Abstract
The brain–gut–microbiome axis is a bidirectional communication pathway between the gut microbiota and the central nervous system. The growing interest in the gut microbiota and mechanisms of its interaction with the brain has contributed to the considerable attention given to the potential use of probiotics, prebiotics and postbiotics in the prevention and treatment of depressive disorders. This review discusses the up-to-date findings in preclinical and clinical trials regarding the use of pro-, pre- and postbiotics in depressive disorders. Studies in rodent models of depression show that some of them inhibit inflammation, decrease corticosterone level and change the level of neurometabolites, which consequently lead to mitigation of the symptoms of depression. Moreover, certain clinical studies have indicated improvement in mood as well as changes in biochemical parameters in patients suffering from depressive disorders.
1. Introduction
The microbiome consists of the microorganisms (bacteria, archaea, viruses, protists and fungi), their genomes, and their surrounding environment, including the gastrointestinal tract, oral mucosa, urogenital and respiratory systems, and the skin surface [1]. Bacteria are the dominant group of microorganisms that make up the microbiome [2]. It is estimated that their number in the entire human body is of the same order as the number of human cells [3]. The majority of bacteria reside in the intestines [4]. Due to their huge number and variety, they can significantly affect normal physiology and modify the host’s susceptibility to diseases [5]. Multiple roles of bacteria in the gut include digestion, taking part in the production of short-chain fatty acids, vitamins synthesis, immune system maintenance, and influence on the permeability of the mucosal barrier [6,7]. Furthermore, additional administration of probiotic bacteria may provide health benefits to the host [5]. Amongst many different strains of probiotics, there are psychobiotic bacteria that have a beneficial effect on mental health when ingested in sufficient amounts [8]. It has been demonstrated that these bacteria have a significant impact on metabolism and central nervous system function, consequently influencing mental health [9,10], thanks to the brain–gut–microbiome (BGM) axis via neuronal, endocrine, and immune mechanisms [11,12]. The impact of probiotics on human neurometabolism can also be promoted by prebiotics, which stimulates the proper growth of probiotic bacteria and can support the gut–brain interaction [13]. Moreover, recent studies indicate the participation of postbiotics in the modulation of the BGM axis [14].
This review describes the properties of probiotics, prebiotics and postbiotics, focusing on their beneficial effect on human health in a regular diet. We discuss the ways through which the gut microbiota communicates with the brain. Furthermore, we focus on recent studies in both animals and humans investigating pro-, pre- and postbiotic supplementation in depressive disorders. We also discuss the shortcomings of these clinical trials. The increasing amount of research on the microbiome suggests that this is an extremely important issue and further investigations of the application of probiotics, prebiotics and postbiotics in the prevention and treatment of depression are essential.
2. Probiotics
The term “probiotic” originates etymologically from the words “pro bios” meaning “for life” and the beneficial effect of lactic acid fermentation products on human health has ancient roots [15]. The original theory is attributed to microbiologist and Nobel laureate Élie Metchnikoff, who was a co-author of many pioneering studies concerning the role and function of probiotic bacteria [16]. He assigns potential life-lengthening properties to lactic acid bacteria present in the human colon [16,17]. Nowadays, probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [18]. These include mainly Lactobacillus and Bifidobacterium strains, as well as some Streptococcus and Enterococcus strains [18,19]. The beneficial effects associated with probiotics include antiallergic action [20], improvement of intestinal health (e.g., elimination of dysbiosis and sealing the intestinal epithelium) [21,22], enhancement of the immune response [23,24], inhibition of lactose intolerance [25], prevention of cancer [26,27], and a beneficial impact on mental health [28,29,30]. It should be emphasized that the majority of bacteria is first acquired at birth and is maintained and extended by diet [31,32]. In healthy people who maintain a healthy diet, there is a beneficial balance of microbiota. Otherwise, additional administration of probiotic bacteria may be considered [18]. Dinan et al. [8] have defined psychobiotics to be probiotics that, when ingested in adequate amounts, produce a health benefit in patients suffering from psychiatric illness [8]. In addition to a positive effect on intestines, psychobiotics contribute to changes in concentrations of brain neurotransmitters and proteins, reduction of cortisol levels, and alterations in serum cytokine levels, which consequently lead to behavioral changes, as demonstrated in animal and clinical studies [13].
Many bacteria can regulate neuroactive metabolites such as gamma-aminobutyric acid (GABA), 5-hydroxytryptamine (5-HT) and catecholamines that play an important role in brain and mental health [33,34,35]. GABA is an amino acid that inhibits synaptic conduction by hyperpolarization of neuronal cell membranes and consequently decreases activity in the central nervous system [33]. GABAergic system dysfunctions are strongly correlated with mood disorders [33]. It has been shown that depression and anxiety disorders are associated with decreased gamma-aminobutyric acid levels in the brain [36,37]. Among GABA-regulating bacteria, there are food-derived Lactobacillus strains like Lactobacillus plantarum [38,39,40,41], Lactobacillus paracasei, Lactobacillus rhamnosus [37,38,39,42], and Lactobacillus brevis [43,44,45,46,47,48]. Yunes et al. [49] have screened 135 human-derived Bifidobacterium and Lactobacillus strains for their ability to produce GABA. Some bacterial strains can also affect serotonin levels [50]. Serotonin is a monoamine neurotransmitter synthetized from tryptophan. Most 5-HT is produced in the enterochromaffin cells in the gastrointestinal tract [34]. The function of serotonin is very complex as it takes part in the regulation of mood, cognition, and several physiological processes [34]. A disrupted serotonergic system is one of the main causes of depression [34]. Bacteria strains that affect the 5-HT pathway are Escherichia coli [51], Klebsiella pneumoniae, Morganella morganii [52], Lactobacillus plantarum, Lactococcus lactis subsp. cremoris, Streptococcus thermophilus [53]. Bacteria that regulate catecholamines (adrenaline, noradrenaline and dopamine) include Bacillus spp., Escherichia coli, Staphylococcus aureus [50,51], Klebsiella pneumoniae and Morganella morganii [52]. Some of the these strains have been incorporated in improved health-promoting functional foods, which have a considerable effect on the regulation of neurometabolites [54].
3. Prebiotics
At first, prebiotics were defined as a “non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria already resident in the colon” [55]. Over time and with the advancement of our knowledge, however, the definition has been modified to include not only stimulation of bacteria residing in the colon but also other bacteria in the human body [56,57]. The International Scientific Association for Probiotics and Prebiotics proposes the following definition of prebiotic: “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [58]. Prebiotics are nondigestible polysaccharides such as oligosaccharides, fructans (fructooligosaccharides, inulin) and galactooligosaccharides that can be found in many natural products and dietary ingredients [59] and are listed in Table 1. Bacterial fermentation of prebiotic carbohydrates results in the production of short-chain fatty acids (SCFAs) such as butyric acid, acetic acid or propionic acid [22]. Prebiotics and the SCFAs are crucial for intestinal health, stimulate the immune system, can be a source of energy for gut microbiota, and have antagonistic properties to detrimental gut bacteria [60,61,62]. Moreover, numerous experimental studies have proved that prebiotics can help decrease the severity of particular diseases such as mental disorders, diabetes, irritable bowel syndrome (IBS), infectious diseases and reduce the colon cancer risk [63,64].

Table 1.
Prebiotic sources.
4. Postbiotics
The term postbiotic has appeared in the literature for over 20 years, however, research on the effects of postbiotics has intensified over the last five years [81]. The International Scientific Association for Probiotics and Prebiotics proposed the following definition of postbiotics: “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [81]. The concept of non-living microorganisms that could promote or preserve health is not new, other terms that have been used to describe such substances include “paraprobiotics” [82,83], “heat-killed probiotics” [84,85], “metabiotics” [86] and “bacterial lysates” [87]. In order to provide a clear definition, a panel of experts have defined the scope of postbiotics to be deliberately inactivated microbial cells, with or without metabolites or cell components, that contribute to demonstrated health benefits [81]. They note that bacterial metabolites (e.g., lactic acid, proteins, vitamins, SCFAs) or cell components (including pili, cell wall components) would not qualify as postbiotics in their own right, although some might be present in postbiotic preparations [81]. Potential mechanisms for the mediation of health effects by postbiotics are similar to those of probiotics [14] and include enhancement of epithelial barrier function, modulation of host-microbiota, modulation of immune responses, modulation of systemic metabolism and signaling via the nervous system [81,88].
