Atelocollagen-Embedded Chondrocyte Precursors as a Treatment for Grade-4 Cartilage Defects of the Femoral Condyle: A Case Series with up to 9-Year Follow-Up
Abstract
:1. Introduction
2. Results
2.1. Demography
2.2. CP Derivation
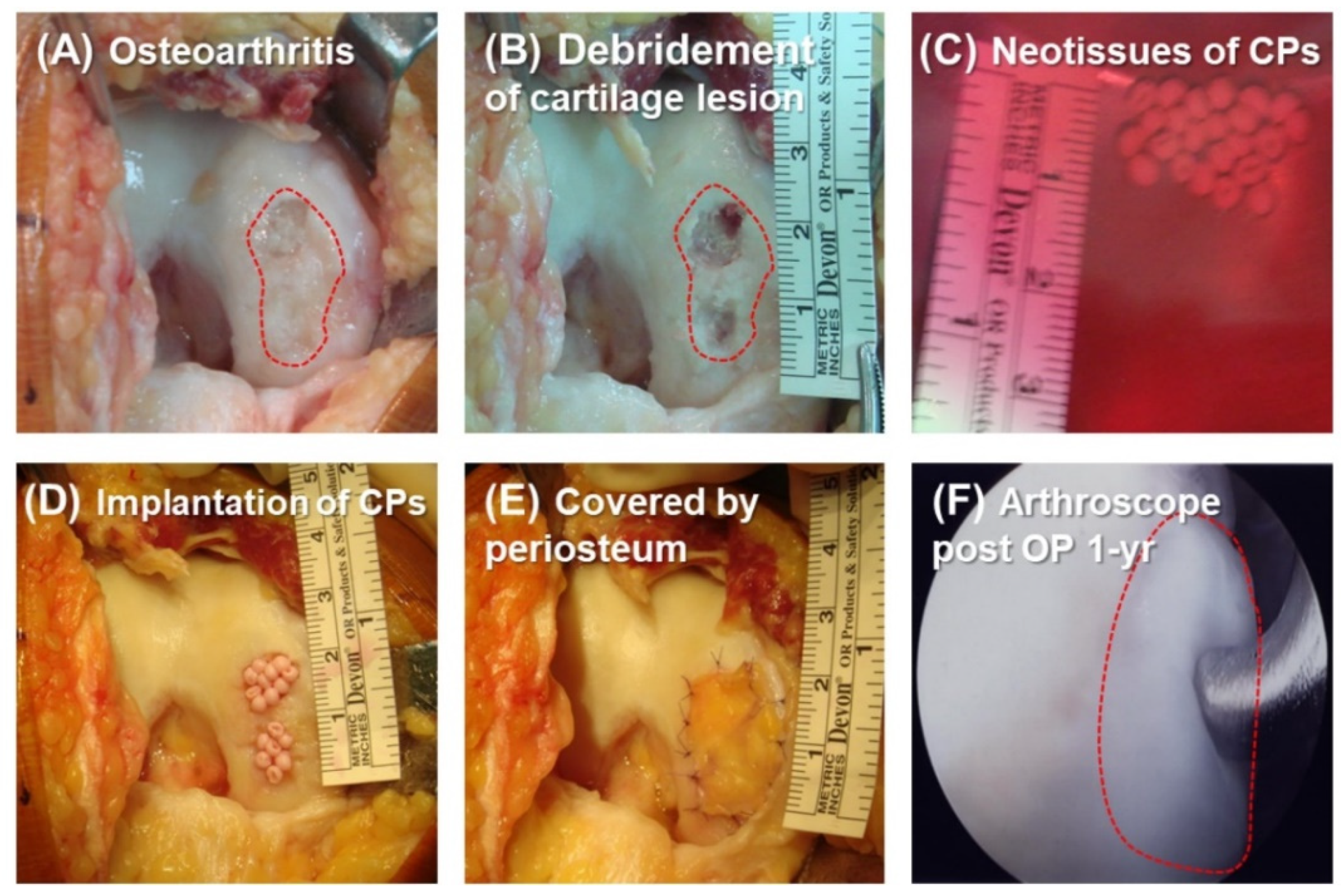
2.3. Patient Characteristics before and after CP Therapy
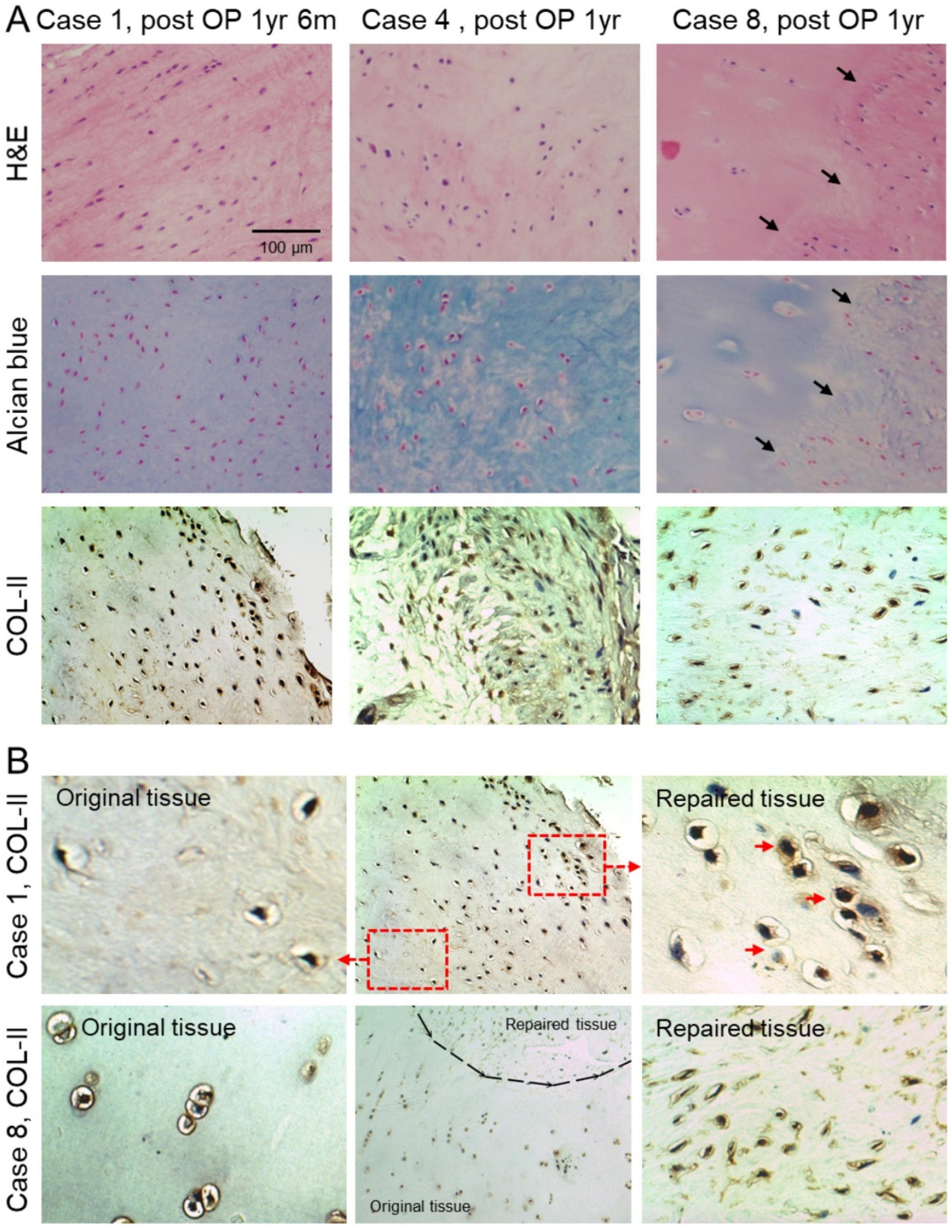
2.4. Histologic Analysis of Repaired Tissue
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W. Of the structure and disease of articulating cartilages. Clin. Orthop. Relat. Res. 1995, 317, 3–6. [Google Scholar]
- Hunter, W. VI. Of the structure and diseases of articulating cartilages. Philos. Trans. 1743, 42, 514–521. [Google Scholar]
- Johnson, L.L. Arthroscopic abrasion arthroplasty: A review. Clin. Orthop. Relat. Res. 2001, 391, S306–S317. [Google Scholar] [CrossRef]
- Mithoefer, K.; Williams, R.J., III; Warren, R.F.; Potter, H.G.; Spock, C.R.; Jones, E.C.; Wickiewicz, T.L.; Marx, R.G. The microfracture technique for the treatment of articular cartilage lesions in the knee: A prospective cohort study. JBJS 2005, 87, 1911–1920. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Singleton, S.B.; Briggs, K.K. Microfracture technique forfull-thickness chondral defects: Technique and clinical results. Oper. Tech. Orthop. 1997, 7, 300–304. [Google Scholar] [CrossRef]
- Hangody, L.; Kish, G.; Kárpáti, Z.; Udvarhelyi, I.; Szigeti, I.; Bély, M. Mosaicplasty for the treatment of articular cartilage defects: Application in clinical practice. Orthopedics 1998, 21, 751–756. [Google Scholar] [CrossRef]
- Bugbee, W.D.; Convery, F.R. Osteochondral allograft transplantation. Clin. Sports Med. 1999, 18, 67–75. [Google Scholar] [CrossRef]
- Hunziker, E.B. Articular cartilage repair: Are the intrinsic biological constraints undermining this process insuperable? Osteoarthr. Cartil. 1999, 7, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Oussedik, S.; Tsitskaris, K.; Parker, D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: A systematic review. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.J.; Pascual-Garrido, C.; Grumet, R.C. Surgical management of articular cartilage defects in the knee. JBJS 2009, 91, 1778–1790. [Google Scholar]
- Nakagawa, Y.; Mukai, S.; Yabumoto, H.; Tarumi, E.; Nakamura, T. Serial changes of the cartilage in recipient sites and their mirror sites on second-look imaging after mosaicplasty. Am. J. Sports Med. 2016, 44, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Alparslan, L.; Winalski, C.S.; Boutin, R.D.; Minas, T. Postoperative Magnetic Resonance Imaging of Articular Cartilage Repair. In Seminars in Musculoskeletal Radiology, 2001; Copyright© 2001 by Thieme Medical Publishers, Inc.: New York, NY, USA, 2001; pp. 345–364. [Google Scholar]
