Evidence That Artificial Light at Night Induces Structure-Specific Changes in Brain Plasticity in a Diurnal Bird
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Melatonin Levels in Plasma
2.3. BrdU Administration and Immunohistochemistry
2.4. Mapping and Quantification
2.5. Statistical Analysis
3. Results
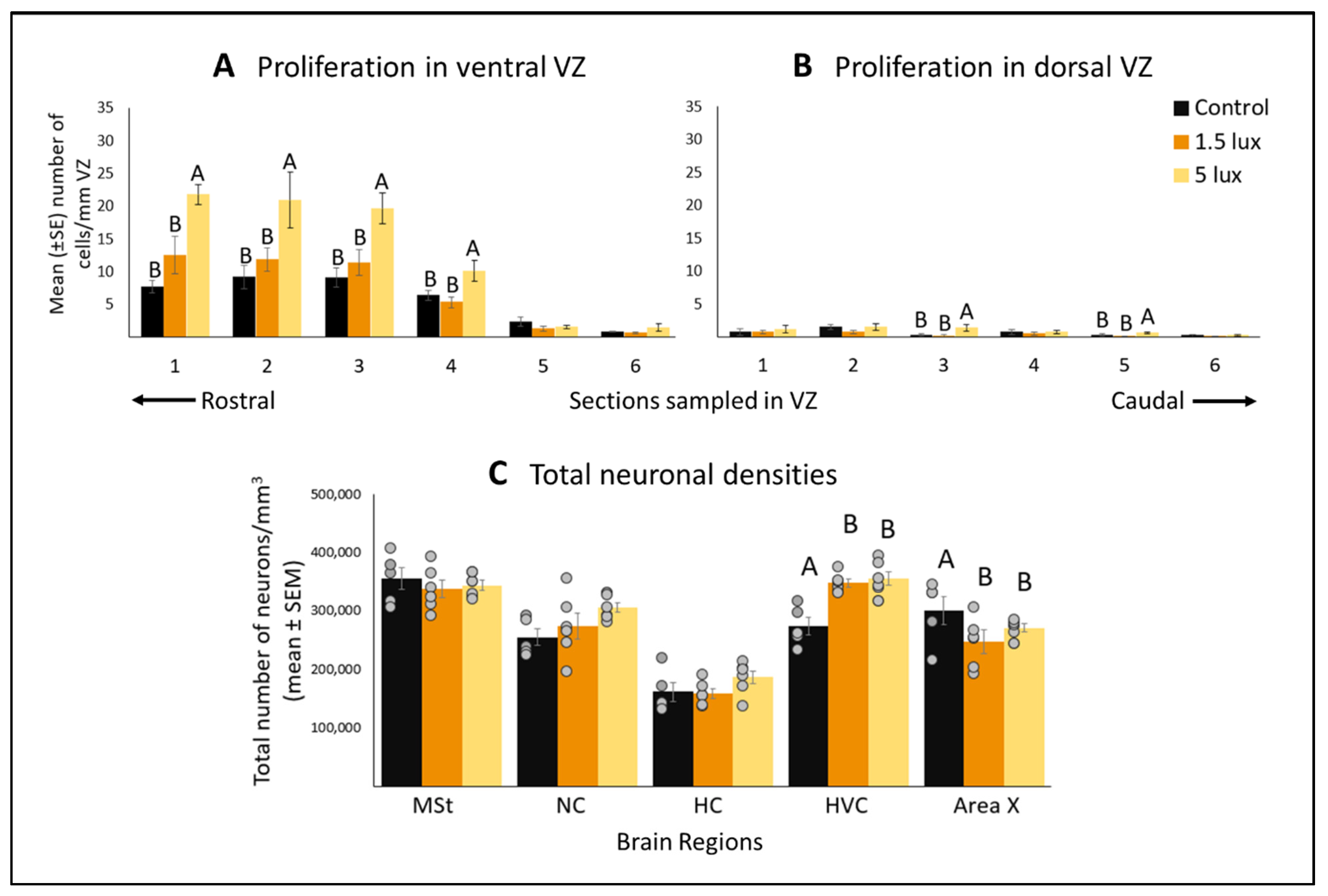
3.1. ALAN Increases Cell Proliferation in the VZ
3.2. ALAN Exposure Differentially Affects Total Neuronal Densities in Some Brain Regions after Short Term Exposure
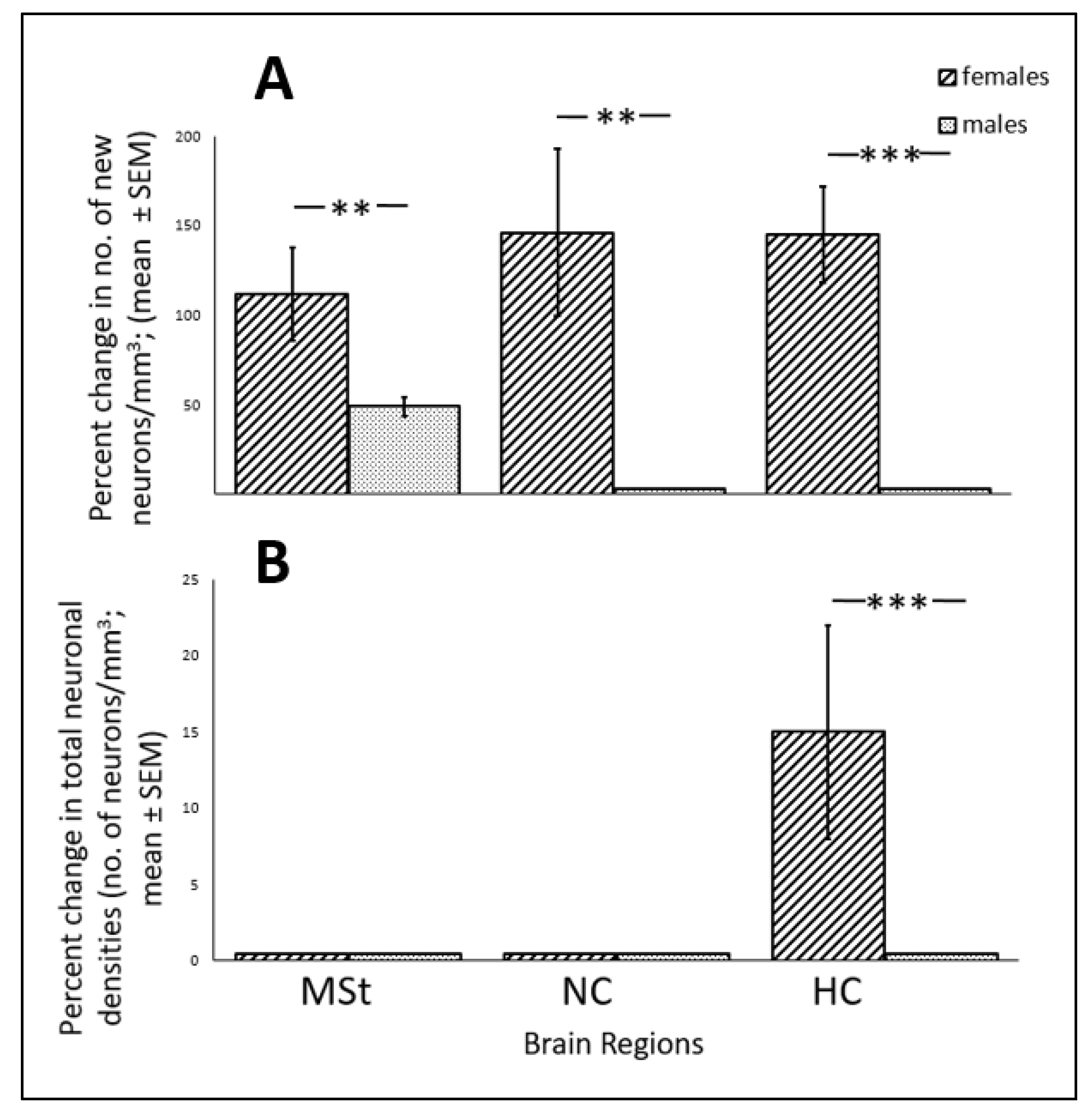
3.3. ALAN Increases New Neuronal Recruitment in Some Brain Regions and Subregions
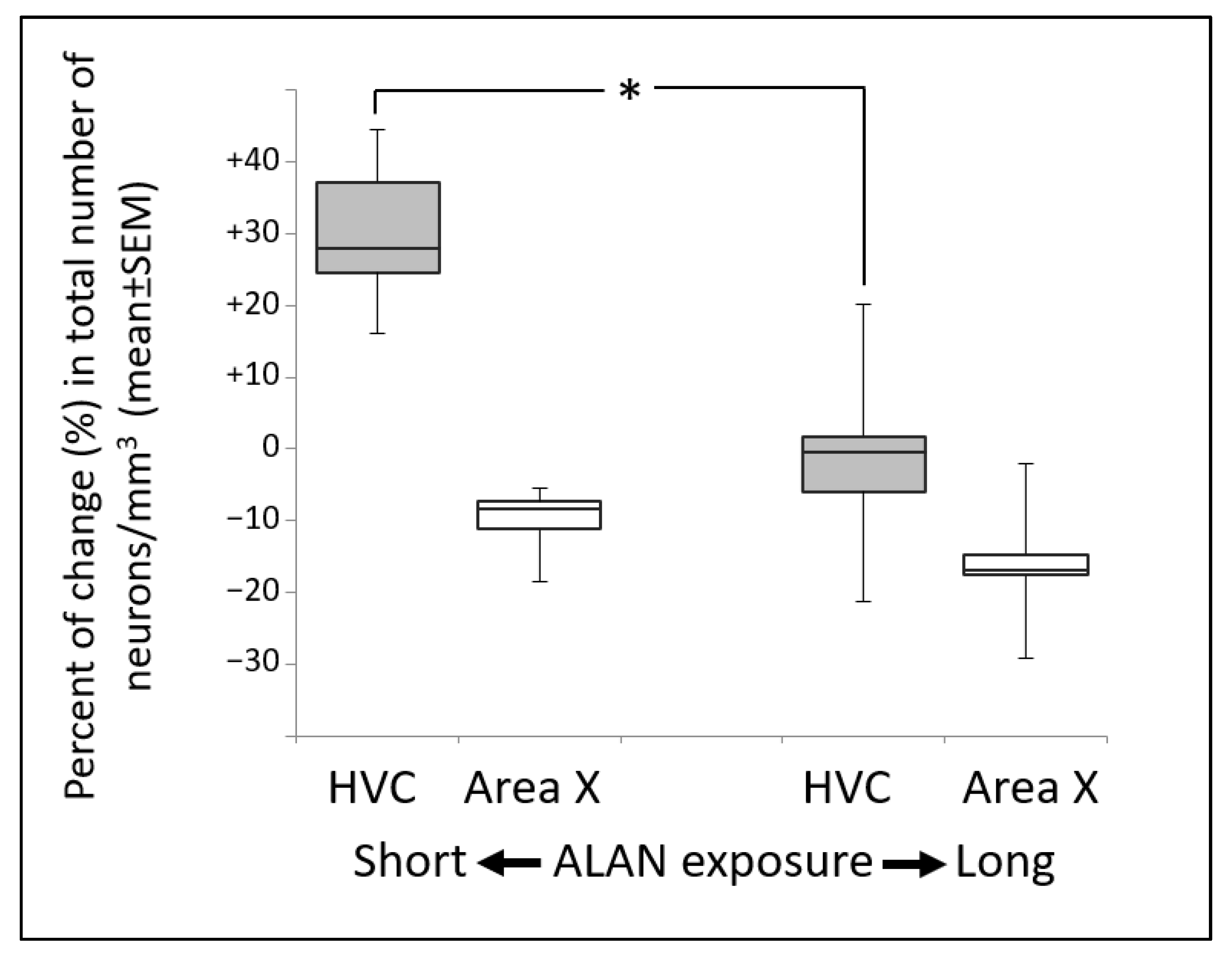
3.4. ALAN Decreases Total Neuronal Densities But Only in Area X after Long Term Exposure
3.5. Total Neuronal Densities Relative to the Control Decreased in the HVC as a Function of ALAN Exposure Duration
3.6. ALAN Reduces Nocturnal Melatonin Levels
3.7. ALAN Does Not Affect Body Mass
4. Discussion
4.1. ALAN Increases Cell Proliferation in the VZ
4.2. ALAN Increases Neuronal Recruitment in Some Brain Regions
4.3. Differential Effects of the Duration of Exposure to ALAN on Total Neuronal Densities
4.4. Effects of ALAN on Nocturnal Melatonin Levels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Grubisic, M.; Haim, A.; Bhusal, P.; Dominoni, D.M.; Gabriel, K.; Jechow, A.; Kupprat, F.; Lerner, A.; Marchant, P.; Riley, W. Light pollution, circadian photoreception, and melatonin in vertebrates. Sustainability 2019, 11, 6400. [Google Scholar] [CrossRef]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef]
- Dominoni, D.; Quetting, M.; Partecke, J. Artificial light at night advances avian reproductive physiology. Phil. Trans. R. Soc. B 2013, 280, 20123017. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.M.; Partecke, J. Does light pollution alter daylength? A test using light loggers on free-ranging European blackbirds (Turdus merula). Philos. Trans. R. Soc. B 2015, 370, 20140118. [Google Scholar] [CrossRef]