5. Brain-Gut-Microbiome Communication Routes
Human organisms are under a significant stimulus from microorganisms inhabiting the intestines and from the metabolites produced by the microbiota [89,90]. Conversely, it is understood that the brain regulates the function of the gut and the structure of the gut microbial community via the autonomic nervous system by modulating intestinal transit, gut motility, secretion and gut permeability [91]. The bidirectional communication pathway between gut microbiota and the gut, and their interaction with the central nervous system, has been termed the brain–gut–microbiome (BGM) axis [11]. Investigations of the BGM axis involve animal studies in germ-free (GF) animals [92], prebiotic and probiotic studies in rodent models [93,94,95], probing the effects of antibiotics [96], fecal transplantation [97] and cultured gut organ systems [98]. These approaches allow for the identification of neuronal, neuroendocrine and neuroimmune routes of BGM mechanisms (Figure 1) [11].
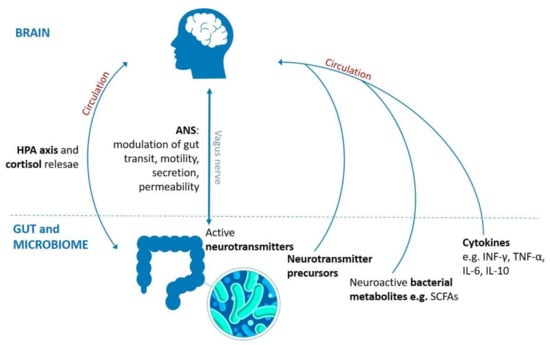
Figure 1.
Routes involved in bidirectional communication pathway between brain, gut and gut microbiota. It comprises neuronal (vagus nerve, enteric nervous system, neurotransmitters), endocrine (HPA axis and stress hormones, e.g., cortisol), and immune (cytokines) mechanisms. HPA: hypothalamic–pituitary–adrenal axis, ANS: autonomic nervous system, SCFAs: short-chain fatty acids, CNS: central nervous system, INF-γ: interferon γ, TNF-α: tumor necrosis factor α, IL-6: interleukin 6, IL-10: interleukin 10.
5.1. Neuronal Routes
The gut is innervated by the enteric nervous system (ENS), which is responsible for the coordination of intestinal function, e.g., motility, fluid secretion, blood flow and reaction to metabolites formed due to the activity of the intestinal microbiota [91]. The digestive function is controlled by the vagus nerve, pelvic nerve and sympathetic pathways [99]. Research to date suggests that specific bacterial strains may play a critical role in the development and function of the ENS, and exposure to bacteria at birth and during early life is essential for the postnatal development of the enteric nervous system [100]. For example, GF mice have been observed to have an immature intestinal nervous system and a deficit in sensory signaling, while reconstruction of their gut microbiota mitigates those shortcomings [101,102]. Moreover, both ex vivo [103] and germ-free animals studies [104] have shown that bacteria impact gut motility. Studies assessing changes in velocity, amplitude and frequency of contractions in the intact segments of jejunum and colon excised from mice have demonstrated that bacterial microvesicles, which are small lipids and bilayer structures secreted by bacteria, are mainly involved in the modulation of gut motility [105].
The vagus nerve plays a crucial role in communication between the gut and the brain [106]. Signals from the intestines are transmitted directly through the vagus nerve or indirectly through the mediation of enteroendocrine cells and hormonal factors [106]. Bravo et al. have established that dietary supplementation with lactic bacteria in healthy anxiolytic mice has a direct effect on GABA receptors in the CNS and reduces anxiety- and depressive-like behavior, while vagotomized mice do not show either neurochemical or behavioral effects [94]. This confirms the importance of the vagus nerve in the BGM pathway [94]. In addition, it has also been observed that vagotomized mice do not exhibit anxiety-like behavior associated with chronic colitis [93].
Numerous bacterial species present in the human intestine are capable of modulating neurotransmitter levels [50]. Lactobacillus and Bifidobacterium species have been found to produce GABA and histamine [49,107], while Escherichia coli produce serotonin, dopamine, and noradrenaline [51]. Neurometabolites produced by bacteria have the potential to influence the CNS and, consequently, behavior [50]. The mechanisms by which those molecules affect the brain involve vagus nerve signaling and circulation [11,108,109]. GABA, serotonin and dopamine cannot cross the blood–brain barrier (BBB) under normal physiological conditions [110,111,112]. However, some of the neurotransmitters’ precursors do cross the BBB and can then be transformed into active neurotransmitters [113,114]. This is the case with tryptophan, the precursor to serotonin, the availability of which is affected by gut bacteria [34]. It is also known that stress can activate enzymes of the kynurenine pathway, which can reduce the amount of tryptophan available for serotonin synthesis; in consequence, kynurenines are believed to play a significant role in the pathogenesis of depressive disorders [115,116].
The microbiome influences the concentrations of brain-derived neurotrophic factor (BDNF) in the brain [117]. This protein is a widely expressed neurotrophin serving several functions within the CNS, including neuronal differentiation and survival [118], and regulation of BDNF concentration is involved in depression and anxiety [119]. BDNF levels have been observed to be lower in the cortex and hippocampus of GF mice as compared to controls, suggesting that the gut microbiota contributes to the elevation of brain BDNF and may modulate behavior through changes in the BDNF level [92]. Moreover, recent research suggests that changes in the microbiome affect hippocampal neurogenesis and are dependent on age and gender [120,121,122].
5.2. Microbiota and the Hypothalamic–Pituitary–Adrenal Axis
One of the factors influencing the BGM axis along the endocrine pathway is the modulation of the hypothalamic–pituitary–adrenal axis (HPA) [123]. The HPA axis works on the principle of negative feedback and plays a key role in stimulating the body’s stress response and regulating physiological processes, including digestion, the functioning of the immune system, emotions and energy balance [123]. In response to stress in the humoral pathway, the hypothalamus releases corticotropin-releasing hormone (CRH). CRH reaches the pituitary gland via the circulation, which synthesizes adrenocorticotropic hormone (ACTH). ACTH stimulates the adrenal glands to synthesize glucocorticosteroid hormones (stress hormones), e.g., cortisol or corticosterone [124]. Acting systemically, stress hormones cause leakage of tight junctions and thus increase the permeability of the intestinal barrier [125]. This leads to bacterial translocation, which causes HPA axis response and immune activation [123,126]. The HPA axis response to acute stress can be alleviated by dietary probiotic supplementation [127]. It has been shown that the development of the HPA axis depends on postnatal microbial colonization [92]. Disturbances in the functioning of the HPA axis, increased susceptibility to stress and cognitive disorders were shown in germ-free mice [128].
5.3. Immune Routes
Immune mechanisms are relevant to the function of the BGM axis. The intestine and gut-associated lymphoid tissues (GALT) are the largest immune organs in the human body, providing a defensive barrier between external pathogens and the internal environment [129]. Immune cells, such as regulatory T cells (Tregs) and antigen-presenting cells (APC), control the gut and can be transferred from GALT to other peripheral lymphoid sites, including the CNS [130].
The intestinal microbiome modulates immune activity in two different ways [129]. Firstly, short-chain fatty acids and microbiota-derived bacterial fermentation products are produced by intestinal bacteria. These can then be transported to the brain and have a direct effect on neuronal cells and immune cells such as microglia [131]. However, the mechanism by which these molecules cross the blood–brain barrier is not fully understood [129]. It is known that dietary bacterial metabolites such SCFAs may pass from the intestines into the systemic circulation, where they interfere with immune regulation and CNS function [132]. Moreover, it has been reported that butyrate (a short-chain fatty acid) modulates brain function by inhibiting histone deacetylase, and its administration was found to have an antidepressant effect by inducing histone hyperacetylation in mice [133].
