract
:1. Importance of Medicinal Plants
2. The Importance of Polyploidy
2.1. Autopolyploids
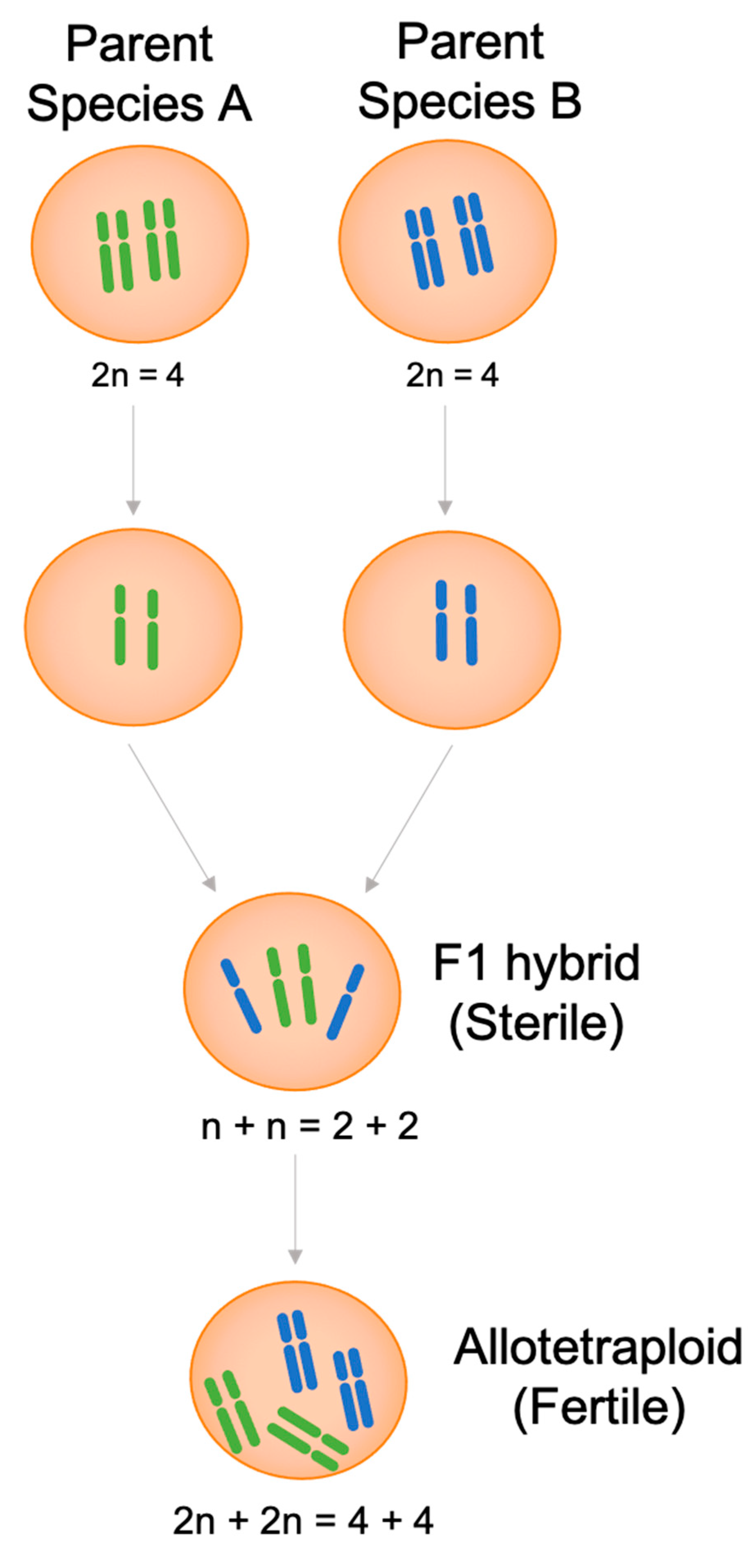
2.2. Allopolyploids
3. The Importance of Polyploidy in Medicinal Plant Breeding
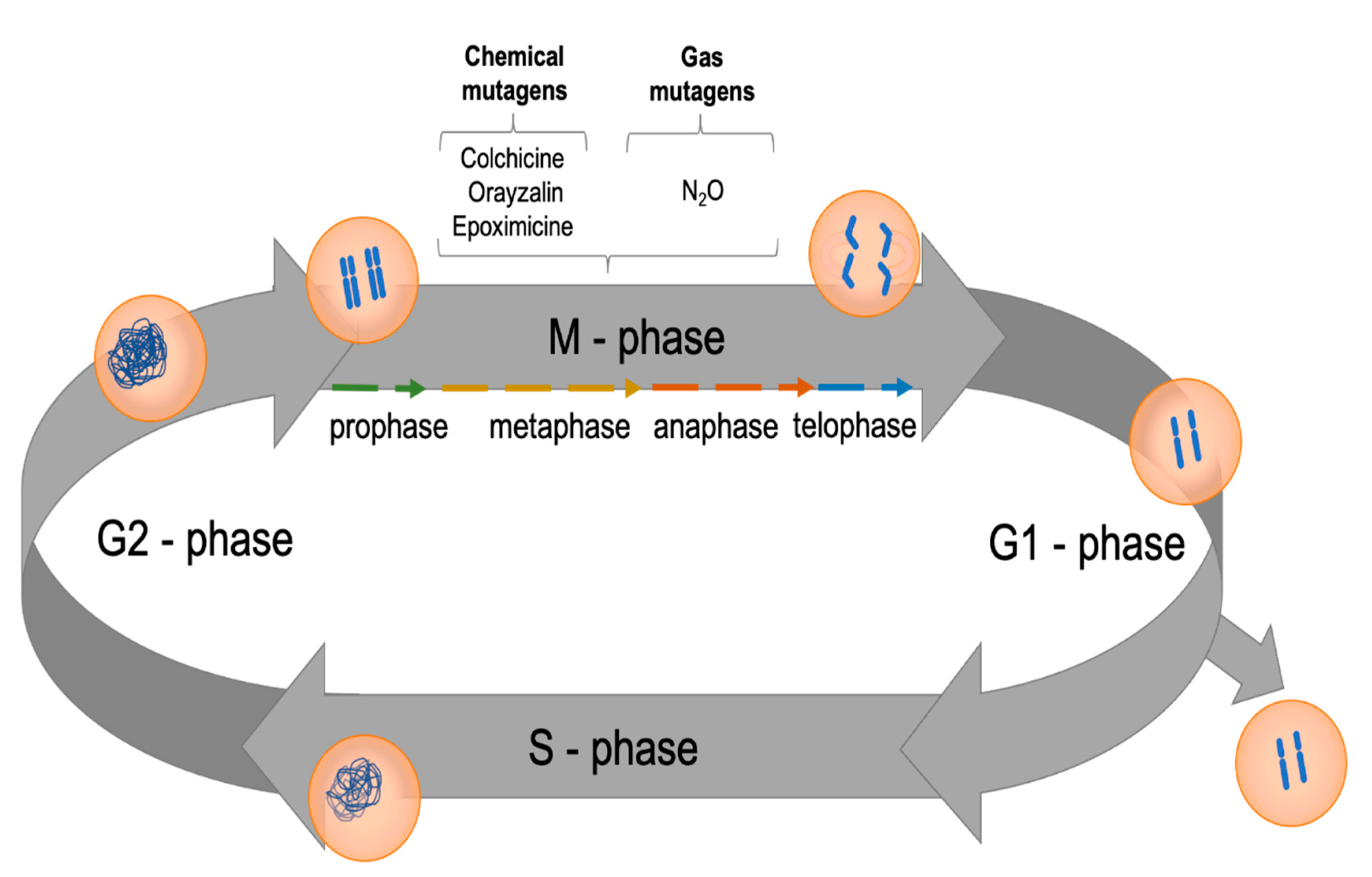
4. Methods of Increasing Ploidy Levels in Plants
5. Polyploidy Effects on Gene Expression and Silencing
6. Polyploidy Effects on Valuable Compounds
6.1. Alkaloids
6.2. Terpenes
6.3. Phenols
7. Limitations
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Che, C.T.; Zhang, H. Plant natural products for human health. Int. J. Mol. Sci. 2019, 20, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Kessler, A.; Kalse, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.; Lewis, N. Natural Products (Secondary Metabolites). Biochem. Mol. Biol. Plants 2000, 24, 1250–1319. [Google Scholar]
- World Health Organization (WHO). WHO Traditional Medicine Strategy 2014–2023; World Health Organization (WHO): Hong Kong SAR, China, 2013; pp. 1–76. [Google Scholar]
- Barnes, P.M.; Bloom, B.; Nahin, R.L.; Stussman, B.J. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl. Health Stat. Rep. 2009, 18, 1–14. [Google Scholar]
- Dehghan, E.; Häkkinen, S.T.; Oksman-Caldentey, K.M.; Ahmadi, F.S. Production of tropane alkaloids in diploid and tetraploid plants and in vitro hairy root cultures of Egyptian henbane (Hyoscyamus muticus L.). Plant. Cell Tissue Organ. Cult. 2012, 110, 35–44. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzym. Microb. Technol. 1995, 17, 674–684. [Google Scholar] [CrossRef]
- Vanisree, M.; Lee, C.Y.; Lo, S.F.; Nalawade, S.M.; Lin, C.Y.; Tsay, H.S. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 2004, 45, 1–22. [Google Scholar]
- Oksman-Caldentey, K.M.; Hiltunen, R. Transgenic crops for improved pharmaceutical products. Field Crop. Res. 1996, 45, 57–69. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- DiCosmo, F.; Misawa, M. Eliciting secondary metabolism in plant cell cultures. Trends Biotechnol. 1985, 3, 318–322. [Google Scholar] [CrossRef]
- Poulev, A.; O’Neal, J.M.; Logendra, S.; Pouleva, R.B.; Timeva, V.; Garvey, A.S.; Raskin, I.; Cragg, G.M. Elicitation, a new window into plant chemodiversity and phytochemical drug discovery. J. Med. Chem. 2003, 46, 2542–2547. [Google Scholar] [CrossRef]
- Sevón, N.; Oksman-Caldentey, K.M. Agrobacterium rhizogenes-mediated transformation: Root cultures as a source of alkaloids. Planta Médica 2002, 68, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Spencer, A.; Hamill, J.D.; Rhodes, M.J.C. In Vitro biosynthesis of monoterpenes by Agrobacterium transformed shoot cultures of two Mentha species. Phytochemistry 1993, 32, 911–919. [Google Scholar] [CrossRef]
- Fujita, Y.; Tabata, M. Secondary metabolites from plant cells: Pharmaceutical applications and progress in commercial production. Plant Biol. 1987, 3, 169–185. [Google Scholar]
