Role of White Matter Hyperintensities and Related Risk Factors in Vascular Cognitive Impairment: A Review
Abstract
1. Introduction
2. Relationship between WMHs and Cognitive Impairment
2.1. White-Matter Lesions: Severity
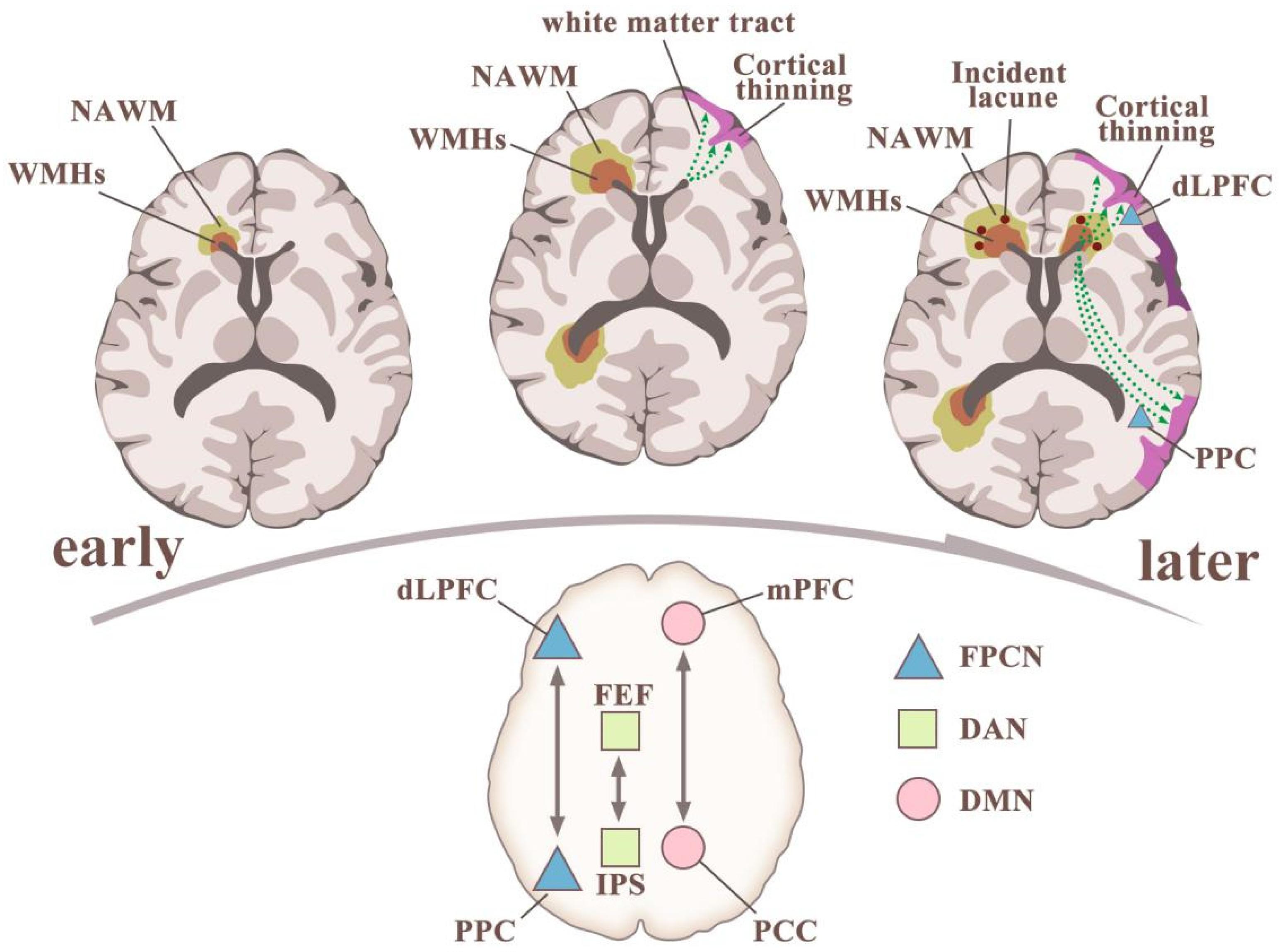
2.2. White-Matter Lesions: Location
3. Risk Factors Linking WMHs to VCI
3.1. Blood-Brain-Barrier (BBB) Function
3.2. Inflammatory Biomarkers
3.3. Cerebral Aβ and Tau Burden
3.4. Metabolic Abnormalities
3.5. Other Vascular Risk Factors for WMHs-Related Cognitive Deficit
4. Treatment of Cognitive Impairment Associated with WMHs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Leeuw, F.E.; De Groot, J.C.; De Achten, E.; Oudkerk, M.; Ramos, L.M.; Heijboer, R.; Hofman, A.; Jolles, J.; Gijn, J.; Van Breteler, M.M. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 2001, 70, 2. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- de Leeuw, F.E.; de Groot, J.C.; Oudkerk, M.; Witteman, J.C.; Hofman, A.; van Gijn, J.; Breteler, M.M. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 2002, 125, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.J.; Zonneveld, H.I.; Hofman, A.; van der Lugt, A.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A.; Heart-Brain Connection Collaborative Research Group. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation 2017, 136, 719–728. [Google Scholar] [CrossRef]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef]
- Schenk, C.; Wuerz, T.; Lerner, A.J. Small vessel disease and memory loss: What the clinician needs to know to preserve patients’ brain health. Curr. Cardiol. Rep. 2013, 15, 427. [Google Scholar] [CrossRef]
- Habes, M.; Erus, G.; Toledo, J.B.; Zhang, T.; Bryan, N.; Launer, L.J.; Rosseel, Y.; Janowitz, D.; Doshi, J.; Van der Auwera, S.; et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016, 139, 1164–1179. [Google Scholar] [CrossRef]
- Inzitari, D.; Pracucci, G.; Poggesi, A.; Carlucci, G.; Barkhof, F.; Chabriat, H.; Erkinjuntti, T.; Fazekas, F.; Ferro, J.M.; Hennerici, M.; et al. Changes in white matter as determinant of global functional decline in older independent outpatients: Three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 2009, 339, b2477. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 4, 18003. [Google Scholar] [CrossRef]
- Jokinen, H.; Kalska, H.; Ylikoski, R.; Madureira, S.; Verdelho, A.; van der Flier, W.M.; Scheltens, P.; Barkhof, F.; Visser, M.C.; Fazekas, F.; et al. Longitudinal cognitive decline in subcortical ischemic vascular disease—The LADIS Study. Cerebrovasc. Dis. 2009, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- van Rooden, S.; van Opstal, A.M.; Labadie, G.; Terwindt, G.M.; Wermer, M.J.; Webb, A.G.; Middelkoop, H.A.; Greenberg, S.M.; van der Grond, J.; van Buchem, M.A. Early Magnetic Resonance Imaging and Cognitive Markers of Hereditary Cerebral Amyloid Angiopathy. Stroke 2016, 47, 3041–3044. [Google Scholar] [CrossRef] [PubMed]
- van Opstal, A.M.; van Rooden, S.; van Harten, T.; Ghariq, E.; Labadie, G.; Fotiadis, P.; Gurol, M.E.; Terwindt, G.M.; Wermer, M.J.H.; van Buchem, M.A.; et al. Cerebrovascular function in presymptomatic and symptomatic individuals with hereditary cerebral amyloid angiopathy: A case-control study. Lancet Neurol. 2017, 16, 115–122. [Google Scholar] [CrossRef]
- Pavlovic, A.M.; Pekmezovic, T.; Tomic, G.; Trajkovic, J.Z.; Sternic, N. Baseline predictors of cognitive decline in patients with cerebral small vessel disease. J. Alzheimer’s Dis. 2014, 42 (Suppl. S3), S37–S43. [Google Scholar] [CrossRef]
- Englund, E. Neuropathology of white matter lesions in vascular cognitive impairment. Cerebrovasc. Dis. 2002, 13 (Suppl. S2), 11–15. [Google Scholar] [CrossRef]
- de Groot, M.; Verhaaren, B.F.; de Boer, R.; Klein, S.; Hofman, A.; van der Lugt, A.; Ikram, M.A.; Niessen, W.J.; Vernooij, M.W. Changes in normal-appearing white matter precede development of white matter lesions. Stroke 2013, 44, 1037–1042. [Google Scholar] [CrossRef]
- Sam, K.; Crawley, A.; Poublanc, J.; Conklin, J.; Sobczyk, O.; Mandell, D.; Duffin, J.; Venkatraghavan, L.; Fisher, J.; Black, S. Vascular dysfunction in leukoaraiosis. Am. J. Neuroradiol. 2016, 37, 2258–2264. [Google Scholar] [CrossRef]
