Sirtuin 1, Visfatin and IL-27 Serum Levels of Type 1 Diabetic Females in Relation to Cardiovascular Parameters and Autoimmune Thyroid Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Data Collection
2.3. Echocardiography and Electrocardiography
2.4. Measurement of Carotid Intima-Media Thickness and Thyroid Assessment
2.5. Biochemical Analysis
2.6. Sirtuin 1, Visfatin, and IL-27 Measurements
2.7. Statistical Analysis
3. Results
3.1. Study Population
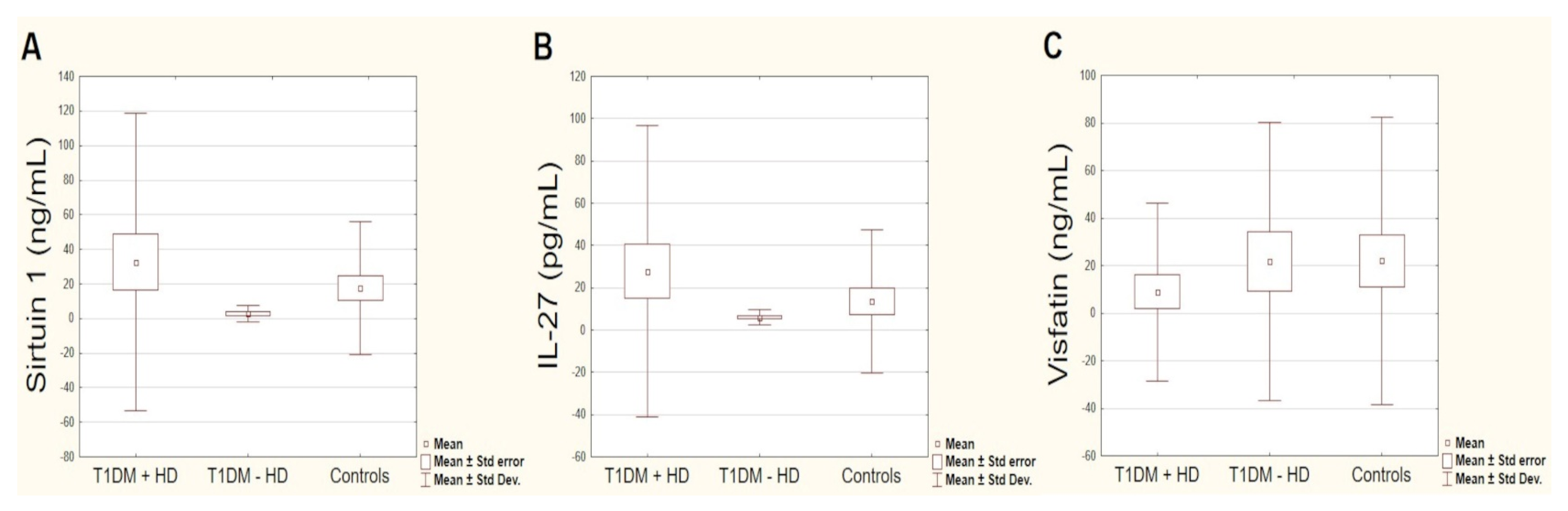
3.2. Circulating Sirtuin 1, IL-27 and Visfatin Levels
3.3. Correlation Analysis between the Circulating Markers and Other Studied Variables Using Spearman’s Correlation (r) (Supplementary Tables S1–S3)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef]
- Wang, Y.; O’Neil, A.; Jiao, Y.; Wang, L.; Huang, J.; Lan, Y.; Zhu, Y.; Yu, C. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Komorita, Y.; Peters, S.A.E.; Woodward, M. Diabetes as a risk factor for heart failure in women and men: A systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 2019, 62, 1550–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.-M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, E.D.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2019, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Zore, T.; Palafox, M.; Reue, K. Sex differences in obesity, lipid metabolism, and inflammation—A role for the sex chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef]
- Díaz, A.; Lopez-Grueso, R.; Gambini, J.; Monleón, D.; Mas-Bargues, C.; Abdelaziz, K.M.; Viña, J.; Borrás, C. Sex Differences in Age-Associated Type 2 Diabetes in Rats-Role of Estrogens and Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Razavi, A.C.; Potts, K.S.; Kelly, T.N.; Bazzano, L.A. Sex, gut microbiome, and cardiovascular disease risk. Biol. Sex Differ. 2019, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Madonna, R.; Balistreri, C.R.; Geng, Y.-J.; De Caterina, R. Diabetic microangiopathy: Pathogenetic insights and novel therapeutic approaches. Vasc. Pharmacol. 2017, 90, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sousa, G.; Pober, D.; Galderisi, A.; Lv, H.; Yu, L.; Pereira, A.C.; Doria, A.; Kosiborod, M.; Lipes, M.A. Glycemic Control, Cardiac Autoimmunity, and Long-Term Risk of Cardiovascular Disease in Type 1 Diabetes Mellitus. Circulation 2019, 139, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Głowinska-Olszewska, B.; Borysewicz-Sańczyk, H.; Sawicka, B.; Klonowska, B.; Charemska, D.; Żelazowska-Rutkowska, B.; Bossowski, A. Does Hashimoto’s Thyroiditis Increase the Risk of Cardiovascular Disease in Young Type 1 Diabetic Patients? Front. Endocrinol. 2020, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD + in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Dong, L.; Li, Y.; Liu, G. SIRT1 and HIF1α signaling in metabolism and immune responses. Cancer Lett. 2018, 418, 20–26. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.-G.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2016, 8, 1845–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of FOXOs and p53 by SIRT1 Modulators under Oxidative Stress. PLoS ONE 2013, 8, e73875. [Google Scholar] [CrossRef]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Wang, H.; Farmer, S.R. Adiponectin Secretion Is Regulated by SIRT1 and the Endoplasmic Reticulum Oxidoreductase Ero1-Lα. Mol. Cell. Biol. 2007, 27, 4698–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revollo, J.R.; Grimm, A.A.; Imai, S.-I. The NAD Biosynthesis Pathway Mediated by Nicotinamide Phosphoribosyltransferase Regulates Sir2 Activity in Mammalian Cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [Green Version]