- Eilert, U.; Kurz, W.G.W.; Constabel, F. Stimulation of sanguinarine accumulation in Papaver somniferum cell cultures by fungal elicitors. J. Plant. Physiol. 1985, 119, 65–76. [Google Scholar] [CrossRef]
- Ulbrich, B.; Wiesner, W.; Arens, H. Large-Scale Production of Rosmarinic Acid from Plant Cell Cultures of Coleus blumei Benth. In Primary and Secondary Metabolism of Plant Cell Cultures; Springer: Belin/Heidelberg, Germany, 1985; ISBN 978-3-642-70717-9. [Google Scholar]
- Henry, R.J. Plant Diversity and Evolution: Genotypic and Phenotypic Variation in Higher Plants; CABI Publishing: Wallingford, UK, 2005; pp. 97–139. ISBN 9780851999043. [Google Scholar]
- Ravandi, E.G.; Rezanejad, F.; Dehghan, E. In vitro regeneration ability of diploid and autotetraploid plants of Cichorium intybus L. Cytol. Genet. 2014, 48, 166–170. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, X.; Hao, B.; Ge, S.; Luo, J. Duplication and DNA segmental loss in the rice genome: Implications for diploidization. New Phytol. 2005, 165, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Wendel, J.F. Polyploidy and genome evolution in plants. Curr. Opin. Plant. Biol. 2005, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.L.; Zhu, D.N.; Cai, Z.H.; Xu, D.R. Autotetraploid plants from colchicine-treated bud culture of Salvia miltiorrhiza Bge. Plant. Cell Tissue Organ. Cult. 1996, 47, 73–77. [Google Scholar] [CrossRef]
- Goeden-Kallemeyn, Y.C.; Chen, C.H. Consistency of the chromosome constitution in plants derived from a long-term daylily callus culture. Proc. S. Dak. Acad. Sci. 1978, 57, 108–112. [Google Scholar]
- Kondorosi, E.; Roudier, F.; Gendreau, E. Plant cell-size control: Growing by ploidy? Curr. Opin. Plant. Biol. 2000, 3, 488–492. [Google Scholar] [CrossRef]
- Warner, D.A.; Edwards, G.E. Effects of polyploidy on photosynthesis. Photosynth. Res. 1993, 35, 135–147. [Google Scholar] [CrossRef]
- Lavania, U.C. Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Plant Genet. Resour. 2005, 3, 170–177. [Google Scholar] [CrossRef]
- Yang, X.M.; Cao, Z.Y.; An, L.Z.; Wang, Y.M.; Fang, X.W. In vitro tetraploid induction via colchicine treatment from diploid somatic embryos in grapevine (Vitis vinifera L.). Euphytica 2006, 152, 217–224. [Google Scholar] [CrossRef]
- Wehner, T.C. Watermelon. In Vegetables I; Springer: New York, NY, USA, 2008; Volume 1, Chapter 17; ISBN 978-038-730-443-4. [Google Scholar]
- Compton, M.E.; Gray, D.J.; Elmstrom, G.W. Identification of tetraploid regenerants from cotyledons of diploid watermelon cultured in vitro. Euphytica 1996, 87, 165–172. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef] [Green Version]
- Bellostas, N.; Sørensen, A.D.; Sørensen, J.C.; Sørensen, H. Genetic variation and metabolism of glucosinolates. Adv. Bot. Res. 2007, 45, 369–415. [Google Scholar] [CrossRef]
