Chlorophyllides: Preparation, Purification, and Application
Abstract
:1. Definition
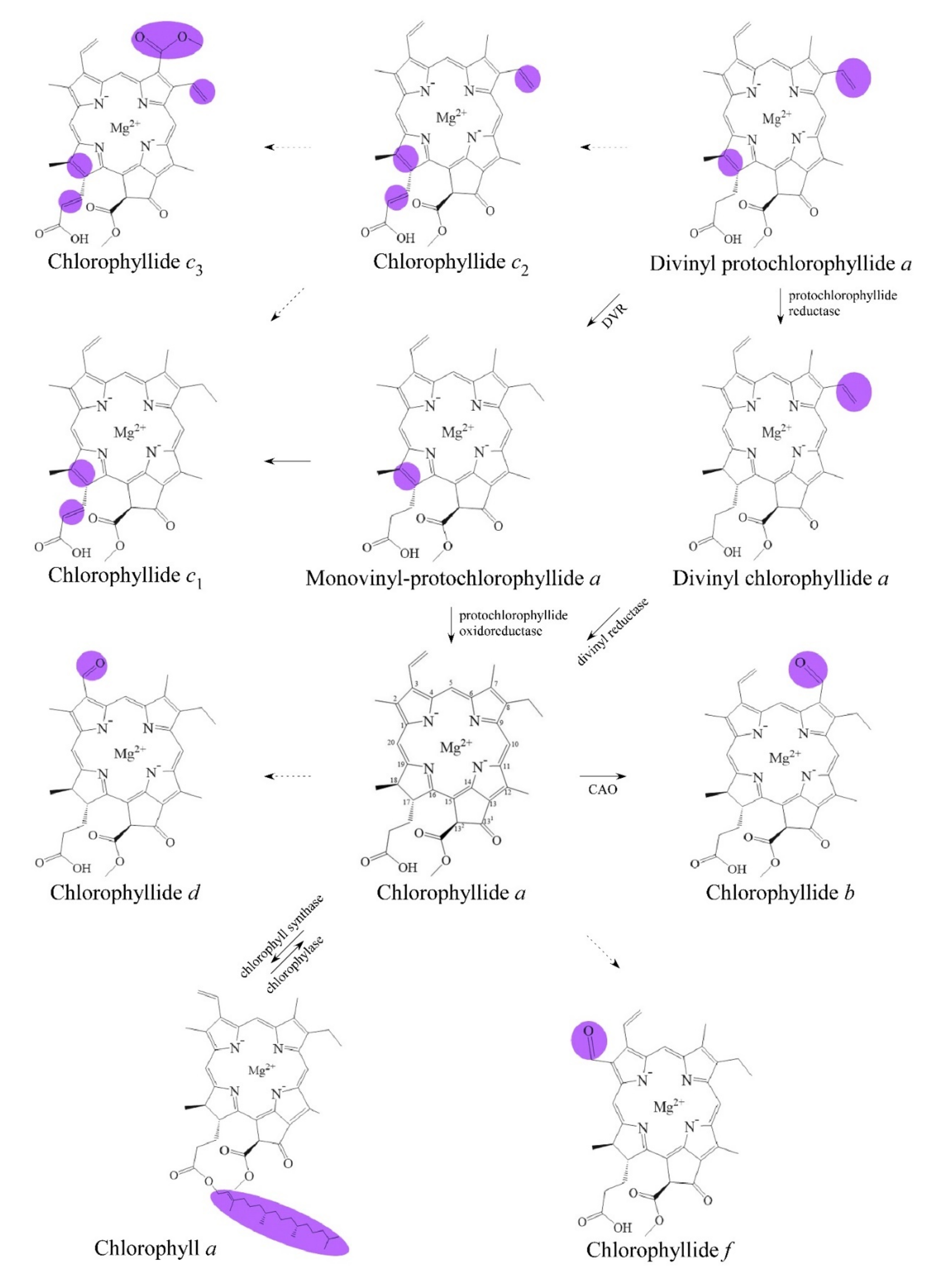
2. Biosynthetic Routes of Chlorophyllides
2.1. From Protochlorophyllide a to Chlorophyllide a
2.2. From Divinyl-Chlorophyllide a to Chlorophyllide a
2.3. From Chlorophyll to Chlorophyllide a by Chlorophyllase
3. Purification of Chlorophyllides
3.1. Purification Using Solvents
3.2. Purification Using Chromatography
4. Applications of Chlorophyllides
4.1. Various Biological Activities
4.2. Applications in Photoactivity
- New recombinant enzymes with high catalytic activity should be developed.
- Isolation and purification of chlorophyllides should be optimized.
- New preservation technology for chlorophyllides should be developed.
- The detailed mechanism of chlorophyllides interacting with other proteins or compounds should be investigated, which might help us to understand the activity of chlorophyllides (e.g., cytotoxicity or antiviral activity).
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| bp | base pairs |
| CAO | chlorophyllide a oxygenase |
| chlorophyllase | chlorophyll chlorophyllidohydrolase |
| DVR | 3,8-divinyl protochlorophyllide a 8-vinyl reductase |
| DPOR | dark-operative protochlorophyllide oxidoreductase |
| FDA | food and drug administration |
| FNR | ferredoxin–NADP+ oxidoreductase |
| GA3 | gibberellin-A3 |
| HPLC | high-performance liquid chromatography |
| kDa | kilodalton |
| LC | liquid chromatography |
| LPOR | light-operative protochlorophyllide oxidoreductase |
| protochlorophyllide a | monovinyl protochlorophyllide a |
| nm | nanometer |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| ppm | part per million |
| UV–Vis | ultraviolet–visible |
References
- Chen, M. Chlorophylls☆,1. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Bryant, D.A.; Hunter, C.N.; Warren, M.J. Biosynthesis of the modified tetrapyrroles-the pigments of life. J. Biol. Chem. 2020, 295, 6888–6925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, H.A.W. Chlorophyllides in green and etiolated leaves. Phytochemistry 1968, 7, 885–886. [Google Scholar] [CrossRef]
- Rise, M.; Goldschmidt, E.E. Occurrence of chlorpphyllides in developing, light grown leaves of several plant species. Plant Sci. 1990, 71, 147–151. [Google Scholar] [CrossRef]
- Hinterstoisser, B.; Missbichler, A.; Pineau, B.; Peschek, G.A. Detection of chlorophyllide in chlorophyll-free plasma membrane preparations from Anacystis nidulans. Biochem. Biophys. Res. Commun. 1988, 154, 839–846. [Google Scholar] [CrossRef]
- Bednarik, D.P.; Hoober, J.K. Biosynthesis of a chlorophyllide b-like pigment in phenanthroline-treated Chlamydomonas reinhardtii y-1. Arch. Biochem. Biophys. 1985, 240, 369–379. [Google Scholar] [CrossRef]
- Rebeiz, C.A.; Wu, S.M.; Kuhadja, M.; Daniell, H.; Perkins, E.J. Chlorophylla biosynthetic routes and chlorophylla chemical heterogeneity in plants. Mol. Cell. Biochem. 1983, 57, 97–125. [Google Scholar] [CrossRef]
- Lilijenberg, C. The effect of light on the phytolization of chlorophyllide α and the spectral dependence of the process. Physiol. Plant 1966, 19, 848–853. [Google Scholar] [CrossRef]
- Willstätter, R.; Stoll, A. Untersuchungen über Chlorophyll. XI. Über Chlorophyllase. Justus Liebigs Ann. Der Chem. 1911, 378, 18–72. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, L.; Stasiek, M.; Myśliwa-Kurdziel, B.; Strzałka, K. Phytol as one of the determinants of chlorophyll interactions in solution. Photosynth. Res. 2003, 78, 47–57. [Google Scholar] [CrossRef]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini. Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef] [Green Version]
- Ghebreamlak, S.M.; Mansoorabadi, S.O. Divergent Members of the Nitrogenase Superfamily: Tetrapyrrole Biosynthesis and Beyond. Chembiochem 2020, 21, 1723–1728. [Google Scholar] [CrossRef]
- Azizullah, A.; Rehman, Z.U.; Ali, I.; Murad, W.; Muhammad, N.; Ullah, W.; Häder, D.P. Chlorophyll derivatives can be an efficient weapon in the fight against dengue. Parasitol. Res. 2014, 113, 4321–4326. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green natural colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef] [Green Version]
- Orzeł, Ł.; Szmyd, B.; Rutkowska-Żbik, D.; Fiedor, L.; van Eldik, R.; Stochel, G. Fine tuning of copper(ii)–chlorophyll interactions in organic media. Metalation versus oxidation of the macrocycle. Dalton Trans. 2015, 44, 6012–6022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczygieł, M.; Urbańska, K.; Jurecka, P.; Stawoska, I.; Stochel, G.; Fiedor, L. Central metal determines pharmacokinetics of chlorophyll-derived xenobiotics. J. Med. Chem. 2008, 51, 4412–4418. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Huang, K.-S.; Wang, Y.-T.; Shaw, J.-F. A Review of Bacteriochlorophyllides: Chemical Structures and Applications. Molecules 2021, 26, 1293. [Google Scholar] [CrossRef] [PubMed]
- Willstätter, R.; Stoll, A. Untersuchungen Über Chlorophyll; Springer: Berlin/Heidelberg, Germany, 1913. [Google Scholar] [CrossRef]
- Holt, A.S.; Jacobs, E.E. Spectroscopy of plant pigments I. Ethyl chlorophyllide a and b and their pheophorbides. Am. J. Bot. 1954, 41, 710–717. [Google Scholar] [CrossRef]
- Duggan, J.X.; Rebeiz, C.A. Chloroplast biogenesis. 38. Quantitative detection of a chlorophyllide b pool in higher plants. Biochim. Biophys. Acta (BBA) Bioenerg. 1982, 679, 248–260. [Google Scholar] [CrossRef]
- Reinbothe, C.; Bartsch, S.; Eggink, L.L.; Hoober, J.K.; Brusslan, J.; Andrade-Paz, R.; Monnet, J.; Reinbothe, S. A role for chlorophyllide a oxygenase in the regulated import and stabilization of light-harvesting chlorophyll a/b proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 4777–4782. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Ito, H.; Tanaka, R.; Tanaka, N.K.; Yoshida, K.; Okada, K. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc. Natl. Acad. Sci. USA 1998, 95, 12719–12723. [Google Scholar] [CrossRef] [Green Version]
