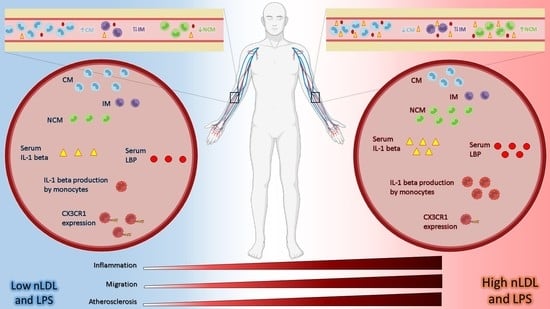
Native Low-Density Lipoproteins Act in Synergy with Lipopolysaccharide to Alter the Balance of Human Monocyte Subsets and Their Ability to Produce IL-1 Beta, CCR2, and CX3CR1 In Vitro and In Vivo: Implications in Atherogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Culture of Primary Human Monocytes
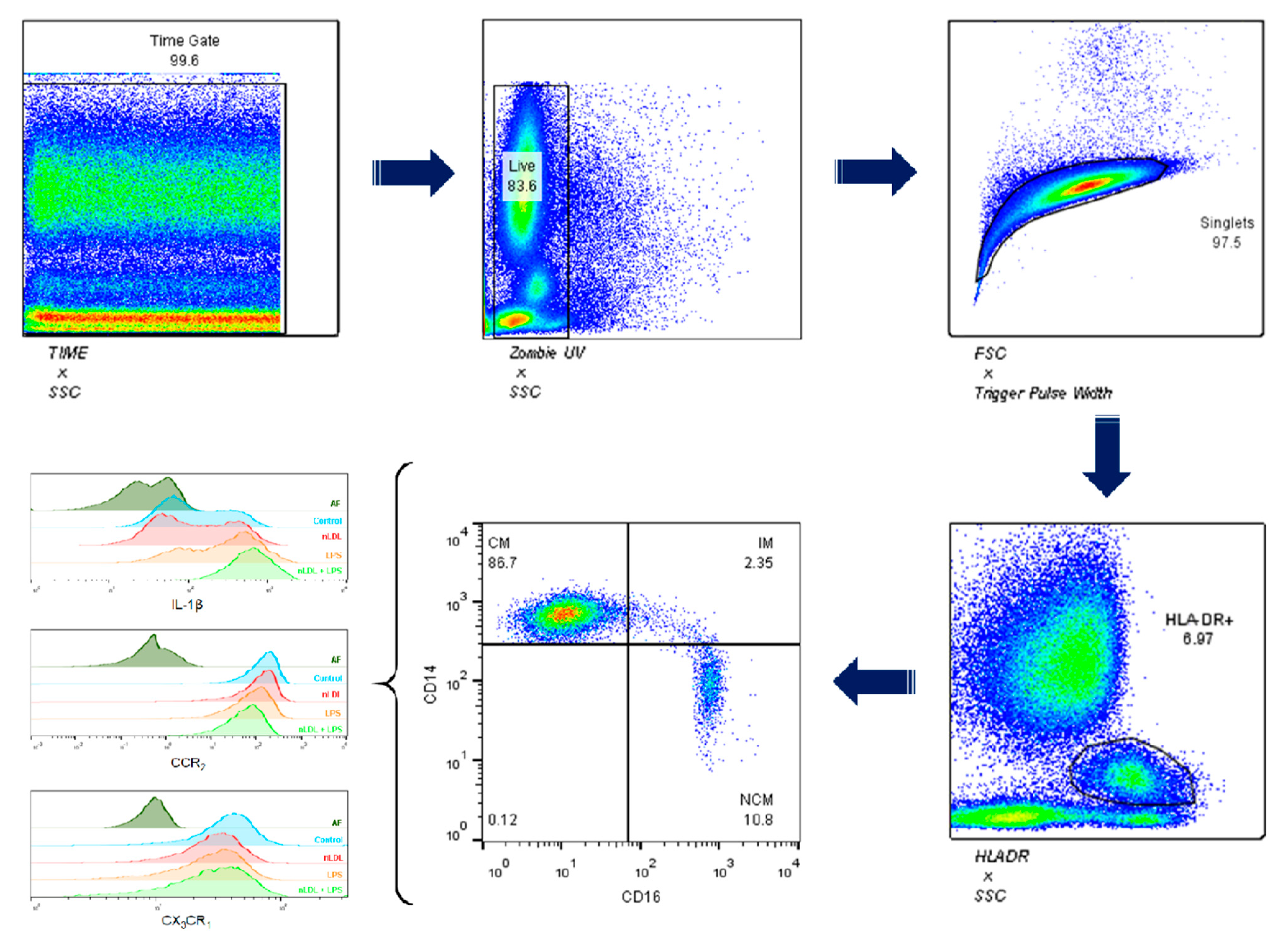
2.2. Flow Cytometry