- Choi, Y.S.; Potter, H.G.; Chun, T.J. MR imaging of cartilage repair in the knee and ankle. Radiographics 2008, 28, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous chondrocyte implantation: A systematic review. JBJS 2010, 92, 2220–2233. [Google Scholar] [CrossRef]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Iwasa, J.; Engebretsen, L.; Shima, Y.; Ochi, M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 561–577. [Google Scholar] [CrossRef] [Green Version]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, Y.-G.; Choi, Y.-J.; Kwon, S.-K.; Kim, Y.-S.; Yeo, J.-E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1308–1316. [Google Scholar] [CrossRef]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin. Orthop. Relat. Res. 2015, 473, 2316–2326. [Google Scholar] [CrossRef] [Green Version]
- Saw, K.-Y.; Anz, A.; Siew-Yoke Jee, C.; Merican, S.; Ching-Soong Ng, R.; Roohi, S.A.; Ragavanaidu, K. Articular Cartilage Regeneration With Autologous Peripheral Blood Stem Cells Versus Hyaluronic Acid: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.J.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Uncovering the periosteum for skeletal regeneration: The stem cell that lies beneath. Bone 2015, 70, 10–18. [Google Scholar] [CrossRef]
- Liu, H.-C. Tissue engineering of cartilage: The road a group of researchers have traveled. J. Orthop. Sci. 2008, 13, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21. [Google Scholar] [CrossRef]
- Ochiya, T.; Takahama, Y.; Nagahara, S.; Sumita, Y.; Hisada, A.; Itoh, H.; Nagai, Y.; Terada, M. New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: The Minipellet. Nat. Med. 1999, 5, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Uchio, Y.; Ochi, M.; Matsusaki, M.; Kurioka, H.; Katsube, K. Human chondrocyte proliferation and matrix synthesis cultured in Atelocollagen® gel. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 50, 138–143. [Google Scholar] [CrossRef]
- Sakai, D.; Mochida, J.; Yamamoto, Y.; Nomura, T.; Okuma, M.; Nishimura, K.; Nakai, T.; Ando, K.; Hotta, T. Transplantation of mesenchymal stem cells embedded in Atelocollagen® gel to the intervertebral disc: A potential therapeutic model for disc degeneration. Biomaterials 2003, 24, 3531–3541. [Google Scholar] [CrossRef]