- Kotzabasis, K.; Senger, H. Evidence for the presence of chlorophyllide b in the green alga Scenedesmus obliquus in vivo. Bot. Acta 1989, 102, 173–177. [Google Scholar] [CrossRef]
- Wasley, J.W.; Scott, W.T.; Holt, A.S. Chlorophyllides c. Can. J. Biochem. 1970, 48, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-green algae with chlorphyllide C1, 2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Fawley, M.W. A new form of chlorophyll C involved in light-harvesting. Plant Physiol. 1989, 91, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, R.C.; Strain, H.H.; Svec, W.A.; Uphaus, R.A.; Katz, J.J. The structure, properties, and distribution of chlorophyll c. J. Am. Chem. Soc. 1970, 92, 2826–2833. [Google Scholar] [CrossRef] [PubMed]
- Fookes, C.J.R.; Jeffrey, S.W. The structure of chlorophyll c3, a novel marine photosynthetic pigment. J. Chem. Soc. Chem. Commun. 1989, 1827–1828. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Wright, S.W. A new spectrally distinct component in preparations of chlorophyll c from the micro-alga Emiliania huxleyi (Prymnesiophycease). Biochim. Biophys. Acta (BBA) Bioenerg. 1987, 894, 180–188. [Google Scholar] [CrossRef]
- Álvarez, S.; Zapata, M.; Garrido, J.L.; Vaz, B. Characterization of [8-ethyl]-chlorophyll c3 from Emiliania huxleyi. Chem. Commun. (Camb) 2012, 48, 5500–5502. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, S.; Rodríguez, F.; Riobó, P.; Garrido, J.L.; Vaz, B. Chlorophyll c(CS-170) isolated from Ostreococcus sp. is [7-methoxycarbonyl-8-vinyl]protochlorophyllide a. Org. Lett. 2013, 15, 4430–4433. [Google Scholar] [CrossRef]
- Büchel, C. Light harvesting complexes in chlorophyll c-containing algae. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148027. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Mizoguchi, T.; Akimoto, S.; Tomo, T.; Tamiaki, H.; Mimuro, M. Metabolic engineering of the Chl d-dominated cyanobacterium acaryochloris marina: Production of a novel chl species by the introduction of the chlorophyllide a oxygenase gene. Plant Cell Physiol. 2012, 53, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Fukusumi, T.; Matsuda, K.; Mizoguchi, T.; Miyatake, T.; Ito, S.; Ikeda, T.; Tamiaki, H.; Oba, T. Non-enzymatic conversion of chlorophyll-a into chlorophyll-d in vitro: A model oxidation pathway for chlorophyll-d biosynthesis. FEBS Lett. 2012, 586, 2338–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, A.S.; Morley, H.V. A proposed structure for chlorpohyll d. Can. J. Chem. 1959, 37, 507–514. [Google Scholar] [CrossRef]
- Larkum, A.W.; Kuhl, M. Chlorophyll d: The puzzle resolved. Trends Plant Sci. 2005, 10, 355–357. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.L.; Neilan, B.A.; Scheer, H. A red-shifted chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Birch, D.; Willows, R.D. A cyanobacterium that contains chlorophyll f--a red-absorbing photopigment. FEBS Lett. 2012, 586, 3249–3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamijala, S.R.; Periyasamy, G.; Pati, S.K. Computational studies on structural and excited-state properties of modified chlorophyll f with various axial ligands. J. Phys. Chem. A 2011, 115, 12298–12306. [Google Scholar] [CrossRef] [Green Version]
- Willows, R.D. The Mg Branch Chlorophyll Synthesis: Biosynthesis of Chlorophyll a from Protoporphyrin IX, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 90, p. 328. [Google Scholar]
- Tamiaki, H.; Teramura, M.; Tsukatani, Y. Reduction processes in biosynthesis of chlorophyll molecules: Chemical implication of enzymatically regio- and stereoselective hydrogenations in the late stages of their biosynthetic pathway. Bull. Chem. Soc. Jpn. 2016, 89, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Belyaeva, O.B.; Litvin, F.F. Mechanisms of phototransformation of protochlorophyllide into chlorophyllide. Biochemistry 2014, 79, 337–348. [Google Scholar] [CrossRef]
- Erdei, A.L.; Kósa, A.; Böddi, B. Distinct UV-A or UV-B irradiation induces protochlorophyllide photoreduction and bleaching in dark-grown pea (Pisum sativum L.) epicotyls. Photosynth. Res. 2019, 140, 93–102. [Google Scholar] [CrossRef]
- Weast, C.A.; Mackinney, G. Chlorophyllase. J. Biol. Chem. 1940, 133, 551–558. [Google Scholar] [CrossRef]
- Ardao, C.; Vennesland, B. Chlorophyllase activity of spinach chloroplastin. Plant Physiol. 1960, 35, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Holden, M. Chlorophylls; Academic Press: New York, NY, USA, 1976; Volume 2. [Google Scholar]
- Barrett, J.; Jeffrey, S.W. Chlorophyllase and formation of an atypical chlorophyllide in marine algae. Plant Physiol. 1964, 39, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Bacon, M.F.; Holden, M. Changes in chlorophylls resulting from various chemical and physical treatments of leaves and leaf extracts. Phytochemistry 1967, 6, 193–210. [Google Scholar] [CrossRef]
- McFeeters, R.F.; Chichester, C.O.; Whitaker, J.R. Purification and properties of chlorophyllase from Ailanthus altissima (Tree-of-Heaven). Plant Physiol. 1971, 47, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Jia, T.; Hörtensteiner, S.; Tanaka, A.; Tanaka, R. Subcellular localization of chlorophyllase2 reveals it is not involved in chlorophyll degradation during senescence in Arabidopsis thaliana. Plant Sci. 2020, 290, 110314. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Wilk, D.; Holland, D.; Goldschmidt, E.E.; Riov, J.; Eyal, Y. Chlorophyll breakdown by chlorophyllase: Isolation and functional expression of the Chlase1 gene from ethylene-treated Citrus fruit and its regulation during development. Plant J. Cell Mol. Biol. 1999, 20, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Ohta, H.; Okawa, K.; Iwamatsu, A.; Shimada, H.; Masuda, T.; Takamiya, K.-I. Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: Finding of a lipase motif and the induction by methyl jasmonate. Proc. Natl. Acad. Sci. USA 1999, 96, 15362–15367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.C.; Chepyshko, H.; Chen, H.H.; Chu, C.C.; Chou, Y.F.; Akoh, C.C.; Shaw, J.F. Genes and biochemical characterization of three novel chlorophyllase isozymes from Brassica oleracea. J. Agric. Food Chem. 2010, 58, 8651–8657. [Google Scholar] [CrossRef]