- Okuliarova, M.; Mazgutova, N.; Majzunova, M.; Rumanova, V.S.; Zeman, M. Dim light at night impairs daily variation of circulating immune cells and renal immune homeostasis. Front. Immunol. 2021, 11, 3548. [Google Scholar] [CrossRef]
- Moaraf, S.; Vistoropsky, Y.; Pozner, T.; Heiblum, R.; Okuliarová, M.; Zeman, M.; Barnea, A. Artificial light at night affects brain plasticity and melatonin in birds. Neurosci. Lett. 2020, 716, 134639. [Google Scholar] [CrossRef]
- Moaraf, S.; Heiblum, R.; Vistoropsky, Y.; Okuliarová, M.; Zeman, M.; Barnea, A. Artificial Light at Night Increases Recruitment of New Neurons and Differentially Affects Various Brain Regions in Female Zebra Finches. Int. J. Mol. Sci. 2020, 21, 6140. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Bhagat, S.; Bloss, E.B.; Morrison, J.H.; McEwen, B.S. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 1657–1662. [Google Scholar] [CrossRef]
- Kilic, E.; Kilic, Ü.; Bacigaluppi, M.; Guo, Z.; Abdallah, N.B.; Wolfer, D.P.; Bassetti, C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res. 2008, 45, 142–148. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Klempin, F.; Babu, H.; Benitez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef]
- Rennie, K.; De Butte, M.; Pappas, B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal. Res. 2009, 47, 313–317. [Google Scholar] [CrossRef]
- Kirn, J.; O’Loughlin, B.; Kasparian, S.; Nottebohm, F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc. Natl. Acad. Sci. USA 1994, 91, 7844–7848. [Google Scholar] [CrossRef]
- Scharff, C.; Kirn, J.R.; Grossman, M.; Macklis, J.D.; Nottebohm, F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 2000, 25, 481–492. [Google Scholar] [CrossRef]
- Paton, J.A.; Nottebohm, F.N. Neurons generated in the adult brain are recruited into functional circuits. Science 1984, 225, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A.; Nottebohm, F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl. Acad. Sci. USA 1983, 80, 2390–2394. [Google Scholar] [CrossRef] [PubMed]