- Pantoni, L.; Simoni, M. Pathophysiology of cerebral small vessels in vascular cognitive impairment. Int. Psychogeriatr. 2003, 15 (Suppl. S1), 59–65. [Google Scholar] [CrossRef]
- Prins, N.D.; Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 2015, 11, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kloppenborg, R.P.; Nederkoorn, P.J.; Geerlings, M.I.; van den Berg, E. Presence and progression of white matter hyperintensities and cognition: A meta-analysis. Neurology 2014, 82, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, L.; Riaz, P.; Kate, M.; Jeerakathil, T.; Beaulieu, C.; Buck, B.; Camicioli, R.; Butcher, K. White matter hyperintensity volume predicts persistent cognitive impairment in transient ischemic attack and minor stroke. Int. J. Stroke 2017, 12, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Alber, J.; Alladi, S.; Bae, H.J.; Barton, D.A.; Beckett, L.A.; Bell, J.M.; Berman, S.E.; Biessels, G.J.; Black, S.E.; Bos, I.; et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimer’s Dement. 2019, 5, 107–117. [Google Scholar] [CrossRef]
- Schmidt, R.; Berghold, A.; Jokinen, H.; Gouw, A.A.; van der Flier, W.M.; Barkhof, F.; Scheltens, P.; Petrovic, K.; Madureira, S.; Verdelho, A.; et al. White matter lesion progression in LADIS: Frequency, clinical effects, and sample size calculations. Stroke 2012, 43, 2643–2647. [Google Scholar] [CrossRef]
- Group, L.S. 2001–2011: A decade of the LADIS (Leukoaraiosis And DISability) Study: What have we learned about white matter changes and small-vessel disease? Cerebrovasc. Dis. 2011, 32, 577–588. [Google Scholar] [CrossRef]
- Ter Telgte, A.; van Leijsen, E.M.C.; Wiegertjes, K.; Klijn, C.J.M.; Tuladhar, A.M.; de Leeuw, F.E. Cerebral small vessel disease: From a focal to a global perspective. Nat. Rev. Neurol. 2018, 14, 387–398. [Google Scholar] [CrossRef]
- van der Flier, W.M.; van Straaten, E.C.; Barkhof, F.; Ferro, J.M.; Pantoni, L.; Basile, A.M.; Inzitari, D.; Erkinjuntti, T.; Wahlund, L.O.; Rostrup, E.; et al. Medial temporal lobe atrophy and white matter hyperintensities are associated with mild cognitive deficits in non-disabled elderly people: The LADIS study. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1497–1500. [Google Scholar] [CrossRef]
- Jang, J.W.; Kim, S.; Na, H.Y.; Ahn, S.; Lee, S.J.; Kwak, K.H.; Lee, M.A.; Hsiung, G.Y.; Choi, B.S.; Youn, Y.C. Effect of white matter hyperintensity on medial temporal lobe atrophy in Alzheimer’s disease. Eur. Neurol. 2013, 69, 229–235. [Google Scholar] [CrossRef]
- Dey, A.K.; Stamenova, V.; Turner, G.; Black, S.E.; Levine, B. Pathoconnectomics of cognitive impairment in small vessel disease: A systematic review. Alzheimer’s Dement. 2016, 12, 831–845. [Google Scholar] [CrossRef]
- Smith, E.E.; Salat, D.H.; Jeng, J.; McCreary, C.R.; Fischl, B.; Schmahmann, J.D.; Dickerson, B.C.; Viswanathan, A.; Albert, M.S.; Blacker, D.; et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology 2011, 76, 1492–1499. [Google Scholar] [CrossRef]
- Nekovarova, T.; Fajnerova, I.; Horacek, J.; Spaniel, F. Bridging disparate symptoms of schizophrenia: A triple network dysfunction theory. Front. Behav. Neurosci. 2014, 8, 171. [Google Scholar] [CrossRef]
- Mascalchi, M.; Ginestroni, A.; Toschi, N.; Poggesi, A.; Cecchi, P.; Salvadori, E.; Tessa, C.; Cosottini, M.; De Stefano, N.; Pracucci, G.; et al. The burden of microstructural damage modulates cortical activation in elderly subjects with MCI and leuko-araiosis. A DTI and fMRI study. Hum. Brain Mapp. 2014, 35, 819–830. [Google Scholar] [CrossRef]
- Duering, M.; Finsterwalder, S.; Baykara, E.; Tuladhar, A.M.; Gesierich, B.; Konieczny, M.J.; Malik, R.; Franzmeier, N.; Ewers, M.; Jouvent, E.; et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimer’s Dement. 2018, 14, 764–774. [Google Scholar] [CrossRef]
- Tomimoto, H. White matter integrity and cognitive dysfunction: Radiological and neuropsychological correlations. Geriatr. Gerontol. Int. 2015, 15 (Suppl. S1), 3–9. [Google Scholar] [CrossRef]