- Chou, Y.L.; Ko, C.Y.; Chen, L.F.; Yen, C.C.; Shaw, J.F. Purification and immobilization of the recombinant Brassica oleracea Chlorophyllase 1 (BoCLH1) on DIAION(R)CR11 as potential biocatalyst for the production of chlorophyllide and phytol. Molecules 2015, 20, 3744–3757. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.L.; Ko, C.Y.; Yen, C.C.; Chen, L.F.; Shaw, J.F. A novel recombinant chlorophyllase1 from Chlamydomonas reinhardtii for the production of chlorophyllide derivatives. J. Agric. Food Chem. 2015, 63, 9496–9503. [Google Scholar] [CrossRef]
- Chou, Y.L.; Lee, Y.L.; Yen, C.C.; Chen, L.F.; Lee, L.C.; Shaw, J.F. A novel recombinant chlorophyllase from cyanobacterium Cyanothece sp. ATCC 51142 for the production of bacteriochlorophyllide a. Biotechnol. Appl. Biochem. 2016, 63, 371–377. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Kalinin, A.A. Chapter Two—Quinoxaline Macrocycles. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 112, pp. 51–115. [Google Scholar]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Whyte, B.J.; Griffiths, W.T. 8-vinyl reduction and chlorophyll a biosynthesis in higher plants. Biochem. J. 1993, 291, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Hortensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Bogorad, L. Biosynthesis of Chlorophylls; Academic Press: Cambridge, MA, USA, 1976; Volume 1. [Google Scholar]
- Beale, S.I. Biosynthesis of Photosynthetic Pigments; Elsevier: Amsterdam, The Netherlands, 1984; Volume 5, pp. 134–205. [Google Scholar]
- Porra, R.J. Recent Progress in Porphyrin and Chlorophyll Biosynthesis. Photochem. Photobiol. 1997, 65, 492–516. [Google Scholar] [CrossRef]
- Goodwin, T.W. Chemistry and Biochemistry of Plant Pigments; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Ito, H.; Tanaka, A. Evolution of a new chlorophyll metabolic pathway driven by the dynamic changes in enzyme promiscuous activity. Plant Cell Physiol. 2014, 55, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Zapata, M.; Garrido, J.L.; Jeffrey, S.W. Chlorophyll c pigments: Current Status. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 39–53. [Google Scholar] [CrossRef]
- Swingley, W.D.; Chen, M.; Cheung, P.C.; Conrad, A.L.; Dejesa, L.C.; Hao, J.; Honchak, B.M.; Karbach, L.E.; Kurdoglu, A.; Lahiri, S.; et al. Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina. Proc. Natl. Acad. Sci. USA 2008, 105, 2005–2010. [Google Scholar] [CrossRef] [Green Version]
- Reinbothe, C.; Bakkouri, M.E.; Buhr, F.; Muraki, N.; Nomata, J.; Kurisu, G.; Fujita, Y.; Reinbothe, S. Chlorophyll biosynthesis: Spotlight on protochlorophyllide reduction. Trends Plant Sci. 2010, 15, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B. The protochlorophyllide-chlorophyllide cycle. Photosynth. Res. 2001, 70, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Koski, V.M. Chlorophyll formation in seedlings of Zea mays L. Arch. Biochem. 1950, 29, 339–343. [Google Scholar]
- Klein, S.; Bryan, G.; Bogorad, L. Early stages in the development of plastid fine structure in red and far-red light. J. Cell Biol. 1964, 22, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlasenok, L.I.; Fradkin, L.I.; Shlyk, A.A. Chlorophyllide as an intermediary in the conversion of protochlorophyllide into chlorophyllide a in green leaves. Photochem. Photobiol. 1965, 4, 385–389. [Google Scholar] [CrossRef]
- Sundqvist, C. The influence of varying light intensities on the photo-transformation of protochlorophyllide 636 in dark grown wheat leaves treated with δ-aminolevulinic acid. Physiol. Plant 1973, 29, 434–439. [Google Scholar] [CrossRef]
- Sironval, C. The Protochlorophyllide-Chlorophyllide Cycle as a Source of Photosyntheticaclly Active Chlorophylls; Balaban International Science Services: Philadelphia, PA, USA, 1981; Volume 5. [Google Scholar]
- Layer, G.; Krausze, J.; Moser, J. Reduction of chemically stable multibonds: Nitrogenase-like biosynthesis of tetrapyrroles. Adv. Exp. Med. Biol. 2017, 925, 147–161. [Google Scholar] [CrossRef]
- Garrone, A.; Archipowa, N.; Zipfel, P.F.; Hermann, G.; Dietzek, B. Plant Protochlorophyllide Oxidoreductases A and B: Catalytic efficiency and initial reacttion steps. J. Biol. Chem. 2015, 290, 28530–28539. [Google Scholar] [CrossRef] [Green Version]
- Dance, I. Computational Investigations of the Chemical Mechanism of the Enzyme Nitrogenase. Chembiochem 2020, 21, 1671–1709. [Google Scholar] [CrossRef]
- Gabruk, M.; Mysliwa-Kurdziel, B. The origin, evolution and diversification of multiple isoforms of light-dependent protochlorophyllide oxidoreductase (LPOR): Focus on angiosperms. Biochem. J. 2020, 477, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Schneidewind, J.; Krause, F.; Bocola, M.; Stadler, A.M.; Davari, M.D.; Schwaneberg, U.; Jaeger, K.E.; Krauss, U. Consensus model of a cyanobacterial light-dependent protochlorophyllide oxidoreductase in its pigment-free apo-form and photoactive ternary complex. Commun. Biol. 2019, 2, 351. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Heyes, D.J.; Feng, L.; Sun, W.; Johannissen, L.O.; Liu, H.; Levy, C.W.; Li, X.; Yang, J.; Yu, X.; et al. Structural basis for enzymatic photocatalysis in chlorophyll biosynthesis. Nature 2019, 574, 722–725. [Google Scholar] [CrossRef]
- Dong, C.S.; Zhang, W.L.; Wang, Q.; Li, Y.S.; Wang, X.; Zhang, M.; Liu, L. Crystal structures of cyanobacterial light-dependent protochlorophyllide oxidoreductase. Proc. Natl. Acad. Sci. USA 2020, 117, 8455–8461. [Google Scholar] [CrossRef] [PubMed]