- Brentano, F.; Schorr, O.; Ospelt, C.; Stanczyk, J.; Gay, R.E.; Gay, S.; Kyburz, D. Pre–B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007, 56, 2829–2839. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Zhang, Z.; Wang, J.; Yang, H.; Liu, G. Intercellular interplay between Sirt1 signalling and cell metabolism in immune cell biology. Immunology 2015, 145, 455–467. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Le, H.T.; Nguyen, Q.T.; Kim, S.; Lee, J.; Min, B. Cutting Edge: IL-27 Attenuates Autoimmune Neuroinflammation via Regulatory T Cell/Lag3–Dependent but IL-10–Independent Mechanisms In Vivo. J. Immunol. 2019, 202, 1680–1685. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.; Lim, H.; Choi, G.; Park, Y.-J.; Cho, M.; Na, H.; Ahn, C.W.; Kim, Y.C.; Kim, W.-U.; Lee, S.-H.; et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat. Immunol. 2018, 19, 583–593. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef]
- Rasha, F.; Mims, B.M.; Castro-Piedras, I.; Barnes, B.J.; Grisham, M.B.; Rahman, R.L.; Pruitt, K. The Versatility of Sirtuin-1 in Endocrinology and Immunology. Front. Cell Dev. Biol. 2020, 8, 589016. [Google Scholar] [CrossRef]
- Dludla, P.V.; Silvestri, S.; Orlando, P.; Gabuza, K.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Mokgalaboni, K.; Johnson, R.; Muller, C.J.F.; et al. Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-Associated Complications: A Systematic Review of Preclinical Studies. Nutrients 2020, 12, 739. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, J. Regulation and Immune Function of IL-27. Adv. Exp. Med. Biol. 2016, 941, 191–211. [Google Scholar] [CrossRef]
- Ciecko, A.; Foda, B.; Barr, J.Y.; Ramanathan, S.; Atkinson, M.A.; Serreze, D.V.; Geurts, A.M.; Lieberman, S.M.; Chen, Y.-G. Interleukin-27 Is Essential for Type 1 Diabetes Development and Sjögren Syndrome-like Inflammation. Cell Rep. 2019, 29, 3073–3086.e5. [Google Scholar] [CrossRef] [Green Version]
- Phan, W.-L.; Huang, Y.-T.; Ma, M.-C. Interleukin-27 Protects Cardiomyocyte-Like H9c2 Cells against Metabolic Syndrome: Role of STAT3 Signaling. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shahi, A.; Afzali, S.; Salehi, S.; Aslani, S.; Mahmoudi, M.; Jamshidi, A.; Amirzargar, A. IL-27 and autoimmune rheumatologic diseases: The good, the bad, and the ugly. Int. Immunopharmacol. 2020, 84, 106538. [Google Scholar] [CrossRef]