- de Groot, J.C.; de Leeuw, F.E.; Oudkerk, M.; van Gijn, J.; Hofman, A.; Jolles, J.; Breteler, M.M. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann. Neurol. 2000, 47, 145–151. [Google Scholar] [CrossRef]
- Marquine, M.J.; Attix, D.K.; Goldstein, L.B.; Samsa, G.P.; Payne, M.E.; Chelune, G.J.; Steffens, D.C. Differential patterns of cognitive decline in anterior and posterior white matter hyperintensity progression. Stroke 2010, 41, 1946–1950. [Google Scholar] [CrossRef][Green Version]
- Grau-Olivares, M.; Arboix, A. Mild cognitive impairment in stroke patients with ischemic cerebral small-vessel disease: A forerunner of vascular dementia? Expert Rev. Neurother. 2009, 9, 1201–1217. [Google Scholar] [CrossRef]
- Grau-Olivares, M.; Arboix, A.; Bartres-Faz, D.; Junque, C. Neuropsychological abnormalities associated with lacunar infarction. J. Neurol. Sci. 2007, 257, 160–165. [Google Scholar] [CrossRef]
- Grau-Olivares, M.; Arboix, A.; Junque, C.; Arenaza-Urquijo, E.M.; Rovira, M.; Bartres-Faz, D. Progressive gray matter atrophy in lacunar patients with vascular mild cognitive impairment. Cerebrovasc. Dis. 2010, 30, 157–166. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Zhang, X.; Shi, Q.; Yang, S.; Fan, H.; Qin, W.; Yang, L.; Yuan, J.; Jiang, T.; et al. Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden. J. Neurol. 2017, 264, 1474–1481. [Google Scholar] [CrossRef]
- Farrall, A.J.; Wardlaw, J.M. Blood-brain barrier: Ageing and microvascular disease--systematic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Sandercock, P.A.; Dennis, M.S.; Starr, J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003, 34, 806–812. [Google Scholar] [CrossRef]
- Zhang, C.E.; Wong, S.M.; van de Haar, H.J.; Staals, J.; Jansen, J.F.; Jeukens, C.R.; Hofman, P.A.; van Oostenbrugge, R.J.; Backes, W.H. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology 2017, 88, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Nelson, A.R.; Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta 2016, 1862, 887–900. [Google Scholar] [CrossRef]
- Gardener, H.; Scarmeas, N.; Gu, Y.; Boden-Albala, B.; Elkind, M.S.; Sacco, R.L.; DeCarli, C.; Wright, C.B. Mediterranean diet and white matter hyperintensity volume in the Northern Manhattan Study. Arch. Neurol. 2012, 69, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Baskin, D.G.; Schwartz, M.W. Insulin and the blood-brain barrier. Curr. Pharm. Des. 2003, 9, 795–800. [Google Scholar] [CrossRef]
- Gasparovic, C.; Prestopnik, J.; Thompson, J.; Taheri, S.; Huisa, B.; Schrader, R.; Adair, J.C.; Rosenberg, G.A. 1H-MR spectroscopy metabolite levels correlate with executive function in vascular cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2013, 84, 715–721. [Google Scholar] [CrossRef] [PubMed]
- MacGregor Sharp, M.; Saito, S.; Keable, A.; Gatherer, M.; Aldea, R.; Agarwal, N.; Simpson, J.E.; Wharton, S.B.; Weller, R.O.; Carare, R.O. Demonstrating a reduced capacity for removal of fluid from cerebral white matter and hypoxia in areas of white matter hyperintensity associated with age and dementia. Acta Neuropathol. Commun. 2020, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Schuitemaker, A.; Dik, M.G.; Veerhuis, R.; Scheltens, P.; Schoonenboom, N.S.; Hack, C.E.; Blankenstein, M.A.; Jonker, C. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol. Aging 2009, 30, 1885–1889. [Google Scholar] [CrossRef]
- Lue, L.F.; Walker, D.G.; Brachova, L.; Beach, T.G.; Rogers, J.; Schmidt, A.M.; Stern, D.M.; Yan, S.D. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: Identification of a cellular activation mechanism. Exp. Neurol. 2001, 171, 29–45. [Google Scholar] [CrossRef]
- Woodling, N.S.; Colas, D.; Wang, Q.; Minhas, P.; Panchal, M.; Liang, X.; Mhatre, S.D.; Brown, H.; Ko, N.; Zagol-Ikapitte, I.; et al. Cyclooxygenase inhibition targets neurons to prevent early behavioural decline in Alzheimer’s disease model mice. Brain 2016, 139, 2063–2081. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.F. Genetic and hormonal regulation of tryptophan kynurenine metabolism: Implications for vascular cognitive impairment, major depressive disorder, and aging. Ann. N.Y. Acad. Sci. 2007, 1122, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.S.; Biessels, G.-J.; Kim, L.; Nicholas, J.M.; Barber, P.A.; Walsh, P.; Gami, P.; Morris, H.R.; Bastos-Leite, A.J.; Schott, J.M. Genetic determinants of white matter hyperintensities and amyloid angiopathy in familial Alzheimer’s disease. Neurobiol. Aging 2015, 36, 3140–3151. [Google Scholar] [CrossRef]