- Nomata, J.; Kondo, T.; Mizoguchi, T.; Tamiaki, H.; Itoh, S.; Fujita, Y. Dark-operative protochlorophyllide oxidoreductase generates substrate radicals by an iron-sulphur cluster in bacteriochlorophyll biosynthesis. Sci. Rep. 2014, 4, 5455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomata, J.; Terauchi, K.; Fujita, Y. Stoichiometry of ATP hydrolysis and chlorophyllide formation of dark-operative protochlorophyllide oxidoreductase from Rhodobacter capsulatus. Biochem. Biophys. Res. Commun. 2016, 470, 704–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corless, E.I.; Bennett, B.; Antony, E. Substrate recognition induces sequential electron transfer across subunits in the nitrogenase-like DPOR complex. J. Biol. Chem. 2020, 295, 13630–13639. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Melo, A.A.; Kruk, J.; Frost, A.; Gabruk, M. Photocatalytic LPOR forms helical lattices that shape membranes for chlorophyll synthesis. Nat. Plants 2021, 7, 437–444. [Google Scholar] [CrossRef]
- Böddi, B.; Ryberg, M.; Sundqvist, C. Identification of four universal protochlorophyllide forms in dark-grown leaves by analyses of the 77 K fluorescence emission spectra. J. Photochem. Photobiol. B 1992, 12, 389–401. [Google Scholar] [CrossRef]
- Böuddi, B.; Ryberg, M.; Sundqvist, C. The formation of a short-wavelength chlorophyllide form at partial phototransformation of protochlorophyllide in isolated etioplast inner membranes. Photochem. Photobiol. 1991, 53, 667–673. [Google Scholar] [CrossRef]
- Böddi, B.; Kis-Petik, K.; Kaposi, A.D.; Fidy, J.; Sundqvist, C. The two spectroscopically different short wavelength protochlorophyllide forms in pea epicotyls are both monomeric. Biochim. Biophys. Acta 1998, 1365, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Koski, V.M.; Smith, J.H. The isolation and spectral absorption properties of protochlorophyll from etiolated barley seedlings. J. Am. Chem. Soc. 1948, 70, 3558–3562. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B.; Bertrand, M. The formation of chlorophyll from chlorophyllide in leaves containing proplastids is a four-step process. FEBS Lett. 2000, 486, 243–246. [Google Scholar] [CrossRef]
- Yahubyan, G.; Minkov, I.; Sundqvist, C. Carotenoid dependence of the protochlorophyllide to chlorophyllide phototransformation in dark-grown wheat seedlings. J. Photochem. Photobiol. B Biol. 2001, 65, 171–176. [Google Scholar] [CrossRef]
- Moulin, M.; McCormac, A.C.; Terry, M.J.; Smith, A.G. Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc. Natl. Acad. Sci. USA 2008, 105, 15178–15183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.W.; Yuan, S.; Feng, H.; Xu, F.; Cheng, J.; Shang, J.; Zhang, D.W.; Lin, H.H. Transient accumulation of Mg-protoporphyrin IX regulates expression of PhANGs—New evidence for the signaling role of tetrapyrroles in mature Arabidopsis plants. J. Plant Physiol. 2011, 168, 714–721. [Google Scholar] [CrossRef]
- Mochizuki, N.; Tanaka, R.; Tanaka, A.; Masuda, T.; Nagatani, A. The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 15184–15189. [Google Scholar] [CrossRef] [Green Version]
- Le Lay, P.; Böddi, B.; Kovacevic, D.; Juneau, P.; Dewez, D.; Popovic, R. Spectroscopic analysis of desiccation-induced alterations of the chlorophyllide transformation pathway in etiolated barley leaves. Plant Physiol. 2001, 127, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.W.; Li, M.X.; Huang, B.; Feng, L.Y.; Wu, F.; Fu, Y.F.; Zheng, X.J.; Peng, H.Q.; Chen, Y.E.; Yang, H.N.; et al. Nitric oxide regulates chlorophyllide biosynthesis and singlet oxygen generation differently between Arabidopsis and barley. Nitric Oxide 2018, 76, 6–15. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, Y.H.; Chao, P.Y.; Chen, C.M.; Hsieh, L.L.; Hu, S.P. Naturally occurring chlorophyll derivatives inhibit aflatoxin B1-DNA adduct formation in hepatoma cells. Mutat. Res. 2008, 657, 98–104. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yang, C.M.; Chen, C.M.; Chao, P.Y.; Hu, S.P. Effects of chlorophyll-related compounds on hydrogen peroxide induced DNA damage within human lymphocytes. J. Agric. Food Chem. 2005, 53, 2746–2750. [Google Scholar] [CrossRef] [PubMed]
- El-Saht, H.M. Effects of delta-aminolevulinic acid on pigment formation and chlorophyllase activity in French bean leaf. Acta Biol. Hung. 2000, 51, 83–90. [Google Scholar] [CrossRef]
- Harpaz-Saad, S.; Azoulay, T.; Arazi, T.; Ben-Yaakov, E.; Mett, A.; Shiboleth, Y.M.; Hörtensteiner, S.; Gidoni, D.; Gal-On, A.; Goldschmidt, E.E.; et al. Chlorophyllase is a rate-limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell 2007, 19, 1007–1022. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.E.; Hitchcock, A.; Mareš, J.; Gong, Y.; Tichý, M.; Pilný, J.; Kovářová, L.; Zdvihalová, B.; Xu, J.; Hunter, C.N.; et al. Evolution of Ycf54-independent chlorophyll biosynthesis in cyanobacteria. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Parham, R.; Rebeiz, C.A. Chloroplast biogenesis: [4-vinyl] chlorophyllide a reductase is a divinyl chlorophyllide a-specific, NADPH-dependent enzyme. Biochemistry 1992, 31, 8460–8464. [Google Scholar] [CrossRef]
- Nagata, N.; Tanaka, R.; Satoh, S.; Tanaka, A. Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. Plant Cell 2005, 17, 233–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, A.G.; Bryant, D.A. Characterization of a plant-like protochlorophyllide a divinyl reductase in green sulfur bacteria. J. Biol. Chem. 2007, 282, 2967–2975. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Aikawa, S.; Midorikawa, T.; Kashino, Y.; Satoh, K.; Koike, H. slr1923 of Synechocystis sp. PCC6803 Is Essential for Conversion of 3,8-Divinyl(proto)chlorophyll(ide) to 3-Monovinyl(proto)chlorophyll(ide). Plant Physiol 2008, 148, 1068–1081. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Yokono, M.; Tanaka, R.; Tanaka, A. Identification of a novel vinyl reductase gene essential for the biosynthesis of monovinyl chlorophyll in Synechocystis sp. PCC6803. J. Biol. Chem. 2008, 283, 9002–9011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Gao, J.; Wan, C.; Zhang, F.; Xu, Z.; Huang, X.; Sun, X.; Deng, X. Divinyl chlorophyll(ide) a can be converted to monovinyl chlorophyll(ide) a by a divinyl reductase in rice. Plant Physiol. 2010, 153, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wan, C.; Xu, Z.; Wang, P.; Wang, W.; Sun, C.; Ma, X.; Xiao, Y.; Zhu, J.; Gao, X.; et al. One divinyl reductase reduces the 8-vinyl groups in various intermediates of chlorophyll biosynthesis in a given higher plant species, but the isozyme differs between species. Plant Physiol. 2013, 161, 521–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.E.; Hitchcock, A.; Jackson, P.J.; Chaudhuri, R.R.; Dickman, M.J.; Hunter, C.N.; Canniffe, D.P.; Mullineaux, C.W. Two unrelated 8-vinyl reductases ensure production of mature chlorophylls in Acaryochloris marina. J. Bacteriol. 2016, 198, 1393–1400. [Google Scholar] [CrossRef] [Green Version]