- Li, F.; Carlsson, D.; Lohmann, C.; Suuronen, E.; Vascotto, S.; Kobuch, K.; Sheardown, H.; Munger, R.; Nakamura, M.; Griffith, M. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc. Natl. Acad. Sci. USA 2003, 100, 15346–15351. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, H.; Hirachi, A.; Hasegawa, N.; Iwata, T.; Hamaguchi, H.; Shiba, H.; Takata, T.; Kato, Y.; Kurihara, H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J. Periodontol. 2004, 75, 1281–1287. [Google Scholar] [CrossRef]
- Widjaja, W.; Maitz, P. The use of dermal regeneration template (Pelnac®) in acute full-thickness wound closure: A case series. Eur. J. Plast. Surg. 2016, 39, 125–132. [Google Scholar] [CrossRef]
- Matsushita, R.; Nakasa, T.; Ishikawa, M.; Tsuyuguchi, Y.; Matsubara, N.; Miyaki, S.; Adachi, N. Repair of an Osteochondral Defect With Minced Cartilage Embedded in Atelocollagen Gel: A Rabbit Model. Am. J. Sports Med. 2019, 47, 2216–2224. [Google Scholar] [CrossRef]
- Andrades, J.A.; Han, B.; Becerra, J.; Sorgente, N.; Hall, F.L.; Nimni, M.E. A recombinant human TGF-β1 fusion protein with collagen-binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells. Exp. Cell Res. 1999, 250, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Jevotovsky, D.; Alfonso, A.; Einhorn, T.; Chiu, E. Osteoarthritis and stem cell therapy in humans: A systematic review. Osteoarthr. Cartil. 2018, 26, 711–729. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, P.; Pestka, J.M.; Kreuz, P.C.; Erggelet, C.; Schmal, H.; Suedkamp, N.P.; Steinwachs, M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am. J. Sports Med. 2008, 36, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Foldager, C.B.; Gomoll, A.H.; Lind, M.; Spector, M. Cell seeding densities in autologous chondrocyte implantation techniques for cartilage repair. Cartilage 2012, 3, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Hunziker, E.; Quinn, T.; Häuselmann, H.-J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr. Cartil. 2002, 10, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, F.; Giavaresi, G.; Tschon, M.; Borsari, V.; Nicoli Aldini, N.; Fini, M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2012, 22, 181–192. [Google Scholar] [CrossRef]
- Mazor, M.; Lespessailles, E.; Coursier, R.; Daniellou, R.; Best, T.; Toumi, H. Mesenchymal stem-cell potential in cartilage repair: An update. J. Cell. Mol. Med. 2014, 18, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Miot, S.; Brehm, W.; Dickinson, S.; Sims, T.; Wixmerten, A.; Longinotti, C.; Hollander, A.; Mainil-Varlet, P.; Martin, I. Influence of in vitro maturation of engineered cartilage on the outcome of osteochondral repair in a goat model. Eur. Cell Mater. 2012, 23, 222–236. [Google Scholar] [CrossRef]