- Kalheim, L.F.; Bjornerud, A.; Fladby, T.; Vegge, K.; Selnes, P. White matter hyperintensity microstructure in amyloid dysmetabolism. J. Cereb. Blood Flow Metab. 2017, 37, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Weaver, N.A.; Doeven, T.; Barkhof, F.; Biesbroek, J.M.; Groeneveld, O.N.; Kuijf, H.J.; Prins, N.D.; Scheltens, P.; Teunissen, C.E.; van der Flier, W.M.; et al. Cerebral amyloid burden is associated with white matter hyperintensity location in specific posterior white matter regions. Neurobiol. Aging 2019, 84, 225–234. [Google Scholar] [CrossRef]
- Saridin, F.N.; Hilal, S.; Villaraza, S.G.; Reilhac, A.; Gyanwali, B.; Tanaka, T.; Stephenson, M.C.; Ng, S.L.; Vrooman, H.; van der Flier, W.M.; et al. Brain amyloid β, cerebral small vessel disease, and cognition: A memory clinic study. Neurology 2020, 95, e2845–e2853. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.; Cho, H.; Jang, Y.K.; San Lee, J.; Jang, H.; Kim, Y.; Kim, K.W.; Ryu, Y.H.; Choi, J.Y.; et al. Assessment of Extent and Role of Tau in Subcortical Vascular Cognitive Impairment Using 18F-AV1451 Positron Emission Tomography Imaging. JAMA Neurol. 2018, 75, 999–1007. [Google Scholar] [CrossRef]
- Lim, J.S.; Kwon, H.M.; Lee, Y.S. Effect of cholinergic pathway disruption on cortical and subcortical volumes in subcortical vascular cognitive impairment. Eur. J. Neurol. 2020, 27, 210–212. [Google Scholar] [CrossRef]
- Finsterwalder, S.; Vlegels, N.; Gesierich, B.; Araque Caballero, M.; Weaver, N.A.; Franzmeier, N.; Georgakis, M.K.; Konieczny, M.J.; Koek, H.L.; Karch, C.M.; et al. Small vessel disease more than Alzheimer’s disease determines diffusion MRI alterations in memory clinic patients. Alzheimer’s Dement. 2020, 16, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Palau, X.; Guo, X.; Serra, A.; Sze, S.K. Alzheimer’s disease progression characterized by alterations in the molecular profiles and biogenesis of brain extracellular vesicles. Alzheimer’s Res. Ther. 2020, 12, 54. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Q.; Hu, G.; Deng, T.; Wang, Q.; Zhou, J.; Su, X. Extracellular vesicles in mesenchymal stromal cells: A novel therapeutic strategy for stroke. Exp. Ther. Med. 2018, 15, 4067–4079. [Google Scholar] [CrossRef]
- Gallart-Palau, X.; Serra, A.; Hase, Y.; Tan, C.F.; Chen, C.P.; Kalaria, R.N.; Sze, S.K. Brain-derived and circulating vesicle profiles indicate neurovascular unit dysfunction in early Alzheimer’s disease. Brain Pathol. 2019, 29, 593–605. [Google Scholar] [CrossRef]
- Blevins, B.L.; Vinters, H.V.; Love, S.; Wilcock, D.M.; Grinberg, L.T.; Schneider, J.A.; Kalaria, R.N.; Katsumata, Y.; Gold, B.T.; Wang, D.J.J.; et al. Brain arteriolosclerosis. Acta Neuropathol. 2021, 141, 1–24. [Google Scholar] [CrossRef]
- Wang, M.; Norman, J.E.; Srinivasan, V.J.; Rutledge, J.C. Metabolic, inflammatory, and microvascular determinants of white matter disease and cognitive decline. Am. J. Neurodegener. Dis. 2016, 5, 171–177. [Google Scholar] [PubMed]
- Araki, E.; Nishikawa, T. Oxidative stress: A cause and therapeutic target of diabetic complications. J. Diabetes Investig. 2010, 1, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Araki, A. Diabetes mellitus and white matter hyperintensity. Geriatr. Gerontol. Int. 2015, 15 (Suppl. S1), 34–42. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Li, Q.; Miao, K.; Wang, C.; Zhang, D.; Tian, C.; Zhang, S. Presence of White Matter Lesions Associated with Diabetes-Associated Cognitive Decline in Male Rat Models of Pre-Type 2 Diabetes. Med. Sci. Monit. 2019, 25, 9679–9689. [Google Scholar] [CrossRef]