Secondly, the gut microbiome influences peripheral immune cells, which then transmit signals to the brain via cytokines [134], which play a crucial role in neurodevelopment and neuroinflammation [135,136,137]. Additionally, several studies confirm that the microbiome modulates the concentration of anti-inflammatory cytokines and lowers the levels of pro-inflammatory cytokines, e.g., interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) [138]. This effect has been demonstrated in rats supplemented with Bifidobacteria, which contributes to reduced levels of IL-6, INF-γ and TNF-α, as compared to the placebo group [12].
Considering the significance of the brain–gut–microbiome axis in the functioning of the organism, there is an increasing number of studies describing the beneficial effects of probiotics and prebiotics on mental health, the majority of which are preclinical studies in animal models of disease. Below we present recent reports on the use of probiotics and prebiotics in studying depression disorders.
6. Depression
According to the WHO, over 300 million people worldwide suffer from depression [139]. The main symptoms of depression include affective disorders such as sadness, anhedonia, the impression of reduced intellectual performance, cognitive disorders and low self-esteem [140]. Depressed patients display apathy and reduced motivation, withdrawing from social activity and limiting their typical behavior. Somatic symptoms are dominated by changes related to disturbance of sleep, appetite, and energy levels [141]. To explain the pathophysiological mechanisms of depression, various hypotheses including monoamine, genetic, environmental, immunologic, endocrine factors and neurogenesis have been proposed, but the full elucidation of the depression pathophysiology remains challenging [141,142]. Taking into account the existence of the BGM axis, there is growing scientific evidence for a strong link between depressive disorders and the gut microbiome [10,143]. Many studies show the positive effect of probiotics and prebiotics as adjuvant treatment of depressive disorders [144,145]. Conversely, several studies have shown reduced microbiota diversity in patients suffering from depression [143]. Most up-to-date findings from the preclinical and clinical trials of the use of probiotics and prebiotics in depressive disorders are described below and listed in Table 2 and Table 3. The herein mentioned animal studies focus mainly on the adolescent age span. Adolescence comprises the most important period of postnatal neurodevelopment [146]. Considering the multitude of ongoing neurodevelopmental processes in the adolescent brain, it should be noted that most adult neuropsychiatric disorders have their roots exactly during this time span [146,147].

Table 2.
Probiotics, prebiotics and postbiotics in animal studies on models of depression disorders.

Table 3.
Probiotic, prebiotics and postbiotics in human studies.
6.1. Probiotic Studies
6.1.1. Animal Studies
Studies show that dietary supplementation with probiotic bacteria increases the level of neurotransmitters in brain tissues and has a potentially beneficial effect on the prevention and treatment of depression [148,150]. The antidepressant effect of Lactobacillus plantarum DP189 (DP189) isolated from Chinese traditional fermented sauerkraut has been demonstrated in Sprague Dawley rats subjected to a corticosterone-induced model of chronic stress [148]. Rats were orally gavaged for 21 days; followed by behavioral, histopathological and biochemical studies to assess changes in comparison with a control group, and with rats treated with fluoxetine (serotonin re-uptake inhibitor) [148]. Behavioral Morris water maze and sucrose preference tests have shown that the DP189 supplementation improves memory and spatial learning and decreases anhedonia [148]. Similarly, Barros-Santos et al. have used other L. plantarum strains isolated from fermented food and observed behavioral changes manifested in antidepressant- and anxiolytic-like effects in healthy male mice [149]. L. plantarum DP189-treated rats show decreased serum IL-1β and TNF-α concentrations and, histopathologically, lower levels of hippocampal apoptosis of neurons than the stress group [148]. Moreover, the stress group exhibited decreased hippocampal levels of serotonin (5-HT), dopamine (DA), and norepinephrine (NE), which were alleviated by the DP189 supplementation [148]. A similar effect of probiotic treatment has been observed in studies on the same model of depression in mice [150]. The researchers have tested the antidepressant-like effect of live and heat-killed (postbiotic) Lactobacillus paracasei PS23 (PS23) [150]. In open-field and sucrose preference tests, they show that both live and heat-killed PS23 are able to reverse chronic corticosterone-induced anxiety- and depressive-like behaviors and reverse corticosterone-reduced BDNF protein levels in the hippocampus [150]. Furthermore, mice treated with live bacteria exhibited elevated serotonin levels in the hippocampus, prefrontal cortex and striatum, compared to the stress group [150]. In turn, the heat-killed bacteria increased dopamine levels in the hippocampus and prefrontal cortex [150]. The authors emphasized that both live and heat-killed PS23 can reverse chronic corticosterone-induced anxiety- and depression-like behaviors, moreover, they note that live probiotics could influence the gastrointestinal microbiota and have immunomodulatory effects, while the components of dead probiotics may exert an anti-inflammatory response [150]. In a study by Janik et al., adult male BALB/c mice were treated with Lactobacillus rhamnosus JB-1-enriched diet (1 × 109 CFU daily for four weeks), and in vivo magnetic resonance spectroscopy (MRS) was used to measure cerebral levels of neurometabolites [168]. Glutamate and glutamine, GABA and N-acetylaspartate (NAA) levels increased after four weeks of bacterial diet compared to baseline concentrations [168]. Changes in glutamate and GABA were confirmed using an enzyme-linked immunosorbent assay (ELISA) [168]. A related study that also involved MRS measurements has shown that dietary supplementation with the same Lactobacillus rhamnosus strain reverses stressed-induced decreases in brain metabolites [37]. In this study, a Wistar rat model of chronic unpredictable mild stress (CUMS) was used. After the stress procedure, the rats were fed a microbiotic diet for four weeks (1.7 × 109 CFU daily by oral gavage) [37]. The elevated plus maze behavioral test showed that treatment with L. rhamnosus JB-1 bacteria resulted in the reduction of stress-induced behavior and restored the levels of GABA, glutamate + glutamine, total N-acetylaspartate and total creatine to concentrations observed in the control group [37]. Another probiotic bacterial strain, Bifidobacterium breve CCFM1025 (CCFM1025), has been investigated in a CUMS model in C57BL/6 mice [151]. After five weeks of oral CCFM1025 supplementation, the mice demonstrated decreased anxiety- and depressive-like behaviors [151]. In addition, the bacterial treatment mitigated hypothalamic–pituitary–adrenal (HPA) axis hyperactivity-induced inflammation: serum corticosterone concentrations were elevated in stressed mice, while all these abnormalities were restored by the treatment with CCFM1025 [151]. Strengthening of the serotonergic system in the gut and brain increased expression of BDNF in the hippocampus—modification of the gut microbial composition and metagenome have also been observed [151]. Elevated brain-derived neurotrophic factor expression in the hippocampus along with reduced expression of TNF-α, interleukin-1b (IL-1b), nuclear factor kappa B (NF-kB) and Iba1 protein, as well as anxiolytic and antidepressant effects in behavioral tests were observed after Bifidobacterium adolescentis treatment in a chronic restraint stress (CRS) model in mice [152].