- Azai, C.; Kobayashi, M.; Mizoguchi, T.; Tamiaki, H.; Terauchi, K.; Tsukatani, Y. Rapid C8-vinyl reduction of divinyl-chlorophyllide a by BciA from Rhodobacter capsulatus. J. Photochem. Photobiol. A 2018, 353, 661–666. [Google Scholar] [CrossRef]
- Nakanishi, H.; Nozue, H.; Suzuki, K.; Kaneko, Y.; Taguchi, G.; Hayashida, N. Characterization of the Arabidopsis thaliana mutant pcb2 which accumulates divinyl chlorophylls. Plant Cell Physiol. 2005, 46, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Bryant, D.A. Multiple types of 8-vinyl reductases for (bacterio)chlorophyll biosynthesis occur in many green sulfur bacteria. J. Bacteriol. 2011, 193, 4996–4998. [Google Scholar] [CrossRef] [Green Version]
- Bryant, D.A.; Liu, Z.; Li, T.; Zhao, F.; Costas, A.M.G.; Klatt, C.G.; Ward, D.M.; Frigaard, N.-U.; Overmann, J. Comparative and functional genomics of anoxygenic green bacteria from the taxa Chlorobi, Chloroflexi, and Acidobacteria. In Functional Genomics and Evolution of Photosynthetic Systems; Springer: Berlin/Heidelberg, Germany, 2012; pp. 47–102. [Google Scholar]
- Canniffe, D.P.; Chidgey, J.W.; Hunter, C.N. Elucidation of the preferred routes of C8-vinyl reduction in chlorophyll and bacteriochlorophyll biosynthesis. Biochem. J. 2014, 462, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, A.H.; Golbeck, J.H.; Bryant, D.A. Characterization of BciB: A ferredoxin-dependent 8-vinyl-protochlorophyllide reductase from the green sulfur bacterium Chloroherpeton thalassium. Biochemistry 2013, 52, 8442–8451. [Google Scholar] [CrossRef] [PubMed]
- Teramura, M.; Tamiaki, H. Semi-synthesis and HPLC analysis of (bacterio)chlorophyllides possessing a propionic acid residue at the C17-position. J. Porphyr. Phthalocyanines 2018, 22, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Ellsworth, R. Studies on chlorophyllase. I. Hydrolytic and esterification activities of chlorophyllase from wheat seedlings. Photosynthetica 1971, 5, 226–232. [Google Scholar]
- Ellsworth, R. Studies on chlorophyllase. II. The effects of changes in reaction conditions on hydrolytic and esterification activities observed in preparations from wheat seedlings. Photosynthetica 1972, 6, 32–40. [Google Scholar]
- Ellsworth, R.; Tsuk, R.; ST Pierre, L. Studies on chlorophyllase. IV. Attribution of hydrolytic and esterifying" chlorophyllase" activities observed in vitro to two enzymes. Photosynthetica 1976, 10, 312–323. [Google Scholar]
- Liljenberg, C. Characterization and properties of a protochlorophyllide ester in leaves of dark grown barley with geranylgeraniol as esterifying alcohol. Physiol. Plant 1974, 32, 208–213. [Google Scholar] [CrossRef]
- Benz, J.; Wolf, C.; Rüdiger, W. Chlorophyll biosynthesis: Hydrogenation of geranylgeraniol. Plant Sci. Lett. 1980, 19, 225–230. [Google Scholar] [CrossRef]
- Wellburn, A.R. Studies on the esterification of chlorophyllides. Phytochemistry 1970, 9, 2311–2313. [Google Scholar] [CrossRef]
- Klein, A.O.; Vishniac, W. Activity and partial purification of chlorophyllase in aqueous systems. J. Biol. Chem. 1961, 236, 2544–2547. [Google Scholar] [CrossRef]
- Rüdiger, W.; Böhm, S.; Helfrich, M.; Schulz, S.; Schoch, S. Enzymes of the last steps of chlorophyll biosynthesis: Modification of the substrate structure helps to understand the topology of the active centers. Biochemistry 2005, 44, 10864–10872. [Google Scholar] [CrossRef]
- McFeeters, R.F. Substrate specificity of chlorophyllase. Plant Physiol 1975, 55, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Minguez-Mosquera, M.I.; Gallardo-Guerrero, L. Role of chlorophyllase in chlorophyll metabolism in olives cv. Gordal. Phytochemistry 1996, 41, 691–697. [Google Scholar] [CrossRef]
- Mayer, H. Untersuchungen über die Chlorophyllase. Planta 1930, 11, 294–330. [Google Scholar] [CrossRef]
- Gu, S.; Dai, X.; Xu, Z.; Niu, Q.; Jiang, J.; Liu, Y. Molecular, structural and biochemical characterization of a novel recombinant chlorophyllase from cyanobacterium Oscillatoria acuminata PCC 6304. Microb. Cell Fact. 2021, 20, 14. [Google Scholar] [CrossRef]
- Moll, W.A.; de Wit, B.; Lutter, R. Chlorophyllase activity in developing leaves of Phaseolus vulgaris L. Planta 1978, 139, 79–83. [Google Scholar] [CrossRef]
- Drazkiewicz, M. Chlorophyllase: Occurrence, functions, mechanism of action, effects of external and internal factors. Photosynthetica 1994, 30, 321–331. [Google Scholar]
- Peschek, G.A.; Hinterstoisser, B.; Wastyn, M.; Kuntner, O.; Pineau, B.; Missbichler, A.; Lang, J. Chlorophyll precursors in the plasma membrane of a cyanobacterium, Anacystis nidulans. J. Biol. Chem. 1989, 264, 11827–11832. [Google Scholar] [CrossRef]
- Müller, A.H.; Gough, S.P.; Bollivar, D.W.; Meldal, M.; Willows, R.D.; Hansson, M. Methods for the preparation of chlorophyllide a: An intermediate of the chlorophyll biosynthetic pathway. Anal. Biochem. 2011, 419, 271–276. [Google Scholar] [CrossRef]
- Araki, S.; Oohusa, T.; Omata, T.; Murata, N. Column chromatographic separation of chlorophyllide α and pheophorbide α. Plant Cell Physiol. 1984, 25, 841–843. [Google Scholar] [CrossRef]