- Vergoossen, L.W.; Schram, M.T.; de Jong, J.J.; Stehouwer, C.D.; Schaper, N.C.; Henry, R.M.; van der Kallen, C.J.; Dagnelie, P.C.; van Boxtel, M.P.; Eussen, S.J.; et al. White Matter Connectivity Abnormalities in Prediabetes and Type 2 Diabetes: The Maastricht Study. Diabetes Care 2020, 43, 201–208. [Google Scholar] [CrossRef]
- Sanjari Moghaddam, H.; Ghazi Sherbaf, F.; Aarabi, M.H. Brain microstructural abnormalities in type 2 diabetes mellitus: A systematic review of diffusion tensor imaging studies. Front. Neuroendocrinol. 2019, 55, 100782. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Fang, F.; Lu, L.; Li, T.; Lian, J.; Xiong, Y.; Kong, D.; Li, K. White matter impairment in type 2 diabetes mellitus with and without microvascular disease. Neuroimage Clin. 2019, 24, 101945. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kuller, L.H.; Lopez, O.L.; Diehr, P.; O’Meara, E.S.; Longstreth, W.T., Jr.; Luchsinger, J.A. Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Arch. Neurol. 2009, 66, 336–342. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Strain, G.; Devlin, M.; Cohen, R.; Paul, R.; Crosby, R.D.; Mitchell, J.E.; Gunstad, J. Improved memory function two years after bariatric surgery. Obesity 2014, 22, 32–38. [Google Scholar] [CrossRef]
- Kullmann, S.; Schweizer, F.; Veit, R.; Fritsche, A.; Preissl, H. Compromised white matter integrity in obesity. Obes. Rev. 2015, 16, 273–281. [Google Scholar] [CrossRef]
- Freeman, L.R.; Zhang, L.; Nair, A.; Dasuri, K.; Francis, J.; Fernandez-Kim, S.O.; Bruce-Keller, A.J.; Keller, J.N. Obesity increases cerebrocortical reactive oxygen species and impairs brain function. Free Radic. Biol. Med. 2013, 56, 226–233. [Google Scholar] [CrossRef]
- Yu, D.; Hennebelle, M.; Sahlas, D.J.; Ramirez, J.; Gao, F.; Masellis, M.; Cogo-Moreira, H.; Swartz, R.H.; Herrmann, N.; Chan, P.C.; et al. Soluble Epoxide Hydrolase-Derived Linoleic Acid Oxylipins in Serum Are Associated with Periventricular White Matter Hyperintensities and Vascular Cognitive Impairment. Transl. Stroke Res. 2019, 10, 522–533. [Google Scholar] [CrossRef]
- Hase, Y.; Horsburgh, K.; Ihara, M.; Kalaria, R.N. White matter degeneration in vascular and other ageing-related dementias. J. Neurochem. 2018, 144, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Lampe, L.; Zhang, R.; Beyer, F.; Huhn, S.; Kharabian Masouleh, S.; Preusser, S.; Bazin, P.L.; Schroeter, M.L.; Villringer, A.; Witte, A.V. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann. Neurol. 2019, 85, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Byeon, K.; Lee, M.J.; Kim, S.H.; Park, H. The orbitofrontal cortex functionally links obesity and white matter hyperintensities. Sci. Rep. 2020, 10, 2930. [Google Scholar] [CrossRef]
- Veldsman, M.; Kindalova, P.; Husain, M.; Kosmidis, I.; Nichols, T.E. Spatial distribution and cognitive impact of cerebrovascular risk-related white matter hyperintensities. Neuroimage Clin. 2020, 28, 102405. [Google Scholar] [CrossRef]
- Schreiber, S.; Bueche, C.Z.; Garz, C.; Braun, H. Blood brain barrier breakdown as the starting point of cerebral small vessel disease?—New insights from a rat model. Exp. Transl. Stroke Med. 2013, 5, 4. [Google Scholar] [CrossRef]
- Kimura, S.; Saito, H.; Minami, M.; Togashi, H.; Nakamura, N.; Nemoto, M.; Parvez, H.S. Pathogenesis of vascular dementia in stroke-prone spontaneously hypertensive rats. Toxicology 2000, 153, 167–178. [Google Scholar] [CrossRef]
- Oh, Y.S. Arterial stiffness and hypertension. Clin. Hypertens. 2018, 24, 17. [Google Scholar] [CrossRef]
- Badji, A.; Noriega de la Colina, A.; Karakuzu, A.; Duval, T.; Desjardins-Crepeau, L.; Parizet, M.; Joubert, S.; Bherer, L.; Lamarre-Cliche, M.; Stikov, N.; et al. Arterial stiffness cut-off value and white matter integrity in the elderly. Neuroimage Clin. 2020, 26, 102007. [Google Scholar] [CrossRef] [PubMed]