6.1.2. Human Studies
Wallace and Milev conducted an open-label pilot study on 10 patients with a current episode of major depressive disorder (MDD) who were not taking any antidepressant drugs [158]. Patients received probiotic supplementation with Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 (CEREBIOME®) at a dose of 3 × 109 CFU once a day for 8 weeks [158]. Clinical data were measured at baseline, week 4, and week 8 using a validated series of clinical scales and self-report questionnaires from the Canadian Biomarker Integration Network in Depression (CAN-BIND) [158]. The results showed that daily supplementation with probiotics significantly reduced anxiety and improved overall mood and anhedonia by week 4 and sleep quality by week 8 [158]. In contrast, a double-blind pilot randomized controlled trial [159] including 40 pregnant women with uncomplicated pregnancies and elevated depressive symptoms and/or anxiety, who orally consumed a probiotic multispecies mixture once a day (Ecologic Barrier; 2.5 × 109 CFU/g) from 26 to 30 gestation weeks until delivery showed no differences in mood between probiotic-supplemented and placebo groups, which may have been related to the demanding inclusion and exclusion criteria of the participants [159]. In another double-blind, randomized, placebo-controlled study, Rudzki et al. investigated the properties of Lactobacillus plantarum 299v (LP299v) [160]. In addition to a validated series of clinical scales and self-report questionnaires, biochemical parameters were assessed [160]. Seventy-nine patients with MDD took part in this study, and sixty completed it [160]. The participants received either a selective serotonin reuptake inhibitor (SSRI) with probiotic LP299v (10 × 109 CFU daily) (n = 30) or SSRI with a placebo (n = 30) for 8 weeks [160]. There were no significant changes in the IL-6, IL-1b, TNF-α, and cortisol levels in either the probiotic or placebo groups. However, a significant decrease in the kynurenine concentration and improvement of cognitive functions was shown in the LP299v group as compared to the placebo group with subsequent improvement of cognitive functions [160]. This study indicates the advisability of enriching standard depression therapy with specific strains of probiotic bacteria [160]. The effect of probiotic and prebiotic supplementation on circulating proinflammatory cytokines and urinary cortisol levels has also been studied by Kazemi et al. [164]. They conducted a double-blind placebo-controlled randomized clinical trial in 110 participants diagnosed with MDD who had been taking antidepressant medications for at least 3 months prior to the trial [164]. They were randomly divided into three groups and received ⩾10 × 109 CFU of L. helveticus R0052 and B. longum R0175 or galactooligosaccharide and 0.2% plum flavor as a prebiotic or placebo for 8 weeks (along with antidepressant drug treatment) [164]. The serum inflammatory cytokines levels were not altered in any groups [164]. However, the probiotic supplementation reduced depression symptoms as measured with the Beck Depression Inventory (BDI) score, and decreased the urinary cortisol levels relative to the control group were observed [164]. Another probiotic strain, Bacillus coagulans MTCC 5856, was tested for its efficiency in MDD in irritable bowel syndrome (IBS) patients [161]. Forty patients, randomly divided into two equal groups, received either the probiotic at a daily dose of 2 × 109 CFU or a placebo for 90 days [161]. Effects on the clinical symptoms of MDD measured pre- and post-intervention with the list of clinical scales and self-report questionnaires, and biochemical parameters were also assessed [161]. The study indicates that Bacillus coagulans MTCC 5856 supplementation significantly reduces clinical depression symptoms as measured by scores of primary efficacy tests (e.g., Hamilton depression rating scale, HDRS) [161]. In addition, the probiotic strain demonstrated a beneficial effect on sleeplessness and decreased the level of myeloperoxidases, which are suggested to regulate the production of free radicals leading to cellular oxidative stress and, consequently, linked with depression and some neurodegenerative diseases [161].
6.2. Prebiotic Studies
6.2.1. Animal Studies
The literature showing the effects of prebiotics on depressive disorders is much less abundant than the research on probiotics. Chi et al. [153] investigated the antidepressant efficacy of fructo-oligosaccharides (FOSs) extracted from Morinda officinalis How., a traditional Chinese herb. They used a chronic unpredictable mild stress model of Sprague Dawley rats and then have introduced FOSs via intragastric gavage [153]. The antidepressive properties were assessed through behavioral tests, intestinal morphology and measurements of corticosterone levels [153]. Additionally, the bacterial genomic DNA from feces was extracted and gut microbiota profiling was carried out [153]. In an open-field test and sucrose preference test, it was reported that the FOS treatment alleviated depression-like behaviors [153]. Rats with CUMS showed activation of the HPA axis, as evidenced by elevated levels of corticosterone. The FOS treatment restored plasma and urine levels of corticosterone to levels observed in control rats and may have repaired the damage to the intestinal epithelium [153]. The gut microbiota profile in the CUMS rats indicated the process of dysbiosis. Compared to the control rats, the percentage of bacteria in the gut belonging to Barnesiella, Acinetobacter, Coprococcus, Lactobacillus and Dialister was reduced, while depression-associated bacteria were present (e.g., Anaerostipes, Streptococcus, Proteobacteria) [153]. It was shown that FOS supplementation promoted the presence of beneficial bacterial strains associated with antidepressant properties [153]. In yet another study, a specific mix of non-digestible galactooligosaccharides (BGOS) was investigated [154]. Male CD1 mice were fed BGOS for 3 weeks, and then anxiety was induced through a single injection of lipopolysaccharide (LPS) [154]. Subsequently, behavior, cytokine expression, serotonin and serotonin byproduct, and 5-hydroxyindole acetic acid (5-HIAA) levels were assessed and compared to the control group [154]. In the light–dark box behavioral test, BGOS-fed mice were observed to be less anxious than those from the control group, but the effect on locomotor activity was ambiguous [154]. A similar relationship was observed for serotonin receptors such 5-HT2A, but not for the 5-HT1A serotonin receptor, NMDA receptor subunits, or 5-HIAA [154]. As mentioned above, both prebiotics, i.e., fructo-oligosaccharides and galacto-oligosaccharides, have potentially antidepressant or anxiolytic properties [153,154]. Burokas et al. [155] investigated supplementation with FOS and GOS as well as a combination of FOS+GOS in C57BL/6J male mice. The probiotics were administered to healthy mice for 3 weeks prior to measurements of corticosterone and SCFA levels, assessment of behavior, and determination of gut microbiota composition [155]. Additionally, the effects of the FOS+GOS treatment in a CUMS model were tested [155]. A series of behavioral tests evaluating anxiolytic and antidepressant properties of the treatment were carried out (open-field, elevated plus maze, defensive marble burying, stress-induced hyperthermia, three-chamber test, female urine sniffing test, novel object recognition test, tail suspension test, hot plate, forced swim test) [155]. The study confirmed that these prebiotics significantly altered the behavior and neurochemistry associated with anxiety and depression in mice. Supplementation with FOS+GOS yielded both antidepressant and anxiolytic outcomes [155]. Moreover, it correlated with lower stress-induced plasma corticosterone and L-tryptophan concentrations, which was especially evident in the FOS+GOS combination and with monoamine level alterations [155]. The hippocampal mRNA levels of BDNF and the GABA(B) subunit receptor were also increased in animals administered with the FOS+GOS combination [155]. Changes in gene expression in the hippocampus were also observed by Neufeld et al. after prebiotic supplementation, as compared to control animals [169]. Furthermore, levels of short-chain fatty acids following FOS+GOS supplementation strongly correlated with positive behavioral effects [155]. Finally, microbiota composition exhibited a decreased Actinobacteria/Proteobacteria ratio after stress, which was normalized by prebiotic supplementation [155].
6.2.2. Human Studies
Similar changes in the composition of the gut microbiome has been observed in patients with depression [170]. Several studies also show positive neurobehavioral effects of the combination of probiotics and prebiotics in the treatment of depression disorders [169,171]. In a clinical trial, Ghorbani et al. [163] investigated the effects of specific probiotics and a fructo-oligosaccharide prebiotic (Familact H®) as an adjuvant therapy to fluoxetine. Patients received fluoxetine (20 mg/d) for four weeks before entering the study. In this double-blind, multicenter trial, 40 patients with moderate depression were assessed [163]. The main measure outcome score of the HDRS (Hamilton Depression Rating Scale) was reported [163]. Then patients were randomly divided into a Familact group, which was given two capsules of the symbiotic (plus fluoxetine) for six weeks and a placebo group receiving placebo capsules (plus fluoxetine) for the same time [163]. The study showed that the pro- and prebiotic group had significantly decreased HDRS scores compared to the placebo group, which emphasizes the usefulness of the symbiotic as a complementary therapy [163]. In contrast, another randomized clinical trial did not reveal any significant effects of prebiotic supplementation in patients with MDD who took antidepressant drugs (sertraline, fluoxetine, citalopram, or amitriptyline) for at least three months before the trial [165]. In this study, 110 patients were divided into three groups-receiving a probiotic (Lactobacillus helveticus and Bifidobacterium longum), a prebiotic (galactooligosaccharides) or a placebo for eight weeks (all patients received antidepressant drugs simultaneously) [165]. While the supplementation of patients with MDD with the probiotic improved the Beck Depression Inventory (BDI) score in comparison with the group receiving the placebo, the prebiotic supplementation did not influence the BDI scale results in severe cases. Results may have been affected by the fact that the antidepressant drugs the participants had taken were not identical. [165]. Vaghef-Mehrabany et al. [162] have conducted a study of prebiotics combined with calorie restriction on metabolic and clinical response in 62 obese women with major depressive disorder. Half of the patients received an inulin prebiotic (10 g/day) and the other half were treated with placebo (maltodextrin, 10 g/day) for eight weeks [162]. Additionally, all the participants were on a 25% calorie-restricted diet [162]. Depression symptoms were evaluated by HDRS and Beck Depression Inventory (BDI-II) scales before and after the intervention, and anthropometric and biochemical parameters were also evaluated [162]. There were no statistically significant differences in depression symptoms between the prebiotic and placebo groups after the supplementation [162]. However, the study shows that while the administration of the prebiotic had little effect on metabolic changes, the reduced calorie content and subsequent weight loss had a more significant effect on the improvement of the well-being of obese patients with MDD [162].