- Nottebohm, F.; Arnold, A.P. Sexual dimorphism in vocal control areas of the songbird brain. Science 1976, 194, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Fusani, L.; Gahr, M. Differential expression of melatonin receptor subtypes MelIa, MelIb and MelIc in relation to melatonin binding in the male songbird brain. Brain Behav. Evol. 2015, 85, 4–14. [Google Scholar] [CrossRef]
- Nottebohm, F. The discovery of replaceable neurons. Neurosci. Birdsong 2008, 36, 405–448. [Google Scholar]
- Scharff, C.; Nottebohm, F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J. Neurosci. 1991, 11, 2896–2913. [Google Scholar] [CrossRef]
- Brainard, M.S. The anterior forebrain pathway and vocal plasticity. In Neuroscience of Birdsong; Zeigler, H.P., Marler, P., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 240–255. [Google Scholar]
- Ballentine, B.; Hyman, J.; Nowicki, S. Vocal performance influences female response to male bird song: An experimental test. Behav. Ecol. 2004, 15, 163–168. [Google Scholar] [CrossRef]
- Katz, A.; Mirzatoni, A.; Zhen, Y.; Schlinger, B.A. Sex differences in cell proliferation and glucocorticoid responsiveness in the zebra finch brain. Eur. J. Neurosci. 2008, 28, 99–106. [Google Scholar] [CrossRef][Green Version]
- Mirzatoni, A.; Dong, S.M.; Guerra, M.; Zhen, Y.; Katz, A.; Schlinger, B.A. Steroidal and gonadal effects on neural cell proliferation in vitro in an adult songbird. Brain Res. 2010, 1351, 41–49. [Google Scholar] [CrossRef]
- Vates, G.E.; Broome, B.M.; Mello, C.V.; Nottebohm, F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata). J. Comp. Neurol. 1996, 366, 613–642. [Google Scholar] [CrossRef]
- Mello, C.V.; Clayton, D.F. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J. Neurosci. 1994, 14, 6652–6666. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.V.; Vates, E.; Okuhata, S.; Nottebohm, F. Descending auditory pathways in the adult male zebra finch (Taeniopygia guttata). J. Comp. Neurol. 1998, 395, 137–160. [Google Scholar] [CrossRef]