- Hanamoto, C.M.; Castelfranco, P.A. Separation of monovinyl and divinyl protochlorophyllides and chlorophyllides from etiolated and phototransformed cucumber cotyledons. Plant Physiol. 1983, 73, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Mantoura, R.F.C.; Llewellyn, C.A. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Anal. Chim. Acta 1983, 151, 297–314. [Google Scholar] [CrossRef]
- Shioi, Y.; Beale, S.I. Polyethylene-based high-performance liquid chromatography of chloroplast pigments: Resolution of mono- and divinyl chlorophyllides and other pigment mixtures. Anal. Biochem. 1987, 162, 493–499. [Google Scholar] [CrossRef]
- Zapata, M.; Ayala, A.M.; Franco, J.M.; Garrido, J.L. Separation of chlorophylls and their degradation products in marine phytoplankton by reversed-phase high-performance liquid chromatography. Chromatographia 1987, 23, 26–30. [Google Scholar] [CrossRef]
- Schoefs, B.; Bertrand, M.; Lemoine, Y. Separation of photosynthetic pigments and their precursors by reversed-phase high-performance liquid chromatography using a photodiode-array detector. J. Chromatogr. A 1995, 692, 239–245. [Google Scholar] [CrossRef]
- Darko, E.; Schoefs, B.t.; Lemoine, Y. Improved liquid chromatographic method for the analysis of photosynthetic pigments of higher plants. J. Chromatogr. A 2000, 876, 111–116. [Google Scholar] [CrossRef]
- Garrido, J.L.; Rodríguez, F.; Campaña, E.; Zapata, M. Rapid separation of chlorophylls a and b and their demetallated and dephytylated derivatives using a monolithic silica C18 column and a pyridine-containing mobile phase. J. Chromatogr. A 2003, 994, 85–92. [Google Scholar] [CrossRef]
- Kruk, J.; Myśliwa-Kurdziel, B. Separation of Monovinyl and Divinyl Protochlorophyllides Using C30 Reverse Phase High Performance Liquid Chromatography Column: Analytical and Preparative Applications. Chromatographia 2004, 60, 117–123. [Google Scholar] [CrossRef]
- Loh, C.H.; Inbaraj, B.S.; Liu, M.H.; Chen, B.H. Determination of chlorophylls in Taraxacum formosanum by high-performance liquid chromatography-diode array detection-mass spectrometry and preparation by column chromatography. J. Agric. Food Chem. 2012, 60, 6108–6115. [Google Scholar] [CrossRef]
- Chen, K.; Ríos, J.J.; Roca, M.; Pérez-Gálvez, A. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (II) Dephytylated derivatives. J. Chromatogr. A 2015, 1412, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senge, M.; Ryan, A.; Letchford, K.; Macgowan, S.; Mielke, T. Chlorophylls, Symmetry, Chirality, and Photosynthesis. Symmetry 2014, 6, 781–843. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Oba, T.; Tamiaki, H.; Watanabe, T. Effect of C132-Stereochemistry on the Molecular Properties of Chlorophylls. Bull. Chem. Soc. Jpn. 2000, 73, 1341–1351. [Google Scholar] [CrossRef]
- Ogasawara, S.; Egami, Y.; Hirose, M.; Tamiaki, H. Synthesis of chlorophyll-a homologs by C132-substitutions and their physico- and biochemical properties. Bioorg. Chem. 2020, 94, 103383. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Del Pozo, M.; Gallardo-Guerrero, L.; Gandul-Rojas, B. Influence of alkaline treatment on structural modifications of chlorophyll pigments in NaOH-treated table olives preserved without fermentation. Foods 2020, 9, 701. [Google Scholar] [CrossRef]
- Hynninen, P.H.; Hyvärinen, K. Tracing the allomerization pathways of chlorophylls by (18)O-labeling and mass spectrometry. J. Org. Chem. 2002, 67, 4055–4061. [Google Scholar] [CrossRef]
- Hynninen, P.H. Mechanism of the Allomerization of Chlorophyll: Inhibition of the Allomerization by Carotenoid Pigments. Z. Für Nat. B 1981, 36, 1010–1016. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Gallardo-Guerrero, L. Pigment changes during processing of green table olive specialities treated with alkali and without fermentation. Food Res. Int. 2014, 65, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Gandul-Rojas, B.; Gallardo-Guerrero, L. Pigment changes during preservation of green table olive specialities treated with alkali and without fermentation: Effect of thermal treatments and storage conditions. Food Res. Int. 2018, 108, 57–67. [Google Scholar] [CrossRef]
- Hayes, M.; Ferruzzi, M.G. Update on the bioavailability and chemopreventative mechanisms of dietary chlorophyll derivatives. Nutr. Res. 2020, 81, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Yeh, T.H.; Huang, M.Y.; Hu, S.P.; Chao, P.Y.; Yang, C.M. Organ-specific distribution of chlorophyll-related compounds from dietary spinach in rabbits. Indian J. Biochem. Biophys 2014, 51, 388–395. [Google Scholar]
- Gandul-Rojas, B.; Gallardo-Guerrero, L.; Mínguez-Mosquera, M.I. Influence of the Chlorophyll Pigment Structure on Its Transfer from an Oily Food Matrix to Intestinal Epithelium Cells. J. Agric. Food Chem. 2009, 57, 5306–5314. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Roca, M. In vitro bioavailability of chlorophyll pigments from edible seaweeds. J. Funct. Foods 2018, 41, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Bird, A.; Kopec, R.E. The metabolism and potential bioactivity of chlorophyll and metallo-chlorophyll derivatives in the gastrointestinal tract. Mol. Nutr. Food Res. 2021, 65, 2000761. [Google Scholar] [CrossRef]
- Blaauw-Jansen, G. Chlorophyllide, the probable precursor of a growth inhibitor. Nature 1954, 174, 312–313. [Google Scholar] [CrossRef]
- Hayashita, K. Red fluorescent protein in the digestive juice of the silkworm larvae fed on host-plant mulberry leaves. Entomol. Exp. Appl. 1978, 24, 428–436. [Google Scholar] [CrossRef]
- Pandian, G.N.; Ishikawa, T.; Togashi, M.; Shitomi, Y.; Haginoya, K.; Yamamoto, S.; Nishiumi, T.; Hori, H. Bombyx mori midgut membrane protein P252, which binds to Bacillus thuringiensis Cry1A, is a chlorophyllide-binding protein, and the resulting complex has antimicrobial activity. Appl. Environ. Microbiol. 2008, 74, 1324–1331. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yang, R.; Hu, X.L.; Xiang, X.W.; Wu, X.F. Expression analysis of chlorophyllid α binding protein, a secretory, red fluorescence protein in the midgut of silkworm, Bombyx mori. Insect Sci. 2014, 21, 20–30. [Google Scholar] [CrossRef]