6.3. Postbiotic Studies
6.3.1. Animal Studies
Studies showing the beneficial effects of postbiotics on mental health are limited as little attention has been paid to the potential influence of non-viable bacterial cells and their components on the gut–brain interaction. However, some recent studies demonstrate the beneficial effects of postbiotics on depression. The antidepressant effects of heat-killed Lactobacillus helveticus strain MCC1848 in a mouse model of subchronic and mild social defeat stress (sCSDS) were investigated [156]. Heat-killed cells were prepared by treatment at 90 °C for 15 min. The dose of bacteria was 1.0 × 109 organisms/day for 24 days [156]. Series of behavioral tests, fecal microbiota analysis and gene expression profiles in the nucleus accumbens were performed [156]. Significantly increased interaction time in the social interaction test and sucrose preference ratio in the sucrose preference test were observed, indicating anxiolytic- or antidepressant-like effects in the sCSDS mouse model. Additionally, gene expression in the nucleus accumbens, which is a stress-relevant brain region, was modulated by this postbiotic [156]. Anti-anxiety effects of heat-killed bacteria were also observed in healthy rodents [85,157]. Male C57BL/6J mice were fed with heat-killed Enterococcus faecalis strain EC-12 (EC-12) (diet enriched with 0.125% concentration of heat-killed EC-12 for 4 weeks) [85]. Behavioral tests (open-field, elevated plus-maze and forced swim tests) showed that mice supplemented with EC-12 had decreased anxiety-like and depression-like behavior [85]. Moreover, EC-12 supplementation modified the gene expression profile in the prefrontal cortex and significantly increased Butyricicoccus and Enterococcus composition in the gut compared to the control group [85]. Warda et al. investigated the postbiotic ADR-159, containing a co-fermentate of heat-killed Lactobacillus fermentum and Lactobacillus delbrueckii on male C57BL/6 mice [157]. ADR-159 was incorporated into standard mice chow to a concentration of 5% (3 × 109 cell bodies/gram of chow) for three weeks [157] followed by a battery of behavior tests, and measurement of microbiota concentrations and corticosterone levels. They show that postbiotic ADR-159 diet supplementation subtly but distinctly changed the composition of the microbiota, decreasing locomotor activity in the open-field test and reducing the baseline corticosterone levels, which may indicate action on the HPA axis [157]. Although both studies are promising, further studies in depression models are required to elucidate the exact mechanisms of the effect of the heat-killed bacteria on the brain.
6.3.2. Human Studies
In humans, the efficacy and health benefits of the long-term supplementation with heat-inactivated, washed Lactobacillus gasseri CP2305 (CP2305) was investigated in a group of 60 young adult students preparing for the national examination for medical practitioners (this model has been used in multiple studies of chronic psychological stress and is considered appropriate) [166]. In a double-blind, placebo-controlled, parallel-group clinical trial, 41 men and 19 women ingested CP2305-containing (1 × 1010 bacterial cells pre 2 tablets) or placebo tablets once daily for 24 weeks [166]. With the use of questionnaires assessing mental and physical states, the trial demonstrated that CP2305 significantly reduced anxiety and sleep disturbance compared to placebo. Moreover, fecal microbiota analyses show that diet supplementation with CP2305 attenuated the stress-induced decline of Bifidobacterium spp. and the stress-induced elevation of Streptococcus spp. [166]. While researchers noted the beneficial impact of Lactobacillus gasseri CP2305 for young adults experiencing stressful conditions in this study, the mechanism underlying the stress-relieving effects remains unclear and future studies are needed [166]. In another study, the effects of the postbiotic Lactobacillus paracasei MCC1849 (LAC-Shield™) supplementation on mood and symptoms of the common cold in healthy adults was shown [167]. Two hundred forty-one participants were randomized to receive 1 × 1010 heat-killed L. paracasei MCC1849 cell powder (10LP), 3 × 1010 heat-killed L. paracasei MCC1849 cell powder (30LP), or a placebo once a day for 12 weeks [167]. They demonstrated in the profile of mood states 2 (POMS 2) questionnaire (standard validated psychological test used to assess transient mood states) that the level of deterioration in the positive mood state caused by stress was decreased in the MCC1849-intake group relative to the placebo group. Moreover, no adverse effects associated with the postbiotic supplementation were observed during the study [167].
7. Conclusions and Future Directions
The data presented provide ample evidence for the beneficial role of probiotics, prebiotics and postbiotics on the brain-gut-microbiota axis in animal models of depression. In clinical studies, probiotics demonstrated a greater potential than prebiotics or postbiotics in reducing symptoms of depression in patients. More studies are needed to fully evaluate the therapeutic potential of pre- and postbiotics. Though most of the studies mentioned focused mainly on live probiotics, the use of inanimate microorganisms may have some attractive advantages, including a long shelf-life for commercial products and relatively easy standardization. Nevertheless, because of the novelty of postbiotic interventions, their safety and potential dangers remain poorly understood, hence, further research is necessary.
Although animal studies in the field of pro-, pre- and postbiotics are promising, clinical trial results are slightly disappointing. For this reason, certain considerations should be taken. Initially, the small sample size of some clinical studies undermines the statistical interpretation and generalizability [158,159,161,163]. Demanding inclusion and exclusion criteria [159,161] or various degrees of depression, and the type of basic treatment [164,165] may also be responsible. It is worth noting that changes are compared in groups and not in individual study participants, which may also affect the results. Additionally, while each study includes a validated series of clinical scales and self-report questionnaires, not all of these clinical trials included biochemical measurements, which could improve interpretation of the results [158,159,163]. Another issue is the use of a single bacterial strain [160,161]. Due to the specific mechanisms of bacterial activity, it could be beneficial to test a multi-species probiotic or a synbiotic, which could result in a better therapeutic effect. Furthermore, the interactions of the administered bacterial strains and prebiotics with the native microbiota and its variability may also affect the reactions of patients to the treatment. In the future, it would be reasonable to consider fecal microbiome analysis, which has not been included by any of the studies discussed here. Also missing is the duration of the clinical effects and whether the administration of the treatment could be extended. Therefore, clinical trials with extended follow-up durations are needed. Finally, this research supports the importance of possible new therapeutic targets in the field of nutritional neuropsychopharmacology, however, more clinical trials are required.
Author Contributions
Conceptualization, A.C.; writing—original draft preparation, A.C.; writing—review and editing, A.O., R.R. and G.J.S.; supervision, R.R. and G.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Science Center, Poland, grant no. 2015/17/B/NZ4/02986.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 2018, 200, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Forsythe, P.; Sudo, N.; Dinan, T.; Taylor, V.H.; Bienenstock, J. Mood and gut feelings. Brain Behav. Immun. 2010, 24, 9–16. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772. [Google Scholar] [CrossRef]
- Bravo, J.A.; Julio-Pieper, M.; Forsythe, P.; Kunze, W.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Communication between Gastrointestinal Bacteria and the Nervous System. Curr. Opin. Pharmacol. 2012, 12, 667–672. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [Green Version]
- Mörkl, S.; Butler, M.I.; Cichini, F.; Cryan, J.F.; Dinan, T.G. Psychobiotics. Available online: https://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780190931544.001.0001/oxfordhb-9780190931544-e-7 (accessed on 13 June 2021).