- Kuenzel, W.J.; Medina, L.; Csillag, A.; Perkel, D.J.; Reiner, A. The avian subpallium: New insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res. 2011, 1424, 67–101. [Google Scholar] [CrossRef]
- Watanabe, S. Effects of lobus parolfactorius lesions on repeated acquisition of spatial discrimination in pigeons. Brain Behav. Evol. 2001, 58, 333–342. [Google Scholar] [CrossRef]
- Matsushima, T.; Izawa, E.I.; Aoki, N.; Yanagihara, S. The mind through chick eyes: Memory, cognition and anticipation. Zool. Sci. 2003, 20, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Perkel, D.J.; Bruce, L.L.; Butler, A.B.; Csillag, A.; Kuenzel, W.; Jarvis, E.D. The avian brain nomenclature forum: Terminology for a new century in comparative neuroanatomy. J. Comp. Neurol. 2004, 473, E1. [Google Scholar] [CrossRef]
- Sherry, D.F.; Vaccarino, A.L.; Buckenham, K.; Herz, R.S. The hippocampal complex of food-storing birds. Brain Behav. Evol. 1989, 34, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Shettleworth, S.J.; Krebs, J.R.; Healy, S.D.; Thomas, C.M. Spatial memory of food-storing tits (Parus ater and P. atricapillus): Comparison of storing and nonstoring tasks. J. Comp. Psychol. 1990, 104, 71. [Google Scholar] [CrossRef]
- Smulders, T.V. The avian hippocampal formation and the stress response. Brain Behav. Evol. 2017, 90, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N. Birds as models of aging in biomedical research. Lab. Anim. Res. 1997, 38, 137–140. [Google Scholar] [CrossRef]
- Heldmaier, G.; Werner, D. Environmental signal processing and adaptation. In Environmental Signal Processing and Adaptation; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–8. [Google Scholar]
- Maldonado, K.E.; Cavieres, G.; Veloso, C.; Canals, M.; Sabat, P. Physiological responses in rufous-collared sparrows to thermal acclimation and seasonal acclimatization. J. Comp. Physiol. B 2009, 179, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Gwinner, E. Ontogeny of the rhythmic melatonin production in a precocial and an altricial bird, the Japanese quail and the European starling. J. Comp. Physiol. 1993, A 172, 333–338. [Google Scholar] [CrossRef]