- Firbank, M.J.; Wiseman, R.M.; Burton, E.J.; Saxby, B.K.; O’Brien, J.T.; Ford, G.A. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J. Neurol. 2007, 254, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, X.; Liu, X.; Ortega, D.; Chen, L.; Chen, Z.; Sun, J.; Wang, L.; Hatsukami, T.S.; Yuan, C.; et al. Uncontrolled hypertension associates with subclinical cerebrovascular health globally: A multimodal imaging study. Eur. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front. Aging Neurosci. 2013, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Peters, J.; Booth, A.; Anstey, K.J. Trajectory of blood pressure, body mass index, cholesterol and incident dementia: Systematic review. Br. J. Psychiatry 2020, 216, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Amier, R.P.; Marcks, N.; Hooghiemstra, A.M.; Nijveldt, R.; van Buchem, M.A.; de Roos, A.; Biessels, G.J.; Kappelle, L.J.; van Oostenbrugge, R.J.; van der Geest, R.J.; et al. Hypertensive Exposure Markers by MRI in Relation to Cerebral Small Vessel Disease and Cognitive Impairment. JACC Cardiovasc. Imaging 2021, 14, 176–185. [Google Scholar] [CrossRef]
- Lane, C.A.; Barnes, J.; Nicholas, J.M.; Sudre, C.H.; Cash, D.M.; Parker, T.D.; Malone, I.B.; Lu, K.; James, S.N.; Keshavan, A.; et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): An epidemiological study. Lancet Neurol. 2019, 18, 942–952. [Google Scholar] [CrossRef]
- Power, M.C.; Tingle, J.V.; Reid, R.I.; Huang, J.; Sharrett, A.R.; Coresh, J.; Griswold, M.; Kantarci, K.; Jack, C.R., Jr.; Knopman, D.; et al. Midlife and Late-Life Vascular Risk Factors and White Matter Microstructural Integrity: The Atherosclerosis Risk in Communities Neurocognitive Study. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Kwon, H.M.; Jeong, H.Y.; Park, J.H.; Kwon, H.; Jeong, S.M. Serum homocysteine level is related to cerebral small vessel disease in a healthy population. Neurology 2019, 92, e317–e325. [Google Scholar] [CrossRef]
- Hassan, A.; Hunt, B.J.; O’Sullivan, M.; Bell, R.; D’Souza, R.; Jeffery, S.; Bamford, J.M.; Markus, H.S. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain 2004, 127, 212–219. [Google Scholar] [CrossRef]
- Larsson, S.C.; Traylor, M.; Markus, H.S. Homocysteine and small vessel stroke: A mendelian randomization analysis. Ann. Neurol. 2019, 85, 495–501. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Valenzuela, M.J.; Brodaty, H.; Wang, X.L.; Looi, J.; Lorentz, L.; Howard, L.; Jones, M.; Zagami, A.S.; Gillies, D.; et al. Homocysteine as a risk factor for cognitive impairment in stroke patients. Dement. Geriatr. Cogn. Disord. 2003, 15, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Qu, Q.M. Hypermethylation of ERa-A gene and high serum homocysteine level are correlated with cognitive impairment in white matter hyperintensity patients. QJM 2019, 112, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, Y.; Wang, X.; Zhang, H.; Cui, Y.; Diao, Y.; Xiu, J.; Sun, X.; Jiang, G. Low carotid artery wall shear stress is independently associated with brain white-matter hyperintensities and cognitive impairment in older patients. Atherosclerosis 2016, 247, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Mutsaerts, H.J.; Palm-Meinders, I.H.; de Craen, A.J.; Reiber, J.H.; Blauw, G.J.; van Buchem, M.A.; van der Grond, J.; Box, F.M.; Group, P.S. Diastolic carotid artery wall shear stress is associated with cerebral infarcts and periventricular white matter lesions. Stroke 2011, 42, 3497–3501. [Google Scholar] [CrossRef]
- Kuriyama, N.; Mizuno, T.; Yasuike, H.; Matsuno, H.; Kawashita, E.; Tamura, A.; Ozaki, E.; Matsui, D.; Watanabe, I.; Koyama, T.; et al. CD62-mediated activation of platelets in cerebral white matter lesions in patients with cognitive decline. Arch. Gerontol. Geriatr. 2016, 62, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, S.; Hijazi, B.; Khalila, L.; Blum, A. Diagonal Earlobe Crease (Frank’s Sign): A Predictor of Cerebral Vascular Events. Am. J. Med. 2017, 130, 1324.e1321–1324.e1325. [Google Scholar] [CrossRef]