- Manjunatha, G.K.S.; Peter, A.; Naika, M.B.N.; Niranjana, P.; Shamprasad, P. Identification of in-vitro red fluorescent protein with antipathogenic activity from the midgut of the silkworm (Bombyx Mori L.). Protein Pept. Lett. 2018, 25, 302–313. [Google Scholar] [CrossRef]
- Hu, X.; Makita, S.; Schelbert, S.; Sano, S.; Ochiai, M.; Tsuchiya, T.; Hasegawa, S.F.; Hörtensteiner, S.; Tanaka, A.; Tanaka, R. Reexamination of Chlorophyllase Function Implies Its Involvement in Defense against Chewing Herbivores. Plant Physiol. 2015, 167, 660–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiyama, Y.; Ishizuka, Y.; Terauchi, I.; Matsuda, T.; Maeda, Y.; Yoshino, T.; Matsumoto, M.; Yabuki, A.; Bowler, C.; Tanaka, T. Engineered chlorophyll catabolism conferring predator resistance for microalgal biomass production. Metab. Eng. 2021, 66, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, J.; Mills, C.; Mao, R.; Goddard, C.; Cai, D.; Guo, H.; Cuconati, A.; Block, T.; Lu, X. Screening and identification of compounds with antiviral activity against hepatitis B virus using a safe compound library and novel real-time immune-absorbance PCR-based high throughput system. Antivir. Res. 2013, 98, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Pan, X.; Mao, R.; Zhang, X.; Wang, L.; Lu, X.; Chang, J.; Guo, J.T.; Passic, S.; Krebs, F.C.; et al. Alkylated porphyrins have broad antiviral activity against hepadnaviruses, flaviviruses, filoviruses, and arenaviruses. Antimicrob. Agents Chemother. 2011, 55, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Ou, H.C.; Santos, F.; Raible, D.W.; Simon, J.A.; Rubel, E.W. Drug screening for hearing loss: Using the zebrafish lateral line to screen for drugs that prevent and cause hearing loss. Drug Discov. Today 2010, 15, 265–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musdal, Y.; Hegazy, U.M.; Aksoy, Y.; Mannervik, B. FDA-approved drugs and other compounds tested as inhibitors of human glutathione transferase P1-1. Chem. Biol. Interact. 2013, 205, 53–62. [Google Scholar] [CrossRef]
- Kariola, T.; Brader, G.; Li, J.; Palva, E.T. Chlorophyllase 1, a Damage Control Enzyme, Affects the Balance between Defense Pathways in Plants. Plant Cell 2005, 17, 282–294. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Souid, S.; Elsayed, H.E.; Ebrahim, H.Y.; Mohyeldin, M.M.; Siddique, A.B.; Karoui, H.; El Sayed, K.A.; Essafi-Benkhadir, K. 131 -Oxophorbine protopheophorbide A from Ziziphus lotus as a novel mesenchymal-epithelial transition factor receptor inhibitory lead for the control of breast tumor growth in vitro and in vivo. Mol. Carcinog. 2018, 57, 1507–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Yang, C.H.; Huang, T.Y.; Tai, M.H.; Sie, R.H.; Shaw, J.F. Cytotoxic efects of chlorophyllides in ethanol crude extracts from plant leaves. Evid Based Complement. Altern. Med. 2019, 2019, 9494328. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, Y.P.; Wang, Y.T.; Huang, K.S.; Huang, T.Y.; Tai, M.H.; Lin, Y.M.; Yang, C.H.; Shaw, J.F. Facile Production of Chlorophyllides using Recombinant CrCLH1 and their Cytotoxicity towards Multidrug Resistant Breast Cancer Cell Lines. PLoS ONE 2021. [Google Scholar] [CrossRef] [PubMed]
- Tapper, B.A.; Lohrey, E.; Hove, E.L.; Allison, R.M. Photosensitivity from chlorophyll-derived pigments. J. Sci. Food Agric. 1975, 26, 277–284. [Google Scholar] [CrossRef]
- Gerola, A.P.; Santana, A.; França, P.B.; Tsubone, T.M.; de Oliveira, H.P.M.; Caetano, W.; Kimura, E.; Hioka, N. Effects of metal and the phytyl chain on chlorophyll derivatives: Physicochemical evaluation for photodynamic inactivation of microorganisms. Photochem. Photobiol. 2011, 87, 884–894. [Google Scholar] [CrossRef]
- Gerola, A.P.; Costa, P.F.A.; de Morais, F.A.P.; Tsubone, T.M.; Caleare, A.O.; Nakamura, C.V.; Brunaldi, K.; Caetano, W.; Kimura, E.; Hioka, N. Liposome and polymeric micelle-based delivery systems for chlorophylls: Photodamage effects on Staphylococcus aureus. Colloids Surf. B Biointerfaces 2019, 177, 487–495. [Google Scholar] [CrossRef]
- Tadini-Buoninsegni, F.; Moncelli, M.; Aloisi, G.; Agostiano, A.; Guidelli, R. Photoinduced electroreduction of chlorophyllide on alkanethiol-coated mercury. J. Am. Chem. Soc. 2005, 127, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Carballo, R.R.; Rezzano, I.N. Polyallylamine-chlorophyllide derivatized gold and silver nanoparticles as optical probes for sensor applications. Sens. Actuators B Chem. 2010, 145, 250–253. [Google Scholar] [CrossRef]
- Hamer, M.; Lázaro-Martínez, J.M.; Rezzano, I.N. Fluorescent responsive chlorophyllide-hydrogel for carbon dioxide detection. Sens. Actuators B Chem. 2016, 237, 905–911. [Google Scholar] [CrossRef]
- Sobral, P.J.A.; Carvalho, R.A.; Fávaro-Trindade, C.S. Physical properties of edible gelatin films colored with chlorophyllide. In Food Engineering Interfaces; Aguilera, J., Simpson, R., Welti-Chanes, J., Bermudez-Aguirre, D.G.B.-C., Eds.; Springer: New York, NY, USA, 2011; pp. 661–678. [Google Scholar]
- Tostado-Plascencia, M.M.; Sanchez-Tizapa, M.; Zamudio-Ojeda, A. Synthesis and characterization of multiwalled carbon nanotubes functionalized with chlorophyll-derivatives compounds extracted from Hibiscus tiliaceus. Diam. Relat. Mater. 2018, 89, 151–162. [Google Scholar] [CrossRef]
| Name | Chlorophyllide a | Divinyl Chlorophyllide a | Chlorophyllide b | Chlorophyll(ide) c1 | Chlorophyll(ide) c2 |
| Molecular formula | C35H34MgN4O5 | C35H32MgN4O5 | C35H32MgN4O6 | C35H30MgN4O5 | C35H28MgN4O5 |
| Molecular weight (g/mol) | 615 | 613 | 629 | 611 | 609 |
| Structure |  | 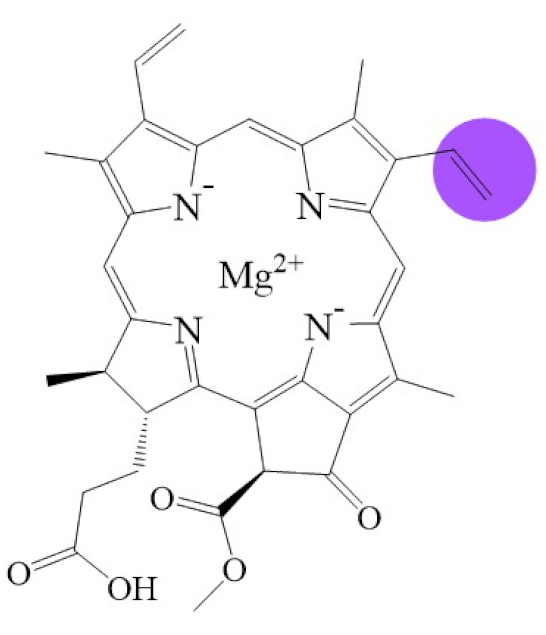 | 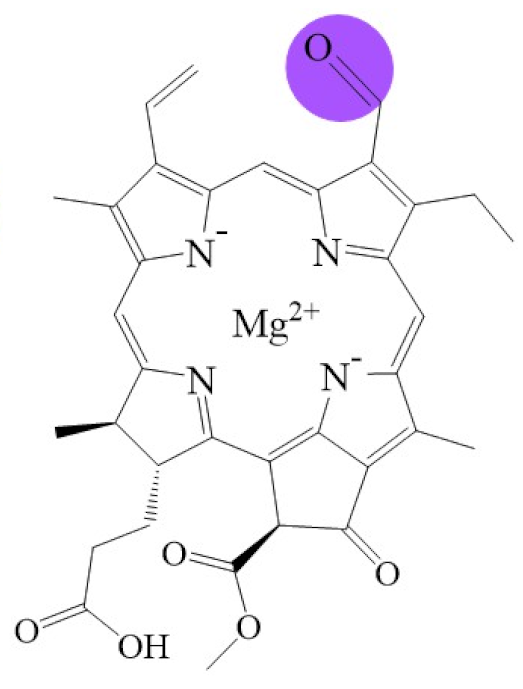 |  | 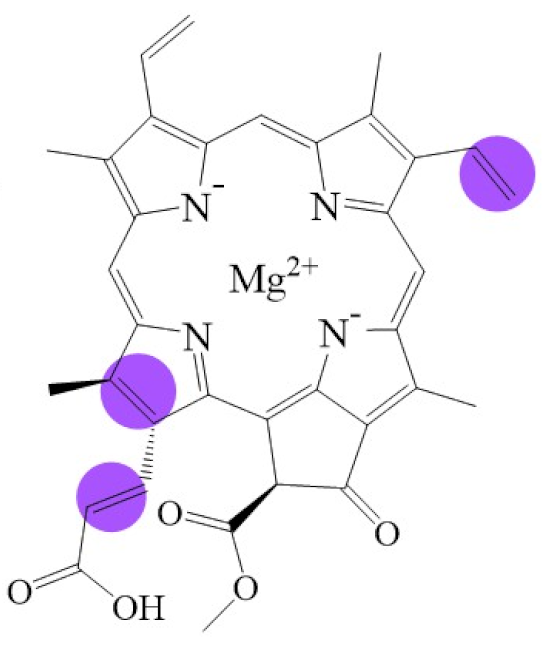 |
| Name | Chlorophyll c3 | 8-ethyl chlorophyll c3 | Chlorophyll cCS-170 | Chlorophyllide d | Chlorophyllide f |