- Van´t Hof, T.J.; Gwinner, E. Development of post-hatching melatonin rhythm in zebra finches (Poephila guttata). Experientia 1996, 52, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Aloni, E.; Shapira, M.; Eldar-Finkelman, H.; Barnea, A. GSK-3β inhibition affects singing behavior and neurogenesis in adult songbirds. Brain Behav. Evol. 2005, 85, 233–244. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Nottebohm, F. Migration of young neurons in adult avian brain. Nature 1988, 335, 353. [Google Scholar] [CrossRef]
- Vistoropsky, Y.; Heiblum, R.; Smorodinsky, N.I.; Barnea, A. Active immunization against vasoactive intestinal polypeptide decreases neuronal recruitment and inhibits reproduction in zebra finches. J. Comp. Neurol. 2016, 524, 2516–2528. [Google Scholar] [CrossRef]
- Stokes, T.M.; Leonard, C.M.; Nottebohm, F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J. Comp. Neurol. 1974, 156, 337–374. [Google Scholar] [CrossRef]
- Barnea, A.; Mishal, A.; Nottebohm, F. Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: An anatomical representation of time? Behav. Brain Res. 2006, 167, 63–74. [Google Scholar] [CrossRef]
- Cattan, A.; Ayali, A.; Barnea, A. The cell birth marker BrdU does not affect recruitment of subsequent cell divisions in the adult avian brain. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Pozner, T.; Vistoropsky, Y.; Moaraf, S.; Heiblum, R.; Barnea, A. Questioning seasonality of neuronal plasticity in the adult avian brain. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Balaram, P.; Young, N.A.; Kaas, J.H. Three counting methods agree on cell and neuron number in chimpanzee primary visual cortex. Front. Neuroanat. 2014, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Barkan, S.; Yom-Tov, Y.; Barnea, A. A possible relation between new neuronal recruitment and migratory behavior in Acrocephalus warblers. Dev. Neurobiol. 2014, 74, 1194–1209. [Google Scholar] [CrossRef] [PubMed]