- Gismondo, M.R.; Drago, L.; Lombardi, A. Review of probiotics available to modify gastrointestinal flora. Int. J. Antimicrob. Agents 1999, 12, 287–292. [Google Scholar] [CrossRef]
- Podolsky, S.H. Metchnikoff and the microbiome. Lancet 2012, 380, 1810–1811. [Google Scholar] [CrossRef]
- Mackowiak, P.A. Recycling metchnikoff: Probiotics, the intestinal microbiome and the quest for long life. Front. Public Health 2013, 1. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Ouwehand, A.C. Antiallergic effects of probiotics. J. Nutr. 2007, 137, 794S–797S. [Google Scholar] [CrossRef] [Green Version]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sheng, J.; Wang, M.; Luo, H.; Zhu, J.; Zhang, B.; Liu, Z.; Yang, X. Combination therapy of TGF-β blockade and commensal-derived probiotics provides enhanced antitumor immune response and tumor suppression. Theranostics 2019, 9, 4115–4129. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K. Probiotics and the immune response. J. Clin. Gastroenterol. 2006, 40, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef]
- Sharma, A. Importance of probiotics in cancer prevention and treatment. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 4; pp. 33–45. ISBN 978-0-12-816328-3. [Google Scholar]
- Panebianco, C.; Latiano, T.; Pazienza, V. Microbiota manipulation by probiotics administration as emerging tool in cancer prevention and therapy. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Hemanth Kumar, B.S.; Mishra, S.K.; Rana, P.; Singh, S.; Khushu, S. Neurodegenerative evidences during early onset of depression in CMS rats as detected by proton magnetic resonance spectroscopy at 7T. Behav. Brain Res. 2012, 232, 53–59. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.V.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.W.; Clemente, J.C.; Peter, I.; Loos, R.J.F. The prenatal gut microbiome: Are we colonized with bacteria in utero? Pediatric Obesity 2017, 12, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H.-Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Brambilla, P.; Perez, J.; Barale, F.; Schettini, G.; Soares, J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatry 2003, 8, 721–737. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Kobayashi, K. Role of catecholamine signaling in brain and nervous system functions: New insights from mouse molecular genetic study. J. Investig. Dermatol. Symp. Proc. 2001, 6, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Sanacora, G.; Mason, G.F.; Rothman, D.L.; Behar, K.L.; Hyder, F.; Petroff, O.A.C.; Berman, R.M.; Charney, D.S.; Krystal, J.H. Reduced Cortical γ-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 1999, 56, 1043–1047. [Google Scholar] [CrossRef] [Green Version]
- Kochalska, K.; Oakden, W.; Słowik, T.; Chudzik, A.; Pankowska, A.; Łazorczyk, A.; Kozioł, P.; Andres-Mach, M.; Pietura, R.; Rola, R.; et al. Dietary supplementation with lactobacillus rhamnosus JB-1 restores brain neurochemical balance and mitigates the progression of mood disorder in a rat model of chronic unpredictable mild stress. Nutr. Res. 2020, 82, 44–57. [Google Scholar] [CrossRef]
- Franciosi, E.; Carafa, I.; Nardin, T.; Schiavon, S.; Poznanski, E.; Cavazza, A.; Larcher, R.; Tuohy, K.M. Biodiversity and γ-aminobutyric acid production by lactic acid bacteria isolated from traditional alpine raw cow’s milk cheeses. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Siragusa, S.; Angelis, M.D.; Cagno, R.D.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of italian cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Kim, D.H.; Kang, H.J.; Shin, M.; Yang, S.-Y.; Yang, J.; Jung, Y.H. Enhanced production of γ-aminobutyric acid (GABA) using lactobacillus plantarum EJ2014 with simple medium composition. LWT 2021, 137, 110443. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lee, J.-W.; Lim, S.-D. The probiotic characteristics and GABA production of Lactobacillus Plantarum K154 isolated from kimchi. Food Sci. Biotechnol. 2014, 23, 1951–1957. [Google Scholar] [CrossRef]
- Lin, Q. Submerged fermentation of Lactobacillus rhamnosus YS9 for γ-aminobutyric acid (GABA) production. Braz.J. Microbiol. 2013, 44, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Villegas, J.M.; Brown, L.; Savoy de Giori, G.; Hebert, E.M. Optimization of batch culture conditions for GABA production by lactobacillus brevis CRL 1942, isolated from quinoa sourdough. LWT Food Sci. Technol. 2016, 67, 22–26. [Google Scholar] [CrossRef]
- Binh, T.T.T.; Ju, W.-T.; Jung, W.-J.; Park, R.-D. Optimization of γ-amino butyric acid production in a newly isolated lactobacillus brevis. Biotechnol. Lett. 2014, 36, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Hsueh, Y.-H.; Kuo, J.-M.; Liu, S.-J. Characterization of a potential probiotic lactobacillus brevis RK03 and efficient production of γ-aminobutyric acid in batch fermentation. Int. J. Mol. Sci. 2018, 19, 143. [Google Scholar] [CrossRef] [Green Version]
- Thangrongthong, S.; Puttarat, N.; Ladda, B.; Itthisoponkul, T.; Pinket, W.; Kasemwong, K.; Taweechotipatr, M. Microencapsulation of probiotic lactobacillus brevis ST-69 producing GABA using alginate supplemented with nanocrystalline starch. Food Sci. Biotechnol. 2020, 29, 1475–1482. [Google Scholar] [CrossRef]
- Lim, H.S.; Cha, I.-T.; Roh, S.W.; Shin, H.-H.; Seo, M.-J. Enhanced production of gamma-aminobutyric acid by optimizing culture conditions of lactobacillus brevis HYE1 isolated from kimchi, a Korean fermented food. J. Microbiol. Biotechnol. 2017, 27, 450–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanklai, J.; Somwong, T.C.; Rungsirivanich, P.; Thongwai, N. Screening of GABA-producing lactic acid bacteria from thai fermented foods and probiotic potential of levilactobacillus brevis F064A for GABA-fermented mulberry juice production. Microorganisms 2021, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.A.; Poluektova, E.U.; Dyachkova, M.S.; Klimina, K.M.; Kovtun, A.S.; Averina, O.V.; Orlova, V.S.; Danilenko, V.N. GABA production and structure of GadB / GadC Genes in lactobacillus and bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Shishov, V.A.; Kirovskaya, T.A.; Kudrin, V.S.; Oleskin, A.V. Amine neuromediators, their precursors, and oxidation products in the culture of escherichia coli K-12. Appl. Biochem. Microbiol. 2009, 45, 494–497. [Google Scholar] [CrossRef]
- Özoğul, F. Production of biogenic amines by morganella morganii, klebsiella pneumoniae and hafnia alvei using a rapid HPLC method. Eur. Food Res. Technol. 2004, 219, 465–469. [Google Scholar] [CrossRef]
- Özoğul, F.; Kuley, E.; Özoğul, Y.; Özoğul, İ. The function of lactic acid bacteria on biogenic amines production by food-borne pathogens in arginine decarboxylase broth. FSTR 2012, 18, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Yunes, R.A.; Poluektova, E.U.; Vasileva, E.V.; Odorskaya, M.V.; Marsova, M.V.; Kovalev, G.I.; Danilenko, V.N. A multi-strain potential probiotic formulation of GABA-Producing lactobacillus plantarum 90sk and bifidobacterium adolescentis 150 with antidepressant effects. Probiotics Antimicro. Prot. 2020, 12, 973–979. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Pineiro, M.; Asp, N.-G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) Consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health Promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef]
- Ahmad, A.; Khalid, S. Therapeutic aspects of probiotics and prebiotics. In Diet, Microbiome and Health; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 3; pp. 53–91. ISBN 978-0-12-811440-7. [Google Scholar]
- Fernández, J.; Moreno, F.J.; Olano, A.; Clemente, A.; Villar, C.J.; Lombó, F. A galacto-oligosaccharides preparation derived from lactulose protects against colorectal cancer development in an animal model. Front. Microbiol. 2018, 9, 2004. [Google Scholar] [CrossRef] [Green Version]
- Bouchaud, G.; Castan, L.; Chesné, J.; Braza, F.; Aubert, P.; Neunlist, M.; Magnan, A.; Bodinier, M. Maternal exposure to GOS/Inulin mixture prevents food allergies and promotes tolerance in offspring in mice. Allergy 2016, 71, 68–76. [Google Scholar] [CrossRef]