- Ofek, G.; Revell, C.M.; Hu, J.C.; Allison, D.D.; Grande-Allen, K.J.; Athanasiou, K.A. Matrix development in self-assembly of articular cartilage. PLoS ONE 2008, 3, e2795. [Google Scholar] [CrossRef] [Green Version]
- Makris, E.A.; Responte, D.J.; Paschos, N.K.; Hu, J.C.; Athanasiou, K.A. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc. Natl. Acad. Sci. USA 2014, 111, E4832–E4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arokoski, J.; Jurvelin, J.; Väätäinen, U.; Helminen, H. Normal and pathological adaptations of articular cartilage to joint loading. Scand. J. Med. Sci. Sports Rev. Article 2000, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. The effect of mechanical loading on articular cartilage. J. Funct. Morphol. Kinesiol. 2016, 1, 154. [Google Scholar] [CrossRef] [Green Version]
- Vanwanseele, B.; Eckstein, F.; Knecht, H.; Spaepen, A.; Stüssi, E. Longitudinal analysis of cartilage atrophy in the knees of patients with spinal cord injury. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2003, 48, 3377–3381. [Google Scholar] [CrossRef]
- Brama, P.; Tekoppele, J.; Bank, R.; Barneveld, A.; Van Weeren, P. Functional adaptation of equine articular cartilage: The formation of regional biochemical characteristics up to age one year. Equine Vet. J. 2000, 32, 217–221. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sah, R.L.Y.; Grodzinsky, A.J.; Plaas, A.H.K.; Sandy, J.D. Mechanical Regulation of Cartilage Biosynthetic Behavior: Physical Stimuli. Arch. Biochem. Biophys. 1994, 311, 1–12. [Google Scholar] [CrossRef]
- Farnsworth, N.L.; Antunez, L.R.; Bryant, S.J. Dynamic compressive loading differentially regulates chondrocyte anabolic and catabolic activity with age. Biotechnol. Bioeng. 2013, 110, 2046–2057. [Google Scholar] [CrossRef]
- Mauck, R.; Yuan, X.; Tuan, R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 2006, 14, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Koga, H.; Engebretsen, L.; Brinchmann, J.E.; Muneta, T.; Sekiya, I. Mesenchymal stem cell-based therapy for cartilage repair: A review. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Yang, K.-C.; Lin, K.-H.; Liu, Y.-L.; Liu, H.-C.; Lin, F.-H. Cartilage regeneration in SCID mice using a highly organized three-dimensional alginate scaffold. Biomaterials 2012, 33, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Feng, Q.; Lin, S.; Yuan, W.; Li, R.; Li, J.; Wei, K.; Chen, X.; Zhang, K.; Yang, Y. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials 2019, 210, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Noshin, M.; Baker, H.B.; Taskoy, E.; Meredith, S.J.; Tang, Q.; Ringel, J.P.; Lerman, M.J.; Chen, Y.; Packer, J.D. 3D printed biofunctionalized scaffolds for microfracture repair of cartilage defects. Biomaterials 2018, 185, 219–231. [Google Scholar] [CrossRef]