- Hui, X.; Zhang, M.; Gu, P.; Li, K.; Gao, Y.; Wu, D.; Wang, Y.; Xu, A. Adipocyte SIRT 1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue. EMBO Rep. 2017, 18, 645–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiroglou, S.G.; Kostopoulos, C.G.; Varakis, J.N.; Papadaki, H.H. Adipokines in periaortic and epicardial adipose tissue: Differential expression and relation to atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Dakroub, A.; Nasser, S.A.; Younis, N.; Bhagani, H.; Al-Dhaheri, Y.; Pintus, G.; Eid, A.A.; El-Yazbi, A.F.; Eid, A.H. Visfatin: A Possible Role in Cardiovasculo-Metabolic Disorders. Cells 2020, 9, 2444. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Marwick, T.H.; Gillebert, T.; Aurigemma, G.; Chirinos, J.; Derumeaux, G.; Galderisi, M.; Gottdiener, J.; Haluska, B.; Ofili, E.; Segers, P.; et al. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE)†. J. Am. Soc. Echocardiogr. 2015, 28, 727–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazett, H.C. An analysis of the time-relations of electrocardiogramS. Ann. Noninvasive Electrocardiol. 1997, 2, 177–194. [Google Scholar] [CrossRef]
- Elwertowski, M.; Małek, G. Standardy badań ultrasonograficznych Polskiego Towarzystwa Ultrasonograficznego–aktualizacja. Badanie Zewnątrzczaszkowych Odcinków Tętnic Szyjnych Oraz Kręgowych. J. Ultrason. 2014, 14, 179–191. [Google Scholar]
- Trzebińska, A.; Dobruch-Sobczak, K.; Jakubowski, W.; Jędrzejowski, M. Standardy badań ultrasonografi cznych Polskiego Towarzystwa Ultrasonografi cznego–aktualizacja. Badanie ultrasonografi czne tarczycy oraz biopsja tarczycy pod kontrolą ultrasonografi i. J. Ultrason. 2014, 14, 49–60. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Braffett, B.H.; Zinman, B.; Martin, C.; White, N.H.; Herman, W.H.; Genuth, S.; Gubitosi-Klug, R. The DCCT/EDIC Research Group Cardiovascular Autonomic Neuropathy and Cardiovascular Outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care 2016, 40, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Lachin, J.M.; Orchard, T.; Nathan, D.M. For the DCCT/EDIC Research Group Update on Cardiovascular Outcomes at 30 Years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2013, 37, 39–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.A.; Zhang, E.; Natarajan, R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 2015, 58, 443–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Lee, S.-M.; Gao, B.; Zhang, J.; Fang, D. Histone Deacetylase Sirtuin 1 Deacetylates IRF1 Protein and Programs Dendritic Cells to Control Th17 Protein Differentiation during Autoimmune Inflammation. J. Biol. Chem. 2013, 288, 37256–37266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, S.; Yu, X.; Shen, L. Autoimmune thyroid diseases and Th17/Treg lymphocytes. Life Sci. 2018, 192, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.-H.; Kurosh, K.; Zahra, A.; Hossein, D.M.; Davood, R.; Ataollahi, M.R. Decreased serum levels of IL-27and IL-35 in patients with Graves disease. Arch. Endocrinol. Metab. 2020, 64, 521–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.Y.; Kim, T.S. Targeting Cytokines of the Interleukin-12 Family in Autoimmunity. Curr. Med. Chem. 2006, 13, 1149–1156. [Google Scholar] [CrossRef]