- Polakowski, C.B.; Kato, M.; Preti, V.B.; Schieferdecker, M.E.M.; Ligocki Campos, A.C. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition 2019, 58, 40–46. [Google Scholar] [CrossRef]
- Maguire, M.; Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci. 2019, 30, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Zagaja, M.; Bryda, J.; Kosikowska, U.; Stępień-Pyśniak, D.; Winiarczyk, S.; Andres-Mach, M. Topinambur–New possibilities for use in a supplementation diet. Ann. Agric. Environ. Med. 2019, 26, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Bornet, F.R.J.; Brouns, F.; Tashiro, Y.; Duvillier, V. Nutritional aspects of short-chain fructooligosaccharides: Natural occurrence, chemistry, physiology and health implications. Dig. Liver Dis. 2002, 34, S111–S120. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, L.; Li, Y.; Cui, Y.; Jiang, S.; Tao, N.; Chen, H.; Zhao, Z.; Xu, J.; Dong, C. A novel inulin-type fructan from asparagus cochinchinensis and its beneficial impact on human intestinal microbiota. Carbohydr. Polym. 2020, 247, 116761. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; He, N.; Croft, K.D. Isolation, characterization, and immunological effects of α-galacto-oligosaccharides from a new source, the herb lycopus lucidus turcz. J. Agric. Food Chem. 2010, 58, 8253–8258. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.D.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [Green Version]
- Skejovic Joehnke, M.; Jeske, S.; Ispiryan, L.; Zannini, E.; Arendt, E.K.; Bez, J.; Christian Sørensen, J.; Lykke Petersen, I. Nutritional and anti-nutritional properties of lentil (Lens Culinaris) protein isolates prepared by pilot-scale processing. Food Chem. X 2021, 100112. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Hess, J.M.; Gould, T.J.; Slavin, J.L. Prebiotic dietary fiber and gut health: Comparing the in vitro fermentations of beta-glucan, inulin and xylooligosaccharide. Nutrients 2017, 9, 1361. [Google Scholar] [CrossRef] [Green Version]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Kalidas, N.R.; Saminathan, M.; Ismail, I.S.; Abas, F.; Maity, P.; Islam, S.S.; Manshoor, N.; Shaari, K. Structural characterization and evaluation of prebiotic activity of oil palm kernel cake mannanoligosaccharides. Food Chem. 2017, 234, 348–355. [Google Scholar] [CrossRef]
- Fernandez, F.; Hinton, M.; Gils, B.V. Dietary mannan-oligosaccharides and their effect on chicken caecal microflora in relation to salmonella enteritidis colonization. Avian Pathol. 2002, 31, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, P.; Chen, C.; Huang, C.; Lin, S.; Zheng, B.; Zhang, Y. Structural properties and prebiotic activities of fractionated lotus seed resistant starches. Food Chem. 2018, 251, 33–40. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Beale, D.J.; Karpe, A.V.; Shastri, S.; Basheer, W.; Southam, B.; Eri, R.; et al. Synbiotic supplementation with prebiotic green banana resistant starch and probiotic bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases. Eur. J. Nutr. 2020, 59, 3669–3689. [Google Scholar] [CrossRef] [Green Version]
- Joshi, D.; Roy, S.; Banerjee, S. Prebiotics: A functional food in health and disease. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 19; pp. 507–523. ISBN 978-0-08-102081-4. [Google Scholar]
- Nooshkam, M.; Babazadeh, A.; Jooyandeh, H. Lactulose: Properties, techno-functional food applications, and food grade delivery system. Trends Food Sci. Technol. 2018, 80, 23–34. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- De Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and paraprobiotics in viral infection: Clinical application and effects on the innate and acquired immune systems. Curr. Pharm. Des. 2018, 24, 710–717. [Google Scholar] [CrossRef]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018, 15, 39. [Google Scholar] [CrossRef] [Green Version]
- Kambe, J.; Watcharin, S.; Makioka-Itaya, Y.; Inoue, R.; Watanabe, G.; Yamaguchi, H.; Nagaoka, K. Heat-killed enterococcus fecalis (EC-12) supplement alters the expression of neurotransmitter receptor genes in the prefrontal cortex and alleviates anxiety-like behavior in mice. Neurosci. Lett. 2020, 720, 134753. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Shukla, G. Administration of metabiotics extracted from probiotic lactobacillus rhamnosus MD 14 inhibit experimental colorectal carcinogenesis by targeting Wnt/β-catenin pathway. Front. Oncol. 2020, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Suárez, N.; Ferrara, F.; Rial, A.; Dee, V.; Chabalgoity, J.A. Bacterial lysates as immunotherapies for respiratory infections: Methods of preparation. Front. Bioeng. Biotechnol. 2020, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. The Immunomodulatory Properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of Microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef] [Green Version]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The Anxiolytic effect of bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Nat. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Yang, X.; Yang, J.; Lai, G.; Yong, T.; Tang, X.; Shuai, O.; Zhou, G.; Xie, Y.; Wu, Q. Prebiotic effect of fructooligosaccharides from morinda officinalis on Alzheimer’s disease in rodent models by targeting the microbiota-gut-brain axis. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Bercik, P.; Collins, S.M. The effects of inflammation, infection and antibiotics on the microbiota-gut-brain axis. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; pp. 279–289. ISBN 978-1-4939-0897-4. [Google Scholar]
- Dinan, T.G.; Cryan, J.F. Brain–Gut–microbiota axis—Mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 69–70. [Google Scholar] [CrossRef]
- Yissachar, N.; Zhou, Y.; Ung, L.; Lai, N.Y.; Mohan, J.F.; Ehrlicher, A.; Weitz, D.A.; Kasper, D.L.; Chiu, I.M.; Mathis, D.; et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 2017, 168, 1135–1148.e12. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Collins, J.; Borojevic, R.; Verdu, E.F.; Huizinga, J.D.; Ratcliffe, E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol. Motil. 2014, 26, 98–107. [Google Scholar] [CrossRef]
- Vadder, F.D.; Grasset, E.; Holm, L.M.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef] [Green Version]
- McVey Neufeld, K.A.; Mao, Y.K.; Bienenstock, J.; Foster, J.A.; Kunze, W.A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013, 25, 183–e88. [Google Scholar] [CrossRef]
- Wu, R.Y.; Pasyk, M.; Wang, B.; Forsythe, P.; Bienenstock, J.; Mao, Y.-K.; Sharma, P.; Stanisz, A.M.; Kunze, W.A. Spatiotemporal maps reveal regional differences in the effects on gut motility for lactobacillus reuteri and rhamnosus strains. Neurogastroenterol. Motil. 2013, 25, e205–e214. [Google Scholar] [CrossRef]
- Husebye, E.; Hellström, P.M.; Midtvedt, T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig. Dis. Sci. 1994, 39, 946–956. [Google Scholar] [CrossRef]
- West, C.L.; Stanisz, A.M.; Mao, Y.-K.; Champagne-Jorgensen, K.; Bienenstock, J.; Kunze, W.A. Microvesicles from lactobacillus reuteri (DSM-17938) completely reproduce modulation of gut motility by bacteria in mice. PLoS ONE 2020, 15, e0225481. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014, 817, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine derived from probiotic lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McVey Neufeld, K.-A.; Bienenstock, J.; Bharwani, A.; Champagne-Jorgensen, K.; Mao, Y.; West, C.; Liu, Y.; Surette, M.G.; Kunze, W.; Forsythe, P. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci. Rep. 2019, 9, 14290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [Green Version]
- Pahuja, R.; Seth, K.; Shukla, A.; Shukla, R.K.; Bhatnagar, P.; Chauhan, L.K.S.; Saxena, P.N.; Arun, J.; Chaudhari, B.P.; Patel, D.K.; et al. Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano 2015, 9, 4850–4871. [Google Scholar] [CrossRef]
- Kuriyama, K.; Sze, P.Y. Blood-brain barrier to h3-γ-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology 1971, 10, 103–108. [Google Scholar] [CrossRef]
- Lee, W.-J.; Hawkins, R.A.; Viña, J.R.; Peterson, D.R. Glutamine Transport by the blood-brain barrier: A possible mechanism for nitrogen removal. Am. J. Physiol. Cell Physiol. 1998, 274, C1101–C1107. [Google Scholar] [CrossRef]