- de Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.; Nizak, R.; van Rijen, M.H.; Saris, D.B. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: A first-in-man trial in 35 patients. Stem Cells 2017, 35, 1984–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, Y.-G.; Kwon, O.-R.; Kim, Y.-S.; Choi, Y.-J.; Tak, D.-H. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 97–109. [Google Scholar] [CrossRef]
- Irrgang, J.J.; Anderson, A.F. Development and validation of health-related quality of life measures for the knee. Clin. Orthop. Relat. Res. 2002, 402, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.; Lawrence, J. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.-F. Reference axes for reconstruction of the knee. Knee 2004, 11, 251–257. [Google Scholar] [CrossRef]
- Marlovits, S.; Singer, P.; Zeller, P.; Mandl, I.; Haller, J.; Trattnig, S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: Determination of interobserver variability and correlation to clinical outcome after 2 years. Eur. J. Radiol. 2006, 57, 16–23. [Google Scholar] [CrossRef]
- Peterson, L.; Minas, T.; Brittberg, M.; Nilsson, A.; Sjögren-Jansson, E.; Lindahl, A. Two-to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Relat. Res. 2000, 374, 212–234. [Google Scholar] [CrossRef] [PubMed]



| Case | Gender | Diagnosis | Age on CPI | Defect Size (cm2) a | No. Implanted CPs (×106) b | Date of CPI | Receive Arthroscopy | ICRS Score by Arthroscopy c PreOP/PostOP | Last Date of MRI | Last Date of X-ray |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | ON | 68 | 2.2 | 6.7 | 20080215 | 20090821 20110701 | 1/- 1/12 | 20120905 | 20131217 |
| 2 | M | OA | 83 | 1.5 | 4.0 | 20080530 | - | 1/- | 20140706 | 20150806 |
| 3 | M | OA | 78 | 1.6 | 9.9 | 20080609 | 20120502 | 3/11 | 20130901 | 20180302 |
| 4 | F | OA | 48 | 1.2 | 8.6 | 20080730 | 20090710 | 2/12 | 20101111 | 20101102 |
| 5 | M | OA | 62 | 2.4 | 13.7 | 20080808 | 20160725 | 2/6 | 20120924 | 20160718 |
| 6 | F | OA | 70 | 3.0 | 13.2 | 20080829 | - | 1/- | - | 20090108 |
| 7 | F | OA | 69 | 3.1 | 8.5 | 20081001 | - | 1/- | 20150830 | 20160801 |
| 8 | M | ON | 60 | 2.4 | 20.6 | 20081031 | 20091119 | 1/11 | 20111128 | 20170216 |
| 9 | F | OA | 64 | 1.3 | 6.3 | 20081022 | - | 1/- | 20160421 | 20160225 |
| 10 | F | OA | 67 | 0.9 | 2.7 | 20081219 | 20100208 | 2/12 | 20150319 | 20161228 |
| 11 | M | ON | 63 | 0.4 | 3.2 | 20100210 | 20110521 | 2/11 | 20171220 | 20180316 |