- Phenekos, C.; Vryonidou, A.; Gritzapis, A.D.; Baxevanis, C.N.; Goula, M.; Papamichail, M. Th1 and Th2 Serum Cytokine Profiles Characterize Patients with Hashimoto’s Thyroiditis (Th1) and Graves’ Disease (Th2). Neuroimmunomodulation 2004, 11, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Meka, R.R.; Venkatesha, S.H.; Dudics, S.; Acharya, B.; Moudgil, K.D. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun. Rev. 2015, 14, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Wang, B.; Mu, K.; Zhang, J.; Yang, Y.; Yao, W.; Li, S.; Zhang, J.-A. Association of single-nucleotide polymorphisms in the IL27 gene with autoimmune thyroid diseases. Endocr. Connect. 2019, 8, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarumaru, M.; Watanabe, M.; Inoue, N.; Hisamoto, Y.; Morita, E.; Arakawa, Y.; Hidaka, Y.; Iwatani, Y. Association between functional SIRT1 polymorphisms and the clinical characteristics of patients with autoimmune thyroid disease. Autoimmunity 2016, 49, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Paulus, W.J. Clinical diabetic cardiomyopathy: A two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015, 36, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Gottumukkala, R.V.; Lv, H.; Cornivelli, L.; Wagers, A.J.; Kwong, R.Y.; Bronson, R.; Stewart, G.C.; Schulze, P.C.; Chutkow, W.; Wolpert, H.A.; et al. Myocardial Infarction Triggers Chronic Cardiac Autoimmunity in Type 1 Diabetes. Sci. Transl. Med. 2012, 4, 138ra80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Mikami, A.; Ogino, A.; Watanabe, T.; Morishita, K.; Okada, H.; Kawasaki, M.; et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 2015, 11, 1146–1160. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, W.H.; Sharma, M.; Cihakova, D.; Talor, M.V.; Rose, N.R.; Mohanakumar, T.; Passmore, G.G. Cardiac antibody production to self-antigens in children and adolescents during and following the correction of severe diabetic ketoacidosis. Autoimmunity 2016, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abaci, N.; Gok, O.; Karaali, Z.; Ergen, A.; Ekmekci, S.S. Serum sirtuin 1 protein as a potential biomarker for type 2 diabetes: Increased expression of sirtuin 1 and the correlation with microRNAs. J. Res. Med. Sci. 2019, 24, 56. [Google Scholar] [CrossRef]
- Rahimi, M.; Ghanbari, S.; Khazaei, M.; Niroman, E. Comparison of sirtuin 1 level and related blood factors in diabetic and healthy subjects. Pediatr. Endocrinol. Diabetes Metab. 2020, 26, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Fathy, S.A.; Ibrahim, D.M.; Elkhayat, W.A.; Ahmed, H.S. Association between serum sirt 1 and advanced glycation end products levels in type 2 diabetic nephropathy patients. Int. J. Biosci. 2017, 10, 398–404. [Google Scholar]
- Xu, W.; Deng, Y.-Y.; Yang, L.; Zhao, S.; Liu, J.; Zhao, Z.; Wang, L.; Maharjan, P.; Gao, S.; Tian, Y.; et al. Metformin ameliorates the proinflammatory state in patients with carotid artery atherosclerosis through sirtuin 1 induction. Transl. Res. 2015, 166, 451–458. [Google Scholar] [CrossRef]
- Packer, M. Cardioprotective Effects of Sirtuin-1 and Its Downstream Effectors: Potential Role in Mediating the Heart Failure Benefits of SGLT2 (Sodium-Glucose Cotransporter 2) Inhibitors. Circ. Heart Fail. 2020, 13, 007197. [Google Scholar] [CrossRef]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Wan, Y.-Z.; Liu, D.-P. Cross-talk between SIRT1 and p66Shc in vascular diseases. Trends Cardiovasc. Med. 2013, 23, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Liao, M.-T.; Hou, Y.-C.; Fang, Y.