| Molecular formula | C36H31MgN4O5 | C36H34MgN4O5 | C36H32MgN4O5 | C34H32MgN4O6 | C35H32MgN4O6 |
| Molecular weight (g/mol) | 653 | 655 | 655 | 617 | 629 |
| Structure | 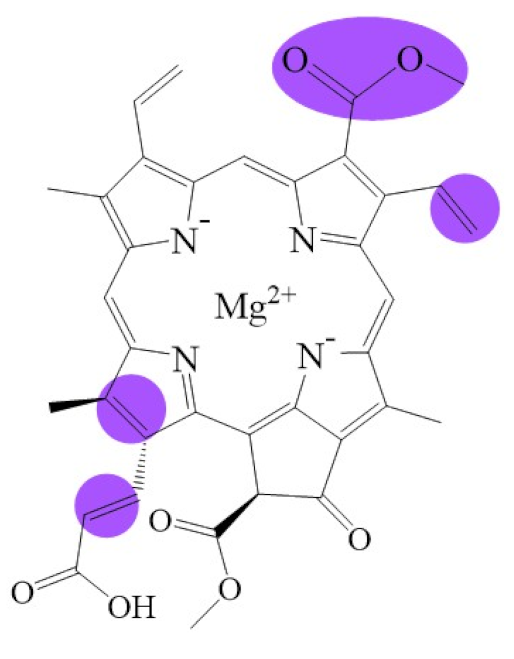 | 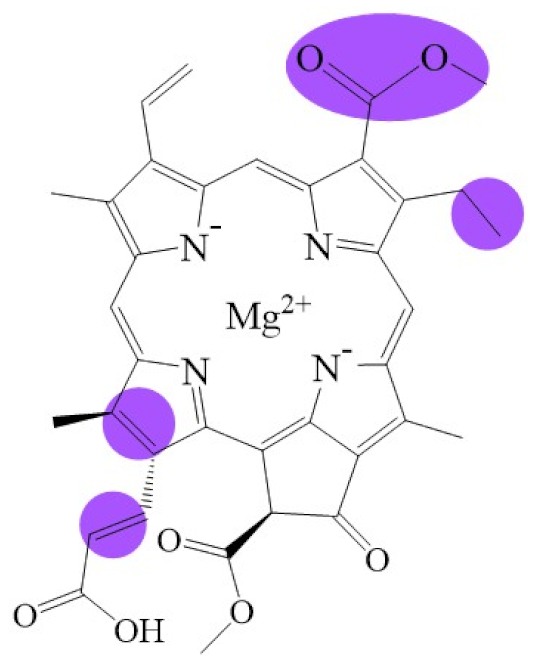 | 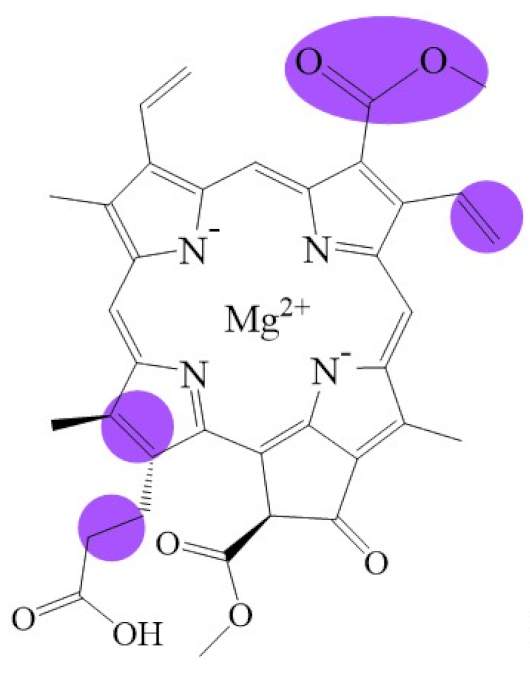 |  | 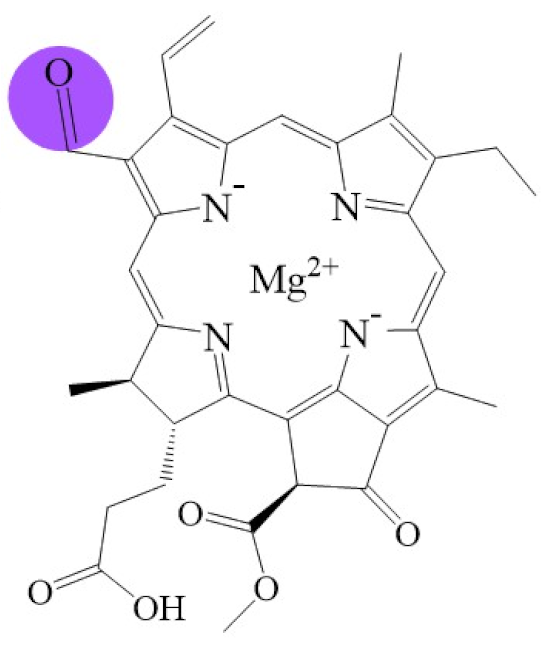 |
| Starting Materials | Enzyme | Plant Source | Affected Factor | Yield | References |
|---|---|---|---|---|---|
| Protochlorophyllide a | NADPH: protochlorophyllide oxidoreductase | Etiolated leaves | Light (+) | N/A | [73] |
| Wheat leaves | δ-aminolevulinic acid (5 mM)/light (+) | 2.5–18 μg/g fresh weight at 1–57 μW/cm−2 of light for 15 min | [75] | ||
| Wheat (Triticum aestivum L., cv. Kosack) seed | Norflurazon, inhibitor of carotenoid biosynthesis (+) | Protochlorophyllide: 39 μg/g at norflurazon-treated leaves (100 μg/L), the ratio of chlide/protochlorophyllide was increased | [93] | ||
| Barley leaves (Hordeum vulgare) | Water (+) on Chlorophyll(ide)684 (−) on Chlorophyll(ide)676 | Chlorophyll(ide)676 was increased 28.4–92.5% in 40% and 78% desiccated leaves | [97] | ||
| Barley seeds (Hordeum vulgare L. Zaoshu No. 3) | Nitric oxide (−) | Nitric oxide inhibited chlorophylls synthesis (49–58% decline in barley and 21–24% decline in Arabidopsis) | [98] | ||
| Chlorophyll | Chlorophyllase | Phaeodactylum tricornutum | 50% acetone at 20 °C (+) | 100% | [49] |
| Heruckum leaves | Heat treatment between 60–75 °C (+) | 10–70% | [50] | ||
| 80% acetone at 1 °C (+) | 100% | ||||
| Ficus macrocarpa leaves | 80% acetone (+) | 95% | [99,100] | ||
| French bean leaves | δ-aminolevulinic acid (+) | N/A | [101] | ||
| Citrus sinensis chlorophyllase | Nicotiana tabacum | 1.8 fold increased | [102] | ||
| Chlamydomonas reinhardtii chlorophyllase | Catalytic efficiency: 9.89 (s−1 μM−1) | [57] | |||
| Brassica oleracea chlorophyllase | Catalytic efficiency: 15.51 × 10−5 (s−1 μM−1) | [56] | |||
| Cyanobacterium Cyanothece sp. ATCC 51142 chlorophyllase | Specific activity of enzyme: 75.60 (U/mg) | [58] | |||
| Oscillatoria acuminata PCC 6304 chlorophyllase | Catalytic efficiency: 15.51 × 10−5 (s−1 µM− 1) | [103] |
| Purification by Solvents | Sources | Yield | References |
|---|---|---|---|
| Membrane suspension was added to a mixture containing acetone (or methanol) and 0.1 M ammonium hydroxide. After being mixed and centrifuged, these combined supernatants was added to petroleum ether or n-hexane. | Plasma membranes and thylakoid membranes of Anucystis nidulans | N/A | [133] |
| Extracted diethyl ether/ethanol (1:1) and repeatedly washed with 20% ethanol (10 mM Tricine–NaOH, pH 8). | R. capsulatus CB1200 cultured in Tween 80 supplemented growth medium | Chlorophyllide a: 7 mg/L | [134] |
| Barley leaves were extracted in 80% acetone (acetone/50 mM Tricine–NaOH, pH 8, 80:20, v/v), and the suspension was repeatedly extracted with n-hexane and diethyl ether/ethanol (1:1). The suspension was then repeatedly washed with 20% ethanol (10 mM Tricine–NaOH, pH 8). | Barley leaves (H. vulgare L.) | Chlorophyllide a: 17 μg/g fresh weightChlorophyllide b: 5 μg/g fresh weight |
| Chromatography | Elution | References |
|---|---|---|
| Column chromatography with sugar or cellulose powder packaged column | N/A | [48] |
| Column chromatography with DEAE-Sepharose CL-6B column | Elution: acetone/H2O (4:1, v/v), 1% ammonium acetate | [135] |
| High-performance liquid chromatography (HPLC), silicic acid column coated with dodecyl residues | Methanol: tetrabutylammonium phosphate: methyl ethyl ketone (70:30:6; v/v/v) Elution: 70% methyl alcohol with methyl ethyl ketone | [136] |
| Reverse-phase HPLC system with column packed with octadecyl-silane bonded 5-μm ODS-Hypersil | Mobile phases contain 1.5% tetrabutylammonium acetate and 7.7% ammonium acetate in water:water:methanol (10:10:80; v/v/v); acetone:methanol (20:80; v/v) in the gradient elution | [137] |
| HPLC with a Shimadzu LC-3A chromatograph using a polyethylene column | Acetone in water: 50% (v/v) | [138] |
| HPLC with reversed-phase Spherisorb ODS-2 column | The mobile phases contained 80% methanol in ammonium acetate solution and 80% methanol in acetone | [139] |
| HPLC with reversed-phase C18 column and photodiode-array detector | The mobile phase contained acetonitrile-methanol (70:30, v/v) and increasing proportions of dichloromethane | [140] |
| HPLC with a monolithic C18-bonded silica rod column | The mobile phases contained 80% methanol in 0.025 M aqueous pyridine solution (pH 5 with acetic acid) and 80% methanol in acetone | [142] |
| HPLC−Diode Array Detection−Mass Spectrometry with an HyPURITY C18 column | The solvent system was consisted of water, methanol, acetonitrile and acetone with a gradient condition at 1 mL/min of flow rate | [144] |
| A high-throughput method that combined with HPLC and time-of-flight mass spectrometry with a C18 Spherisorb ODS-2 column | The mobile phases: water/ion pair reagent/methanol (1:1:8, v/v/v) and methanol/acetone (1:1, v/v), 1 M ammonium acetate in water was used as the ion reagent | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-T.; Yang, C.-H.; Huang, K.-S.; Shaw, J.-F. Chlorophyllides: Preparation, Purification, and Application. Biomolecules 2021, 11, 1115. https://doi.org/10.3390/biom11081115
Wang Y-T, Yang C-H, Huang K-S, Shaw J-F. Chlorophyllides: Preparation, Purification, and Application. Biomolecules. 2021; 11(8):1115. https://doi.org/10.3390/biom11081115
Chicago/Turabian StyleWang, Yi-Ting, Chih-Hui Yang, Keng-Shiang Huang, and Jei-Fu Shaw. 2021. "Chlorophyllides: Preparation, Purification, and Application" Biomolecules 11, no. 8: 1115. https://doi.org/10.3390/biom11081115
APA StyleWang, Y.-T., Yang, C.-H., Huang, K.-S., & Shaw, J.-F. (2021). Chlorophyllides: Preparation, Purification, and Application. Biomolecules, 11(8), 1115. https://doi.org/10.3390/biom11081115