- Koyama, T.; Watanabe, H.; Ito, H. The association of circulating inflammatory and oxidative stress biomarker levels with diagonal earlobe crease in patients with atherosclerotic diseases. J. Cardiol. 2016, 67, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, S.; Kim, H.J.; Kim, Y.; Jang, H.; Kim, K.W.; Rhee, H.Y.; Yoon, S.S.; Hwang, K.J.; Park, K.C.; et al. Diagonal Earlobe Crease is a Visible Sign for Cerebral Small Vessel Disease and Amyloid-beta. Sci. Rep. 2017, 7, 13397. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, I.M.; Pajewski, N.M.; Auchus, A.P.; Chelune, G.; Cheung, A.K.; Cleveland, M.L.; Coker, L.H.; Crowe, M.G.; Cushman, W.C.; Cutler, J.A.; et al. Association of Intensive vs. Standard Blood Pressure Control with Cerebral White Matter Lesions. JAMA 2019, 322, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.D.; Pajewski, N.M.; Auchus, A.P.; Bryan, R.N.; Chelune, G.; Cheung, A.K.; Cleveland, M.L.; Coker, L.H.; Crowe, M.G.; Cushman, W.C.; et al. Effect of Intensive vs. Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019, 321, 553–561. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Wakefield, D.B.; Moscufo, N.; Guttmann, C.R.G.; Kaplan, R.F.; Bohannon, R.W.; Fellows, D.; Hall, C.B.; Wolfson, L. Effects of Intensive Versus Standard Ambulatory Blood Pressure Control on Cerebrovascular Outcomes in Older People (INFINITY). Circulation 2019, 140, 1626–1635. [Google Scholar] [CrossRef]
- Smith, E.E.; Barber, P.; Field, T.S.; Ganesh, A.; Hachinski, V.; Hogan, D.B.; Lanctôt, K.L.; Lindsay, M.P.; Sharma, M.; Swartz, R.H.; et al. Canadian Consensus Conference on Diagnosis and Treatment of Dementia (CCCDTD)5: Guidelines for management of vascular cognitive impairment. Alzheimer’s Dement. 2020, 6, e12056. [Google Scholar] [CrossRef]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef]
- Redel, J.M.; Dolan, L.M.; DiFrancesco, M.; Vannest, J.; Shah, A.S. Youth-Onset Type 2 Diabetes and the Developing Brain. Curr. Diabetes Rep. 2019, 19, 3. [Google Scholar] [CrossRef]
- Roberts, R.O.; Knopman, D.S.; Przybelski, S.A.; Mielke, M.M.; Kantarci, K.; Preboske, G.M.; Senjem, M.L.; Pankratz, V.S.; Geda, Y.E.; Boeve, B.F.; et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014, 82, 1132–1141. [Google Scholar] [CrossRef]
- Groeneveld, O.N.; Moneti, C.; Heinen, R.; de Bresser, J.; Kuijf, H.J.; Exalto, L.G.; Boomsma, J.M.; Kappelle, L.J.; Barkhof, F.; Prins, N.D. The clinical phenotype of vascular cognitive impairment in patients with Type 2 diabetes mellitus. J. Alzheimer’s Dis. 2019, 68, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Trompet, S.; van Vliet, P.; de Craen, A.J.; Jolles, J.; Buckley, B.M.; Murphy, M.B.; Ford, I.; Macfarlane, P.W.; Sattar, N.; Packard, C.J.; et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J. Neurol. 2010, 257, 85–90. [Google Scholar] [CrossRef]
- Collins, R.; Armitage, J.; Parish, S.; Sleight, P.; Peto, R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004, 363, 757–767. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chiu, M.J.; Chen, Y.F.; Cheng, T.W.; Lai, Y.M.; Chen, T.F. The contribution of vascular risk factors in neurodegenerative disorders: From mild cognitive impairment to Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 91. [Google Scholar] [CrossRef]
- Reiman, E.M.; Chen, K.; Langbaum, J.B.; Lee, W.; Reschke, C.; Bandy, D.; Alexander, G.E.; Caselli, R.J. Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer’s disease and normal aging. Neuroimage 2010, 49, 169–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Godin, O.; Tzourio, C.; Maillard, P.; Alperovitch, A.; Mazoyer, B.; Dufouil, C. Apolipoprotein E genotype is related to progression of white matter lesion load. Stroke 2009, 40, 3186–3190. [Google Scholar] [CrossRef]
- Kraut, M.A.; Beason-Held, L.L.; Elkins, W.D.; Resnick, S.M. The impact of magnetic resonance imaging-detected white matter hyperintensities on longitudinal changes in regional cerebral blood flow. J. Cereb. Blood Flow Metab. 2008, 28, 190–197. [Google Scholar] [CrossRef]
- Shu, Z.; Xu, Y.; Shao, Y.; Pang, P.; Gong, X. Radiomics from magnetic resonance imaging may be used to predict the progression of white matter hyperintensities and identify associated risk factors. Eur. Radiol. 2020, 30, 3046–3058. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Zhou, F.; Liang, H.; Xu, Y.; Lou, H. Hypertriglyceridemia Is Associated with Reduced Leukoaraiosis Severity in Patients with a Small Vessel Stroke. Behav. Neurol. 2018, 2018, 1361780. [Google Scholar] [CrossRef]