- Pardridge, W.M. Kinetics of competitive inhibition of neutral amino acid transport across the blood-brain barrier. J. Neurochem. 1977, 28, 103–108. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Evrensel, A.; Ünsalver, B.Ö.; Ceylan, M.E. Immune-kynurenine pathways and the gut microbiota-brain axis in anxiety disorders. In Anxiety Disorders: Rethinking and Understanding Recent Discoveries; Kim, Y.-K., Ed.; Springer: Singapore, 2020; pp. 155–167. ISBN 978-981-329-705-0. [Google Scholar]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef] [Green Version]
- Björkholm, C.; Monteggia, L.M. BDNF–A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, O.F.; Wu, X.; Castren, E. Chronic fluoxetine treatment increases expression of synaptic proteins in the hippocampus of the ovariectomized rat: Role of BDNF signalling. Psychoneuroendocrinology 2009, 34, 367–381. [Google Scholar] [CrossRef]
- Cerdó, T.; Diéguez, E.; Campoy, C. Impact of gut microbiota on neurogenesis and neurological diseases during infancy. Curr. Opin. Pharmacol. 2020, 50, 33–37. [Google Scholar] [CrossRef]
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’Leary, O.F. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry 2015, 78, e7–e9. [Google Scholar] [CrossRef]
- Scott, G.A.; Terstege, D.J.; Vu, A.P.; Law, S.; Evans, A.; Epp, J.R. Disrupted neurogenesis in germ-free mice: Effects of Age and sex. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Weinstock, M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008, 32, 1073–1086. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; Kubera, M.; Leunis, J.-C.; Berk, M.; Geffard, M.; Bosmans, E. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatrica Scand. 2013, 127, 344–354. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; MacQueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.R.; Veiga-Fernandes, H. Neuroimmune circuits in inter-organ communication. Nat. Rev. Immunol. 2020, 20, 217–228. [Google Scholar] [CrossRef]
- Borre, Y.E.; Moloney, R.D.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; pp. 373–403. ISBN 978-1-4939-0897-4. [Google Scholar]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.-H.; May, C.; Wilck, N.; et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef]
- Rojas, O.L.; Pröbstel, A.-K.; Porfilio, E.A.; Wang, A.A.; Charabati, M.; Sun, T.; Lee, D.S.W.; Galicia, G.; Ramaglia, V.; Ward, L.A.; et al. Recirculating intestinal iga-producing cells regulate neuroinflammation via IL-10. Cell 2019, 176, 610–624.e18. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiano, A.J.; Gadani, S.P.; Kipnis, J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat. Rev. Neurosci. 2017, 18, 375–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, S.; Haghikia, A.; Wilck, N.; Müller, D.N.; Linker, R.A. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 2018, 154, 230–238. [Google Scholar] [CrossRef] [Green Version]
- WHO. Depression and Other Common Mental Disorders. Available online: http://www.who.int/mental_health/management/depression/prevalence_global_health_estimates/en/ (accessed on 28 December 2020).
- Belmaker, R.H.; Agam, G. Major depressive disorder. N. Engl. J. Med. 2008, 358, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model-are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Paiva, I.H.R.; Duarte-Silva, E.; Peixoto, C.A. The role of prebiotics in cognition, anxiety, and depression. Eur. Neuropsychopharmacol. 2020, 34, 1–18. [Google Scholar] [CrossRef]
- Vlainić, J.; Šuran, J.; Vlainić, T.; Vukorep, A.L. Probiotics as an adjuvant therapy in major depressive disorder. Curr. Neuropharmacol. 2016, 14, 952–958. [Google Scholar] [CrossRef]
- Casey, B.J.; Jones, R.M.; Hare, T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008, 1124, 111–126. [Google Scholar] [CrossRef]
- Schneider, M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013, 354, 99–106. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, G.; Zhao, Z.; Wang, C.; Duan, C.; Gao, L.; Li, S. Antidepressant-like effects of lactobacillus plantarum DP189 in a corticosterone-induced rat model of chronic stress. Behav. Brain Res. 2020, 395, 112853. [Google Scholar] [CrossRef]
- Barros-Santos, T.; Silva, K.S.O.; Libarino-Santos, M.; Cata-Preta, E.G.; Reis, H.S.; Tamura, E.K.; de Oliveira-Lima, A.J.; Berro, L.F.; Uetanabaro, A.P.T.; Marinho, E.A.V. Effects of chronic treatment with new strains of lactobacillus plantarum on cognitive, anxiety- and depressive-like behaviors in male mice. PLoS ONE 2020, 15, e0234037. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-L.; Wang, S.; Yen, J.-T.; Cheng, Y.-F.; Liao, C.-L.; Hsu, C.-C.; Wu, C.-C.; Tsai, Y.-C. Antidepressant-like activities of live and heat-killed lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019, 1711, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; O’Riordan, K.J.; Lee, Y.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, J.-P.; Deng, K.; Li, X.; Yuan, Y.; Xuan, Q.; Xie, J.; He, X.-M.; Wang, Q.; Li, J.-J.; et al. Prophylactic effects of bifidobacterium adolescentis on anxiety and depression-like phenotypes after chronic stress: A role of the gut microbiota-inflammation axis. Front. Behav. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Chi, L.; Khan, I.; Lin, Z.; Zhang, J.; Lee, M.Y.S.; Leong, W.; Hsiao, W.L.W.; Zheng, Y. Fructo-oligosaccharides from morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine 2020, 67, 153157. [Google Scholar] [CrossRef]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Maehata, H.; Kobayashi, Y.; Mitsuyama, E.; Kawase, T.; Kuhara, T.; Xiao, J.-Z.; Tsukahara, T.; Toyoda, A. Heat-killed lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci. Biotechnol. Biochem. 2019, 83, 1239–1247. [Google Scholar] [CrossRef]
- Warda, A.K.; Rea, K.; Fitzgerald, P.; Hueston, C.; Gonzalez-Tortuero, E.; Dinan, T.G.; Hill, C. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behav. Brain Res. 2019, 362, 213–223. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and tolerability of probiotics on depression: Clinical results from an open-label pilot study. Front. Psychiatry 2021, 12. [Google Scholar] [CrossRef]
- Browne, P.D.; Bolte, A.C.; Besseling-van der Vaart, I.; Claassen, E.; de Weerth, C. Probiotics as a treatment for prenatal maternal anxiety and depression: A double-blind randomized pilot trial. Sci. Rep. 2021, 11, 3051. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Vaghef-Mehrabany, E.; Ranjbar, F.; Asghari-Jafarabadi, M.; Hosseinpour-Arjmand, S.; Ebrahimi-Mameghani, M. Calorie restriction in combination with prebiotic supplementation in obese women with depression: Effects on metabolic and clinical response. Nutr. Neurosci. 2019, 0, 1–15. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Nazari, S.; Etesam, F.; Nourimajd, S.; Ahmadpanah, M.; Razeghi Jahromi, S. The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: A randomized multicenter trial. Arch. Neurosci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: A double-blind, placebo-controlled randomized clinical trial. J. Funct. Foods 2019, 52, 596–602. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health benefits of lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Kondo, J.; Iwabuchi, N.; Takahashi, S.; Yamauchi, K.; Abe, F.; Miura, K. Effects of paraprobiotic lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef. Microbes 2018, 9, 855–864. [Google Scholar] [CrossRef]
- Janik, R.; Thomason, L.A.M.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. NeuroImage 2016, 125, 988–995. [Google Scholar] [CrossRef]
- McVey Neufeld, K.-A.; O’Mahony, S.M.; Hoban, A.E.; Waworuntu, R.V.; Berg, B.M.; Dinan, T.G.; Cryan, J.F. Neurobehavioural Effects of Lactobacillus Rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr. Neurosci. 2019, 22, 425–434. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Mika, A.; Day, H.E.W.; Martinez, A.; Rumian, N.L.; Greenwood, B.N.; Chichlowski, M.; Berg, B.M.; Fleshner, M. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur. J. Neurosci. 2017, 45, 342–357. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).