-W.; Zheng, C.-M.; Liu, W.-C.; Chao, C.-T.; Lu, K.-C.; Ng, Y.-Y. Sirtuin-1 and Its Relevance in Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prola, A.; DA Silva, J.P.; Guilbert, A.; Lecru, L.; Piquereau, J.; Ribeiro, M.; Mateo, P.; Gressette, M.; Fortin, D.; Boursier, C.; et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 2017, 24, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Lu, Q.; Yang, G.; Ma, A. Lin28a protects against postinfarction myocardial remodeling and dysfunction through Sirt1 activation and autophagy enhancement. Biochem. Biophys. Res. Commun. 2016, 479, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 Regulates Aging and Resistance to Oxidative Stress in the Heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Corbi, G.; Polito, M.V.; Ciccarelli, M.; Manzo, V.; Torsiello, M.; De Bellis, E.; D’Auria, F.; Vitulano, G.; Piscione, F.; et al. Sirt1 Activity in PBMCs as a Biomarker of Different Heart Failure Phenotypes. Biomolecules 2020, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, R.; Zeng, L.; Wei, H.; Chen, Y.; Zeng, J. IL-27 genetic variation and susceptibility of dilated cardiomyopathy in Chinese Han population. Pers. Med. 2017, 14, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Grufman, H.; Yndigegn, T.; Gonçalves, I.; Nilsson, J.; Schiopu, A. Elevated IL-27 in patients with acute coronary syndrome is associated with adverse ventricular remodeling and increased risk of recurrent myocardial infarction and cardiovascular death. Cytokine 2019, 122, 154208. [Google Scholar] [CrossRef]
- Kedenko, L.; Lamina, C.; Kedenko, I.; Kollerits, B.; Kiesslich, T.; Iglseder, B.; Kronenberg, F.; Paulweber, B. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med. Genet. 2014, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregersen, I.; Sandanger, Ø.; Askevold, E.T.; Sagen, E.L.; Yang, K.; Holm, S.; Pedersen, T.M.; Skjelland, M.; Krohg-Sørensen, K.; Hansen, T.V.; et al. Interleukin 27 is increased in carotid atherosclerosis and promotes NLRP3 inflammasome activation. PLoS ONE 2017, 12, e0188387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilge, M.; Yesilova, A.; Adas, M.; Helvaci, A. Neutrophil- and Platelet- to Lymphocyte Ratio in Patients with Euthyroid Hashimoto’s Thyroiditis. Exp. Clin. Endocrinol. Diabetes 2018, 127, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Y.; Dini, A.A.; Yang, X.-K.; Li, L.-J.; Wu, G.-C.; Leng, R.-X.; Pan, H.-F.; Ye, D.-Q. Association between serum/plasma adiponectin levels and immune-mediated diseases: A meta-analysis. Arch. Dermatol. Res. 2017, 309, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Di Florio, D.N.; Sin, J.; Coronado, M.J.; Atwal, P.S.; Fairweather, D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020, 31, 101482. [Google Scholar] [CrossRef]
- Rattanasopa, C.; Phungphong, S.; Wattanapermpool, J.; Bupha-Intr, T. Significant role of estrogen in maintaining cardiac mitochondrial functions. J. Steroid Biochem. Mol. Biol. 2015, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chang, D.-M.; Lin, K.-C.; Shin, S.-J.; Lee, Y.-J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef]