- Caniglia, E.C.; Rojas-Saunero, L.P.; Hilal, S.; Licher, S.; Logan, R.; Stricker, B.; Ikram, M.A.; Swanson, S.A. Emulating a target trial of statin use and risk of dementia using cohort data. Neurology 2020, 95, e1322–e1332. [Google Scholar] [CrossRef]
- Veronese, N.; Facchini, S.; Stubbs, B.; Luchini, C.; Solmi, M.; Manzato, E.; Sergi, G.; Maggi, S.; Cosco, T.; Fontana, L. Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 72, 87–94. [Google Scholar] [CrossRef]
- Dao, E.; Barha, C.K.; Best, J.R.; Hsiung, G.Y.; Tam, R.; Liu-Ambrose, T. The Effect of Aerobic Exercise on White Matter Hyperintensity Progression May Vary by Sex. Can. J. Aging 2019, 38, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Suo, C.; Singh, M.F.; Gates, N.; Wen, W.; Sachdev, P.; Brodaty, H.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Singh, N.; et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 2016, 21, 1645. [Google Scholar] [CrossRef] [PubMed]
- Bolandzadeh, N.; Tam, R.; Handy, T.C.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Dao, E.; Beattie, B.L.; Liu-Ambrose, T. Resistance Training and White Matter Lesion Progression in Older Women: Exploratory Analysis of a 12-Month Randomized Controlled Trial. J. Am. Geriatr. Soc. 2015, 63, 2052–2060. [Google Scholar] [CrossRef]
- Wong, A.; Mak, M.K.Y.; Lam, L.C.W.; Mok, V.C.T. Aerobic dance for cognitive and physical functions and mood in older adults with cerebral small vessel disease: Abridged secondary publication. Hong Kong Med. J. 2020, 26 (Suppl. S6), 38–41. [Google Scholar] [PubMed]
- Hsu, C.L.; Best, J.R.; Davis, J.C.; Nagamatsu, L.S.; Wang, S.; Boyd, L.A.; Hsiung, G.R.; Voss, M.W.; Eng, J.J.; Liu-Ambrose, T. Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment. Br. J. Sports Med. 2018, 52, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.; Best, J.R.; Davis, J.C.; Eng, J.J.; Lee, P.E.; Jacova, C.; Boyd, L.A.; Brasher, P.M.; Munkacsy, M.; Cheung, W.; et al. Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology 2016, 87, 2082–2090. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Debette, S.; Jokinen, H.; De Leeuw, F.-E.; Pantoni, L.; Chabriat, H.; Staals, J.; Doubal, F.; Rudilosso, S.; Eppinger, S. ESO Guideline on covert cerebral small vessel disease. Eur. Stroke J. 2021, 23969873211012132. [Google Scholar]
- Blanco-Rojas, L.; Arboix, A.; Canovas, D.; Grau-Olivares, M.; Oliva Morera, J.C.; Parra, O. Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: A comparative study. BMC Neurol. 2013, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Asmaro, K.; Meng, L.; Liu, Y.; Ma, C.; Xi, C.; Li, G.; Ren, C.; Luo, Y.; Ling, F.; et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012, 79, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Ding, Y.; Asmaro, K.; Brogan, D.; Meng, L.; Sui, M.; Shi, J.; Duan, Y.; Sun, Z.; Yu, Y.; et al. Ischemic Conditioning Is Safe and Effective for Octo- and Nonagenarians in Stroke Prevention and Treatment. Neurotherapeutics 2015, 12, 667–677. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, R.; Song, H.; Liu, G.; Hua, Y.; Cui, D.; Zheng, L.; Feng, W.; Liebeskind, D.S.; Fisher, M.; et al. Remote Ischemic Conditioning May Improve Outcomes of Patients with Cerebral Small-Vessel Disease. Stroke 2017, 48, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, X.; Guan, L.; Wang, Y. Role of White Matter Hyperintensities and Related Risk Factors in Vascular Cognitive Impairment: A Review. Biomolecules 2021, 11, 1102. https://doi.org/10.3390/biom11081102
Chen Y, Wang X, Guan L, Wang Y. Role of White Matter Hyperintensities and Related Risk Factors in Vascular Cognitive Impairment: A Review. Biomolecules. 2021; 11(8):1102. https://doi.org/10.3390/biom11081102
Chicago/Turabian StyleChen, Yiyi, Xing Wang, Ling Guan, and Yilong Wang. 2021. "Role of White Matter Hyperintensities and Related Risk Factors in Vascular Cognitive Impairment: A Review" Biomolecules 11, no. 8: 1102. https://doi.org/10.3390/biom11081102
APA StyleChen, Y., Wang, X., Guan, L., & Wang, Y. (2021). Role of White Matter Hyperintensities and Related Risk Factors in Vascular Cognitive Impairment: A Review. Biomolecules, 11(8), 1102. https://doi.org/10.3390/biom11081102