| 12 | M | ON | 65 | 3.0 | 8.0 | 20100401 | - | 3/- | 20160606 | 20171215 |
| 1. Degree of defect repair and filling of the defect | 4. Structure of the repair tissue | ||
| Complete | 7 (63.6%) | Homogenous | 9 (81.8%) |
| Hypertrophy | 2 (18.2%) | Inhomogeneous or cleft formation | 2 (18.2%) |
| Incomplete | 5. Signal intensity of the repair tissue | ||
| >50% of the adjacent cartilage | 1 (9.1%) | Isointense | 8 (72.7%) |
| <50% of the adjacent cartilage | 1 (9.1%) | Moderately hyperintense | 3 (27.3%) |
| Subchondral bone exposed | 0 (0%) | Markedly hyperintense | 0 (0%) |
| 2. Integration to border zone | 6. Subchondral lamina | ||
| Complete | 8 (72.7%) | Intact | 6 (54.5%) |
| Incomplete | Not intact | 5 (45.5%) | |
| Demarcating border visible (split-like) | 1 (9.1%) | 7. Subchondral bone | |
| Defect visible | Intact | 0 (0%) | |
| <50% of the length of the repair tissue | 1 (9.1%) | Non-intact | 11 (100%) |
| >50% of the length of the repair tissue | 1 (9.1%) | 8. Adhesions | |
| 3. Surface of the repair tissue | No | 11 (100%) | |
| Surface intact | 8(72.7%) | Yes | 0 (0%) |
| Surface damaged | 9. Effusion | ||
| <50% of repair tissue depth | 2 (18.2%) | No | 9 (81.8 %) |
| >50% of repair tissue depth or total degeneration | 1 (9.1%) | Yes | 2 (18.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-C.; Liu, T.-S.T.; Liu, Y.-L.; Wang, J.-H.; Chang, C.-H.; Shih, T.T.-F.; Lin, F.-H. Atelocollagen-Embedded Chondrocyte Precursors as a Treatment for Grade-4 Cartilage Defects of the Femoral Condyle: A Case Series with up to 9-Year Follow-Up. Biomolecules 2021, 11, 942. https://doi.org/10.3390/biom11070942
Liu H-C, Liu T-ST, Liu Y-L, Wang J-H, Chang C-H, Shih TT-F, Lin F-H. Atelocollagen-Embedded Chondrocyte Precursors as a Treatment for Grade-4 Cartilage Defects of the Femoral Condyle: A Case Series with up to 9-Year Follow-Up. Biomolecules. 2021; 11(7):942. https://doi.org/10.3390/biom11070942
Chicago/Turabian StyleLiu, Hwa-Chang, Tzu-Shang Thomas Liu, Yen-Liang Liu, Jyh-Horng Wang, Chih-Hung Chang, Tiffany Ting-Fang Shih, and Feng-Huei Lin. 2021. "Atelocollagen-Embedded Chondrocyte Precursors as a Treatment for Grade-4 Cartilage Defects of the Femoral Condyle: A Case Series with up to 9-Year Follow-Up" Biomolecules 11, no. 7: 942. https://doi.org/10.3390/biom11070942
APA StyleLiu, H.-C., Liu, T.-S. T., Liu, Y.-L., Wang, J.-H., Chang, C.-H., Shih, T. T.-F., & Lin, F.-H. (2021). Atelocollagen-Embedded Chondrocyte Precursors as a Treatment for Grade-4 Cartilage Defects of the Femoral Condyle: A Case Series with up to 9-Year Follow-Up. Biomolecules, 11(7), 942. https://doi.org/10.3390/biom11070942