- Hartwell, L.H.; Hood, L.; Goldberg, M.L.; Reynolds, A.E.; Silver, L.M.; Veres, R.C. Genetics from Genes to Genomes; McGraw Hill: Boston, MA, USA, 2004; ISBN 978-007-325-526-6. [Google Scholar]
- De Jesus-Gonzalez, L.; Weathers, P.J. Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep. 2003, 21, 809–813. [Google Scholar] [CrossRef]
- Dhooghe, E.; van Laere, K.; Eeckhaut, T.; Leus, L.; van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ. Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Shahriari-, F.; Dehghan, E.; Farsi, M.; Azizi, M. Tetraploid Induction of Hyoscyamus muticus L. using Colchicine Treatment. Pak. J. Biol. Sci. 2008, 11, 2653–2659. [Google Scholar] [CrossRef] [Green Version]
- Maluszynski, M.; Nichterlein, K.; Zanten, L.; Ahloowalia, B.S. Officially Released Mutant Varieties—The FAO/IAEA Database; INIS-XA--291; International Atomic Energy Agency (IAEA): Vienna, Austria, 2000. Available online: https://www.osti.gov/etdeweb/biblio/20137439 (accessed on 2 May 2021).
- Francis, D. The plant cell cycle-15 years on. New Phytol. 2007, 174, 261–278. [Google Scholar] [CrossRef]
- Dewitte, W.; Murray, J.A.H. The Plant Cell Cycle. Annu. Rev. Plant. Biol. 2003, 54, 235–264. [Google Scholar] [CrossRef] [Green Version]
- Ravandi, E.G.; Rezanejad, F.; Zolala, J.; Dehghan, E. The effects of chromosome-doubling on selected morphological and phytochemical characteristics of Cichorium intybus L. J. Hortic. Sci. Biotechnol. 2015, 88, 701–709. [Google Scholar] [CrossRef]
- Blakeslee, A.F.; Avery, A.G. Methods of inducing doubling of chromosomes in plants: By treatment with colchicine. J. Hered. 1937, 28, 393–411. [Google Scholar] [CrossRef]
- Murashige, T.; Nakano, R. Chromosome complement as a determinant of the morphogenic potential of tobacco cells. Am. J. Bot. 1967, 54, 963–970. [Google Scholar] [CrossRef]
- Pickens, K.A.; Cheng, Z.M.; Kania, S.A. Effects of colchicine and oryzalin on callus and adventitious shoot formation of Euphorbia pulchurrima ‘Winter Rose’. HortScience 2006, 41, 1651–1655. [Google Scholar] [CrossRef] [Green Version]
- Ng, D.W.-K.; Zhang, C.; Miller, M.; Shen, Z.; Briggs, S.P.; Chen, Z.J. Proteomic divergence in Arabidopsis autopolyploids and allopolyploids and their progenitors. Hered. 2012, 108, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollister, J.D.; Arnold, B.J.; Svedin, E.; Xue, K.S.; Dilkes, B.P.; Bomblies, K. Genetic adaptation associated with genome-doubling in autotetraploid Arabidopsis arenosa. PLoS Genet. 2012, 8, e1003093. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.J.; Ni, Z. Mechanisms of genomic rearrangements and gene expression changes in plant polyploids. BioEssays 2006, 28, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Chen, Z.J. Epigenetic and developmental regulation in plant polyploids. Curr. Opin. Plant Biol. 2015, 24, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.L.; Wendel, J.F. Novel patterns of gene expression in polyploid plants. Trends Genet. 2005, 21, 539–543. [Google Scholar] [CrossRef]