- Guillery, R.W.; Herrup, K. Quantification without pontification: Choosing a method for counting objects in sectioned tissues. J. Comp. Neurol. 1997, 386, 2–7. [Google Scholar] [CrossRef]
- Da Silva, A.; Samplonius, J.M.; Schlicht, E.; Valcu, M.; Kempenaers, B. Artificial night lighting rather than traffic noise affects the daily timing of dawn and dusk singing in common European songbirds. Behav. Ecol. 2014, 25, 1037–1047. [Google Scholar] [CrossRef]
- Barker, J.M.; Ball, G.F.; Balthazart, J. Anatomically discrete sex differences and enhancement by testosterone of cell proliferation in the telencephalic ventricle zone of the adult canary brain. J. Chem. Neuroanat. 2014, 55, 1–8. [Google Scholar] [CrossRef]
- Nottebohm, F. Why are some neurons replaced in adult brain? J. Neurosci. 2020, 22, 624–628. [Google Scholar] [CrossRef]
- Li, X.C.; Jarvis, E.D.; Alvarez-Borda, B.; Lim, D.A.; Nottebohm, F. A relationship between behavior, neurotrophin expression, and new neuron survival. Poc. Natl. Acad. Sci. USA 2000, 97, 8584–8589. [Google Scholar] [CrossRef]
- Vellema, M.; Van der Linden, A.; Gahr, M. Area-specific migration and recruitment of new neurons in the adult songbird brain. J. Comp. Neurol. 2010, 518, 1442–1459. [Google Scholar] [CrossRef]
- Gahr, M.; Kosar, E. Identification, distribution, and developmental changes of a melatonin binding site in the song control system of the zebra finch. J. Comp. Neurol. 1996, 367, 308–318. [Google Scholar] [CrossRef]
- Bentley, G.E.; Van’t Hof, T.J.; Ball, G.F. Seasonal neuroplasticity in the songbird telencephalon: A role for melatonin. Poc. Natl. Acad. Sci. USA 1999, 96, 4674–4679. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Rucker, M.G.; Cassone, V.M. Melatonin binding in the house sparrow song control system: Sexual dimorphism and the effect of photoperiod. Horm. Behav. 1996, 30, 528–537. [Google Scholar] [CrossRef]