- Neubauer, K.; Bednarz-Misa, I.; Walecka-Zacharska, E.; Wierzbicki, J.; Agrawal, A.; Gamian, A.; Krzystek-Korpacka, M. Oversecretion and Overexpression of Nicotinamide Phosphoribosyltransferase/Pre-B Colony-Enhancing Factor/Visfatin in Inflammatory Bowel Disease Reflects the Disease Activity, Severity of Inflammatory Response and Hypoxia. Int. J. Mol. Sci. 2019, 20, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farghaly, H.S.; Metwalley, K.A.; Ahmed, F.-A.; Raafat, D.M.; El-Asheer, O.M.; Ali, A.M.; Bahdawy, A.; Zahran, A.M. Visfatin level in children and adolescents with autoimmune thyroiditis. Ther. Adv. Endocrinol. Metab. 2017, 8, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-J.; Wu, C.-S.; Lin, H.; Lee, I.-T.; Tseng, J.-J.; Chou, M.-M.; Sheu, W.H.-H. Visfatin-induced expression of inflammatory mediators in human endothelial cells through the NF-κB pathway. Int. J. Obes. 2009, 33, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, D.G.; Pleiner, J.; Francesconi, M.; Wiesinger, G.F.; Muüller, M.; Wolzt, M. Exercise Training Lowers Plasma Visfatin Concentrations in Patients with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 4702–4704. [Google Scholar] [CrossRef] [Green Version]
- López-Bermejo, A.; Recasens, M.; Casamitjana, R.; Ricart, W.; Chico-Julià, B.; Fernandez-Balsells, M.; Esteve, E.; Fernández-Real, J.M. Serum Visfatin Increases with Progressive -Cell Deterioration. Diabetes 2006, 55, 2871–2875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toruner, F.; Altinova, A.; Bukan, N.; Arslan, E.; Akbay, E.; Ersoy, R.; Arslan, M. Plasma Visfatin Concentrations in Subjects with Type 1 Diabetes Mellitus. Horm. Res. 2009, 72, 33–37. [Google Scholar] [CrossRef]
- El Samahi, M.H.; Ismail, N.; Matter, R.; Selim, A.; Ibrahim, A.; Nabih, W. Study of Visfatin Level in Type 1 Diabetic Children and Adolescents. Open Access Maced. J. Med. Sci. 2017, 5, 299–304. [Google Scholar] [CrossRef]
- Alexiadou, K.; Kokkinos, A.; Liatis, S.; Perrea, D.; Katsilambros, N.; Tentolouris, N. Differences in plasma apelin and visfatin levels between patients with type 1 diabetes mellitus and healthy subjects and response after acute hyperglycemia and insulin administration. Hormones 2012, 11, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawicka-Gutaj, N.; Zybek-Kocik, A.; Klimowicz, A.; Kloska, M.; Mańkowska-Wierzbicka, D.; Sowiński, J.; Ruchała, M. Circulating Visfatin in Hypothyroidism Is Associated with Free Thyroid Hormones and Antithyroperoxidase Antibodies. Int. J. Endocrinol. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tompa, A.; Åkesson, K.; Karlsson, S.; Faresjö, M. Suppressed immune profile in children with combined type 1 diabetes and celiac disease. Clin. Exp. Immunol. 2020, 201, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Hubert, S.; Rissiek, B.; Klages, K.; Huehn, J.; Sparwasser, T.; Haag, F.; Koch-Nolte, F.; Boyer, O.; Seman, M.; Adriouch, S. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2–P2X7 pathway. J. Exp. Med. 2010, 207, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, N.; Yasumizu, Y.; Kitagawa, Y.; Tanaka, A.; Nakamura, Y.; Motooka, D.; Nakamura, S.; Okada, Y.; Sakaguchi, S. Regulatory T Cell-Specific Epigenomic Region Variants Are a Key Determinant of Susceptibility to Common Autoimmune Diseases. Immunity 2020, 52, 1119–1132.