- Yun, D.J.; Hashimoto, T.; Yamada, Y. Metabolic engineering of medicinal plants: Transgenic Atropa belladonna with an improved alkaloid composition. Proc. Natl. Acad. Sci. USA 1992, 89, 11799–11803. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.K.; Pathak, S.; Sharma, A.; Trivedi, P.K.; Shukla, S. Modulated gene expression in newly synthesized auto-tetraploid of Papaver somniferum L. S. Afr. J. Bot. 2010, 76, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Zhou, Y.; Zhang, J.; Lu, X.; Zhang, F.; Shen, Q.; Wu, S.; Chen, Y.; Wang, T.; Tang, K. Enhancement of artemisinin content in tetraploid Artemisia annua plants by modulating the expression of genes in artemisinin biosynthetic pathway. Biotechnol. Appl. Biochem. 2011, 58, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Javadian, N.; Karimzadeh, G.; Sharifi, M.; Moieni, A.; Behmanesh, M. In vitro polyploidy induction: Changes in morphology, podophyllotoxin biosynthesis, and expression of the related genes in Linum album (Linaceae). Planta 2017, 245, 1165–1178. [Google Scholar] [CrossRef]
- Dhawan, O.P.; Lavania, U.C. Enhancing the productivity of secondary metabolites via induced polyploidy: A review. Euphytica 1996, 87, 81–89. [Google Scholar] [CrossRef]
- Levin, D. The Role of Chromosomal Change in Plant Evolution; Oxford University Press: Oxford, UK; New York, NY, USA, 2002; ISBN 978-019-513-860-3. [Google Scholar]
- Thawabeteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The biologival activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aniszewski, T. Evolution of alkaloids and alkaloids in evolution. In Alkaloids, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2015; Chapter 5; pp. 291–344. [Google Scholar] [CrossRef]
- Madani, H.; Hosseini, B.; Dehghan, E.; Rezaei-chiyaneh, E. Enhanced production of scopolamine in induced autotetraploid plants of Hyoscyamus reticulatus L. Acta Physiol. Plant 2015, 37, 55. [Google Scholar] [CrossRef]
- Berkov, S.; Philipov, S. Alkaloid Production in diploid and autotetraploid plants of Datura stramonium. Pharm. Biol. 2002, 40, 617–621. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2000, 21, 73–82. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Chen, Y.; Guan, Z.; Yin, D.; Chen, F. In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci. Hortic. 2011, 127, 411–419. [Google Scholar] [CrossRef]
- Kaensaksiri, T.; Soontornchainaksaeng, P.; Soonthornchareonnon, N.; Prathanturarug, S. In vitro induction of polyploidy in Centella asiatica (L.) Urban. Plant Cell Tissue Organ Cult. 2011, 107, 187–194. [Google Scholar] [CrossRef]