- Bentley, G.E.; Perfito, N.; Calisi, R.M. Season-and context-dependent sex differences in melatonin receptor activity in a forebrain song control nucleus. Horm. Behav. 2013, 63, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Kirn, J.R.; Schwabl, H. Photoperiod regulation of neuron death in the adult canary. J. Neurobiol. 1997, 33, 223–231. [Google Scholar] [CrossRef]
- Derégnaucourt, S.; Saar, S.; Gahr, M. Melatonin affects the temporal pattern of vocal signatures in birds. J. Pineal Res. 2012, 53, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Mishra, I.; Knerr, R.M.; Stewart, A.A.; Payette, W.I.; Richter, M.M.; Ashley, N.T. Light at night disrupts diel patterns of cytokine gene expression and endocrine profiles in zebra finch (Taeniopygia guttata). Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- De Jong, M.; Jening, L.; Ouyang, J.Q.; van Oers, K.; Spoelstra, K.; Visser, M.E. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 2016, 155, 172–179. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moaraf, S.; Heiblum, R.; Okuliarová, M.; Hefetz, A.; Scharf, I.; Zeman, M.; Barnea, A. Evidence That Artificial Light at Night Induces Structure-Specific Changes in Brain Plasticity in a Diurnal Bird. Biomolecules 2021, 11, 1069. https://doi.org/10.3390/biom11081069
Moaraf S, Heiblum R, Okuliarová M, Hefetz A, Scharf I, Zeman M, Barnea A. Evidence That Artificial Light at Night Induces Structure-Specific Changes in Brain Plasticity in a Diurnal Bird. Biomolecules. 2021; 11(8):1069. https://doi.org/10.3390/biom11081069
Chicago/Turabian StyleMoaraf, Stan, Rachel Heiblum, Monika Okuliarová, Abraham Hefetz, Inon Scharf, Michal Zeman, and Anat Barnea. 2021. "Evidence That Artificial Light at Night Induces Structure-Specific Changes in Brain Plasticity in a Diurnal Bird" Biomolecules 11, no. 8: 1069. https://doi.org/10.3390/biom11081069
APA StyleMoaraf, S., Heiblum, R., Okuliarová, M., Hefetz, A., Scharf, I., Zeman, M., & Barnea, A. (2021). Evidence That Artificial Light at Night Induces Structure-Specific Changes in Brain Plasticity in a Diurnal Bird. Biomolecules, 11(8), 1069. https://doi.org/10.3390/biom11081069