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Ding, X.; Zhang, M.; He, M.; Zhao, Y.; Hu, S.; Zhao, F.; Wang, J.; Xie, B.; et al. The proportion of peripheral blood Tregs among the CD4+ T cells of autoimmune thyroid disease patients: A meta-analysis. Endocr. J. 2020, 67, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-H.; Dou, L.-Z.; Gu, C.; Wang, X.-Q. Plasma levels of omentin-1 and visfatin in senile patients with coronary heart disease and heart failure. Asian Pac. J. Trop. Med. 2014, 7, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Lu, N.; Ren, M.; Chen, H. Visfatin associated with major adverse cardiovascular events in patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2020, 20, 271. [Google Scholar] [CrossRef] [PubMed]
- Curat, C.A.; Wegner, V.; Sengenes, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumié, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, T.-Y.; Guan, Y.-F.; Su, D.-F.; Fan, G.-R.; Miao, C.-Y. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: Role of nicotinamide mononucleotide. Cardiovasc. Res. 2008, 81, 370–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malavazos, A.E.; Ermetici, F.; Cereda, E.; Coman, C.; Locati, M.; Morricone, L.; Corsi, M.M.; Ambrosi, B. Epicardial fat thickness: Relationship with plasma visfatin and plasminogen activator inhibitor-1 levels in visceral obesity. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 523–530. [Google Scholar] [CrossRef] [PubMed]


| T1DM (n = 50) | T1DM + HD (n = 28) | T1DM − HD (n = 22) | Controls (n = 30) | p (T1DM vs. Controls) | p (T1DM + HD vs. T1DM − HD) | |
|---|---|---|---|---|---|---|
| Age (years) | 26.2 ± 4.75 | 26.68 ± 4.64 | 25.59 ± 4.92 | 26.7 ± 3.72 | 0.548 | 0.363 |
| BMI (kg/m2) | 22.45 ± 3.14 | 22.78 ± 3.29 | 22.04 ± 2.95 | 22.53 ± 3.03 | 0.941 | 0.481 |
| Diabetes duration (years) | 13.04 ± 6.44 | 14.43 ± 7.20 | 11.27 ± 4.93 | 0.00 | 0.000 | 0.143 |
| Daily insulin dose (units) | 38.48 ± 18.86 | 39.85 ± 21.50 | 36.73 ± 15.15 | 0.00 | 0.000 | 0.837 |
| HD duration (years) | 4.08 ± 5.99 | 7.29 ± 6.40 | 0.00 | 0.00 | 0.000 | 0.000 |
| HbA1c (%) | 7.7 ± 1.33 | 8.03 ± 1.35 | 7.28 ± 1.20 | 5.18 ± 0.28 | 0.000 | 0.071 |
| Total cholesterol (mg/dL) | 173.06 ± 28.27 | 174.57 ± 27.97 | 171.14 ± 29.19 | 172.77 ± 29.23 | 0.917 | 0.647 |
| LDL (mg/dL) | 86.36 ± 25.91 | 87.75 ± 28.61 | 84.59 ± 22.52 | 83.93 ± 28.36 | 0.735 | 0.822 |
| HDL(mg/dL) | 70.66 ± 19.35 | 70.75 ± 18.73 | 70.55 ± 20.54 | 72.57 ± 21.10 | 0.713 | 0.718 |
| TG (mg/dL) | 81.14 ± 33.32 | 80 ± 34.96 | 82.59 ± 31.86 | 82.73 ± 30.36 | 0.651 | 0.777 |
| TSH (µIU/mL) | 2.26 ± 1.95 | 2.53 ± 2.45 | 1.90 ± 0.92 | 2.44 ± 1.15 | 0.127 | 0.718 |
| ft4 (ng/dL) | 1.25 ± 0.23 | 1.28 ± 0.26 | 1.22 ± 0.18 | 1.23 ± 0.17 | 0.608 | 0.200 |
| ft3 (pg/mL) | 2.97 ± 0.46 | 2.94 ± 0.42 | 3.01 ± 0.52 | 3.26 ± 0.53 | 0.007 | 0.992 |
| aTPO IU/mL | 102.51 ± 175.89 | 174.70 ± 209.32 | 10.64 ± 7.68 | 24.85 ± 65.16 | 0.000 | 0.000 |
| aTG IU/mL | 144.51 ± 167.58 | 225.89 ± 168.98 | 40.95 ± 93.53 | 80.88 ± 132.32 | 0.004 | 0.000 |
| Thyroid volume (mL) | 11.48 ± 3.55 | 11.38 ± 3.94 | 11.61 ± 3.08 | 11.52 ± 3.82 | 0.626 | 0.740 |