- Bertea, C.M.; Azzolin, C.M.M.; Bossi, S.; Doglia, G.; Maffei, M.E. Identification of an EcoRl restriction site for a rapid and precise determination of β- asarone-free Acorus calamus cytotypes. Phytochemistry 2005, 66, 507–514. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Yavari, S.; Hassani, M.E.; Yavari, S. Induction of autotetraploidy in dragonhead (Dracocephalum moldavica L.) by colchicine treatment. J. Fruit Ornam. Plant Res. 2010, 18, 23–35. [Google Scholar]
- Saharkhiz, M.J.; Omidbaigi, R.; Sefidkon, F. The Effect of phosphorus and irrigation treatments on the essential oil content and composition of feverfew (Tanacetum parthenium (L.) cv. Zardband). J. Essent. Oil Bear. Plants 2007, 10, 391–398. [Google Scholar] [CrossRef]
- Dijkstra, H.; Speckmann, G.J. Autotetrapliody in Caraway (Carum carvi L.) for the increase of the aetheric oil content of the seed. Euphytica 1980, 29, 89–96. [Google Scholar] [CrossRef]
- Janaki Amal, E.R.; Sobti, S.M. The origin of the Jammu Mint. Curr. Sci. 1962, 31, 387–388. [Google Scholar]
- Gao, S.L.; Chen, B.J.; Zhu, D.N. In vitro production and identification of autotetraploids of Scutellaria baicalensis. Plant Cell Tissue Organ Cult. 2002, 70, 289–293. [Google Scholar] [CrossRef]
- Talebi, S.F.; Saharkhiz, M.J.; Kermani, M.J.; Sharafi, Y.; Fard, R.F. Effect of different antimitotic agents on polyploid induction of anise hyssop (Agastache foeniculum L.). Caryologia 2017, 70, 184–193. [Google Scholar] [CrossRef]
- Tavan, M.; Mirjalili, M.H.; Karimzadeh, G. In vitro polyploidy induction: Changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult. 2015, 122, 573–583. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Cosme, P.; Rodriguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavaliability as a Key Determinant of The Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Wcislo, G.; Szarlej-Wcislo, K. Colorectal Cancer Prevention by Wheat Consumption: A Three-Valued Logic—True, False, or Otherwise? In Wheat and Rice in Disease Prevention and Health; Elsevier: Amsterdam, The Netherlands, 2014; pp. 91–111. ISBN 978-012-401-716-0. [Google Scholar]
- Zahedi, A.A.; Hosseini, B.; Fattahi, M.; Dehghan, E.; Parastar, H.; Madani, H. Overproduction of valuable methoxylated flavones in induced tetraploid plants of Dracocephalum kotschyi Boiss. Bot. Stud. 2014, 55, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Švehlíková, V.; Repčák, M. Variation of Apigenin Quantity in Diploid and Tetraploid Chamomilla recutita (L.) Rauschert. Plant Biol. 2000, 2, 403–407. [Google Scholar] [CrossRef]
- Caruso, I.; Lepore, L.; De Tommasi, N.; Dal Piaz, F.; Frusciante, L.; Aversano, R.; Carputo, D. Secondary metabolite profile in induced tetraploids of wild Solanum commersonii Dun. Chem. Biodivers. 2011, 8, 2226–2237. [Google Scholar] [CrossRef]
- Abdoli, M.; Moieni, A.; Badi, H.N. Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol. Plant 2013, 35, 2075–2083. [Google Scholar] [CrossRef]
- Timko, M.P.; Vasconcelos, A.C.; Fairbrothers, D.E. Euploidy in Ricinus. I. Euploidy and gene dosage effects on cellular proteins. Biochem. Genet. 1980, 18, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Madani, H.; Hosseini, B.; Karimzadeh, G.; Rahimi, A. Enhanced thebaine and noscapine production and modulated gene expression of tyrosine/dopa decarboxylase and salutaridinol 7-O-acetyltransferase genes in induced autotetraploid seedlings of Papaver bracteatum Lindl. Acta Physiol. Plant 2019, 41, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hu, C.G.; Yao, J.L. Tetra ploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J. Plant Physiol. 2010, 167, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Caruso, I.; Dal Piaz, F.; Malafronte, N.; De Tommasi, N.; Aversano, R.; Zottele, C.W.; Scarano, M.-T.; Carputo, D. Impact of ploidy change on secondary metabolites and photochemical efficiency in Solanum Bulbocastanum. Nat. Prod. Commun. 2013. [Google Scholar] [CrossRef] [Green Version]
- Fransz, P.; de Jong, J.H.; Lysak, M.; Castiglione, M.R.; Schubert, I. Interphase chromosomes in Arabidopsis are organized as well defined chromocenters from which euchromatin loops emanate. Proc. Natl. Acad. Sci. USA 2002, 99, 14584–14589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Davis, D.; Birchler, J.A. Dosafe effects on gene expression in a maize ploidy series. Genetics 1996, 142, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Cenci, A.; Hueber, Y.; Zorrilla-Fontanesi, Y.; van Wesemael, J.; Kissel, E.; Gislard, M.; Sardos, J.; Swennen, R.; Roux, N.; Carpentier, S.C.; et al. Effect of paleopolyploidy and allopolyploidy on gene expression in banana. BMC Genom. 2019, 20, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendel, J.F. Genome evolution in poluploids. Plant. Mol. Biol. 2000, 42, 225–249. [Google Scholar] [CrossRef]
- Ranney, T. Polyploidy: From evolution to New Plant Developtment. Proc. Int. Plant Propagators Soc. 2006, 56, 137–142. [Google Scholar]
- Mitchell, C.; Schneper, L.M.; Notterman, D.A. DNA methylation, early life environment, and health outcomes. Pediatr. Res. 2016, 70, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Wang, H.; Dong, B.; Yang, X.; Chen, S.; Jiang, J.; Zhang, Z.; Liu, C.; Zhao, N.; Chen, F. Morphological, Genome and Gene Expression Changes in Newly Induced Autopolyploid Chrysanthemum lavandulifolium (Fisch. ex Trautv.) Makino. Int. J. Mol. Sci. 2016, 17, 1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.Z.; MacQueen, A.; Zheng, G.; Duan, H.; Dore, L.C.; Li, Z.; Liu, J.; Chen, K.; Jia, G.; Bergelson, J.; et al. Unique features of the m6A methylome in Arabidopsis thaliana. Nat. Commun. 2014, 28, 5630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.J.; Krammer, M.C.; Yu, X. N6-Methyladenosine inhibits local ribonucleolytic cleavage to stabilize RNAs in Arabidopsis. Cell Rep. 2018, 25, 1146–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Plant Species | Ploidy Level | The Effect of Induced Polyploidy in Diploid Plants | Reference |