| EKG-HR | 72.86 ± 11.35 | 72.5 ± 10.56 | 73.32 ± 12.53 | 71.53 ± 10.16 | 0.673 | 0.992 |
| EKG-Qtc | 426.38 ± 19.51 | 427.71 ± 19.45 | 424.68 ± 19.91 | 421.27 ± 19.76 | 0.220 | 0.853 |
| cIMT (mm) | 0.66 ± 0.28 | 0.72 ± 0.34 | 0.58 ± 0.12 | 0.58 ± 0.10 | 0.046 | 0.018 |
| EF (%) | 62.27 ± 3.63 | 62.34 ± 3.40 | 62.19 ± 3.99 | 64.53 ± 2.03 | 0.001 | 0.399 |
| LAVI mL/m2 | 22.95 ± 5.3 | 21.51 ± 5.19 | 24.78 ± 4.97 | 24.29 ± 5.88 | 0.398 | 0.032 |
| LVEDd (mm) | 42.9 ± 4.16 | 42.11 ± 4.55 | 43.91 ± 3.45 | 44.57 ± 2.45 | 0.089 | 0.151 |
| IVSD (mm) | 8.56 ± 1.46 | 8.64 ± 1.68 | 8.45 ± 1.14 | 9 ± 1.31 | 0.114 | 0.992 |
| PWD (mm) | 8.92 ± 1.14 | 9.21 ± 1.20 | 8.55 ± 0.96 | 8.93 ± 0.98 | 0.858 | 0.082 |
| LVMI g/m2 | 69.02 ± 11.73 | 68.79 ± 11.76 | 69.32 ± 11.97 | 76.68 ± 10.16 | 0.010 | 0.792 |
| RWT | 0.42 ± 0.10 | 0.45 ± 0.12 | 0.39 ± 0.04 | 0.40 ± 0.04 | 0.592 | 0.040 |
| A wave (ms) | 58.01 ± 14.41 | 59.61 ± 12.16 | 55.98 ± 16.92 | 60.45 ± 15.72 | 0.499 | 0.324 |
| E’sep (cm/s) | 12.34 ± 2.21 | 12.11 ± 2.24 | 12.63 ± 2.17 | 13.20 ± 3.16 | 0.127 | 0.122 |
| DT (ms) | 215.72 ± 42.66 | 211.86 ± 41.78 | 220.63 ± 44.23 | 210.47 ± 40.70 | 0.945 | 0.384 |
| IVRT (ms) | 94.5 ± 13.14 | 96.61 ± 13.66 | 91.82 ± 12.21 | 79.87 ± 8.98 | 0.000 | 0.278 |
| LV GLS | 17.59 ± 1.97 | 16.90 ± 2.12 | 18.32 ± 1.53 | 18.96 ± 2.50 | 0.054 | 0.025 |
| Sirtuin 1 (ng/mL) | 19.51 ± 65.70 | 32.68 ± 86.06 | 2.76 ± 4.88 | 17.51 ± 38.39 | 0.106 | 0.278 |
| Visfatin (ng/mL) | 14.50 ± 47.82 | 8.87 ± 37.47 | 21.66 ± 58.61 | 22.06 ± 60.38 | 0.347 | 0.097 |
| IL-27 (pg/mL) | 18.23 ± 52.33 | 27.82 ± 68.87 | 6.05 ± 3.56 | 13.47 ± 33.88 | 0.622 | 0.132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukawska-Tatarczuk, M.; Franek, E.; Czupryniak, L.; Joniec-Maciejak, I.; Pawlak, A.; Wojnar, E.; Zieliński, J.; Mirowska-Guzel, D.; Mrozikiewicz-Rakowska, B. Sirtuin 1, Visfatin and IL-27 Serum Levels of Type 1 Diabetic Females in Relation to Cardiovascular Parameters and Autoimmune Thyroid Disease. Biomolecules 2021, 11, 1110. https://doi.org/10.3390/biom11081110
Łukawska-Tatarczuk M, Franek E, Czupryniak L, Joniec-Maciejak I, Pawlak A, Wojnar E, Zieliński J, Mirowska-Guzel D, Mrozikiewicz-Rakowska B. Sirtuin 1, Visfatin and IL-27 Serum Levels of Type 1 Diabetic Females in Relation to Cardiovascular Parameters and Autoimmune Thyroid Disease. Biomolecules. 2021; 11(8):1110. https://doi.org/10.3390/biom11081110
Chicago/Turabian StyleŁukawska-Tatarczuk, Magdalena, Edward Franek, Leszek Czupryniak, Ilona Joniec-Maciejak, Agnieszka Pawlak, Ewa Wojnar, Jakub Zieliński, Dagmara Mirowska-Guzel, and Beata Mrozikiewicz-Rakowska. 2021. "Sirtuin 1, Visfatin and IL-27 Serum Levels of Type 1 Diabetic Females in Relation to Cardiovascular Parameters and Autoimmune Thyroid Disease" Biomolecules 11, no. 8: 1110. https://doi.org/10.3390/biom11081110
APA StyleŁukawska-Tatarczuk, M., Franek, E., Czupryniak, L., Joniec-Maciejak, I., Pawlak, A., Wojnar, E., Zieliński, J., Mirowska-Guzel, D., & Mrozikiewicz-Rakowska, B. (2021). Sirtuin 1, Visfatin and IL-27 Serum Levels of Type 1 Diabetic Females in Relation to Cardiovascular Parameters and Autoimmune Thyroid Disease. Biomolecules, 11(8), 1110. https://doi.org/10.3390/biom11081110