|---|---|---|---|
| Acorus calamus | 4x | More than 95% increase in anti-cancer agent beta-asarone | [64] |
| Agastache foeniculum | 4x | Increase in methyl chavicol metabolite content | [70] |
| Artemisia annua | 4x | Artemisinin content increased up to 1.5-fold | [53] |
| Artemisia annua L. | 4x | Artemisinin content increased by 39–56% | |
| Papaver bracteatum | 4x | 5.86- and 30.55-times higher thebaine and noscapine | [81] |
| Centella asiatica | 4x | Enhanced triterpenoid production | [63] |
| Chamomilla recutita | 4x | Increased apigenin content | [77] |
| Cichorium intybus | 4x | Increase in total phenolic compounds and chlorogenic acid | [42] |
| Datura stramonium | 4x | 52–152% increase in alkaloid production | [60] |
| Dioscorea zingiberensis | 4x | Higher concentration of total alkaloids | [82] |
| Dracocephalum kotschyi | 4x | Increase in methoxylated flavones | [76] |
| Dracocephalum moldavica L. | 4x | Higher concentration of essential oil | [65] |
| Echinacea purpurea | 4x | 71 and 45% increase in chlorogenic acid and cichoric acid content | [79] |
| Hyoscyamus muticus | 4x | 200% increase in tropane alkaloids in the fifth generation after tetraploidy | [7] |
| Hyoscyamus reticulatus L. | 4x | 8.8% increase in scopolamine in the plant itself | [59] |
| Linum album | 4x | 1.39- and 1.23-fold increase in podophyllotoxin production | [54] |
| Papaver sominferum. | 4x | Up to 50% increase in morphine content | [52] |
| Scutellaria baicalensis | 4x | 4.6% increase in baicalin production | [69] |
| Solanum commersonii | 4x | Higher concentration of phenylpropanoids | [78] |
| Tanacetum parthenium L. | 4x | Up to 32% increase in essential oil | [66] |
| Thymus persicus | 4x | Increase in betulinic acid (69.73%), oleanolic acid (42.76%), and ursolic acid (140.6%) | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madani, H.; Escrich, A.; Hosseini, B.; Sanchez-Muñoz, R.; Khojasteh, A.; Palazon, J. Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants. Biomolecules 2021, 11, 899. https://doi.org/10.3390/biom11060899
Madani H, Escrich A, Hosseini B, Sanchez-Muñoz R, Khojasteh A, Palazon J. Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants. Biomolecules. 2021; 11(6):899. https://doi.org/10.3390/biom11060899
Chicago/Turabian StyleMadani, Hadi, Ainoa Escrich, Bahman Hosseini, Raul Sanchez-Muñoz, Abbas Khojasteh, and Javier Palazon. 2021. "Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants" Biomolecules 11, no. 6: 899. https://doi.org/10.3390/biom11060899
APA StyleMadani, H., Escrich, A., Hosseini, B., Sanchez-Muñoz, R., Khojasteh, A., & Palazon, J. (2021). Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants. Biomolecules, 11(6), 899. https://doi.org/10.3390/biom11060899