2.3. Subjects for In Vivo Assays
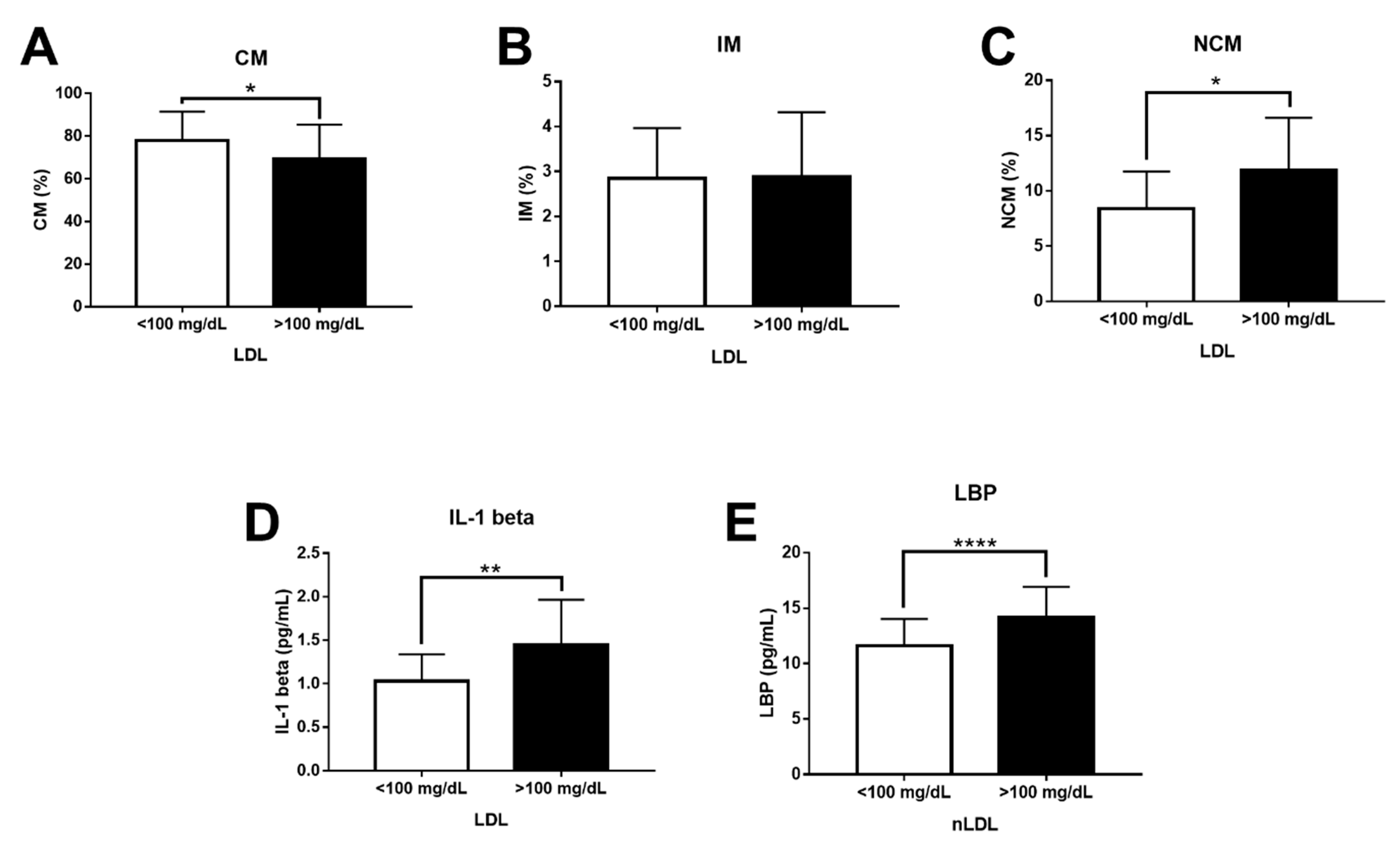
2.4. Effects of LDL and LBP on Monocyte Subpopulations and IL-1 Beta In Vivo
2.5. Statistics
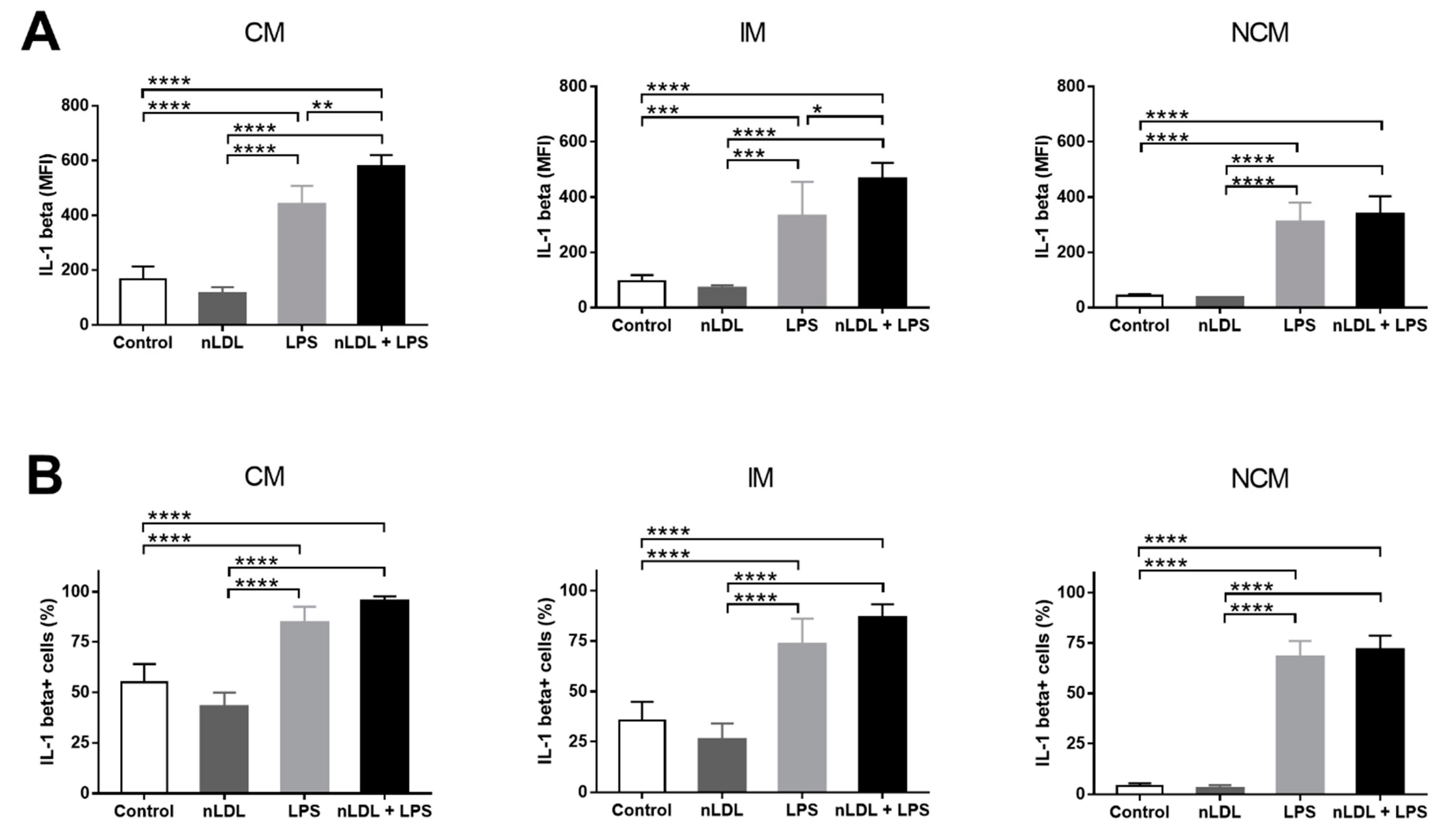
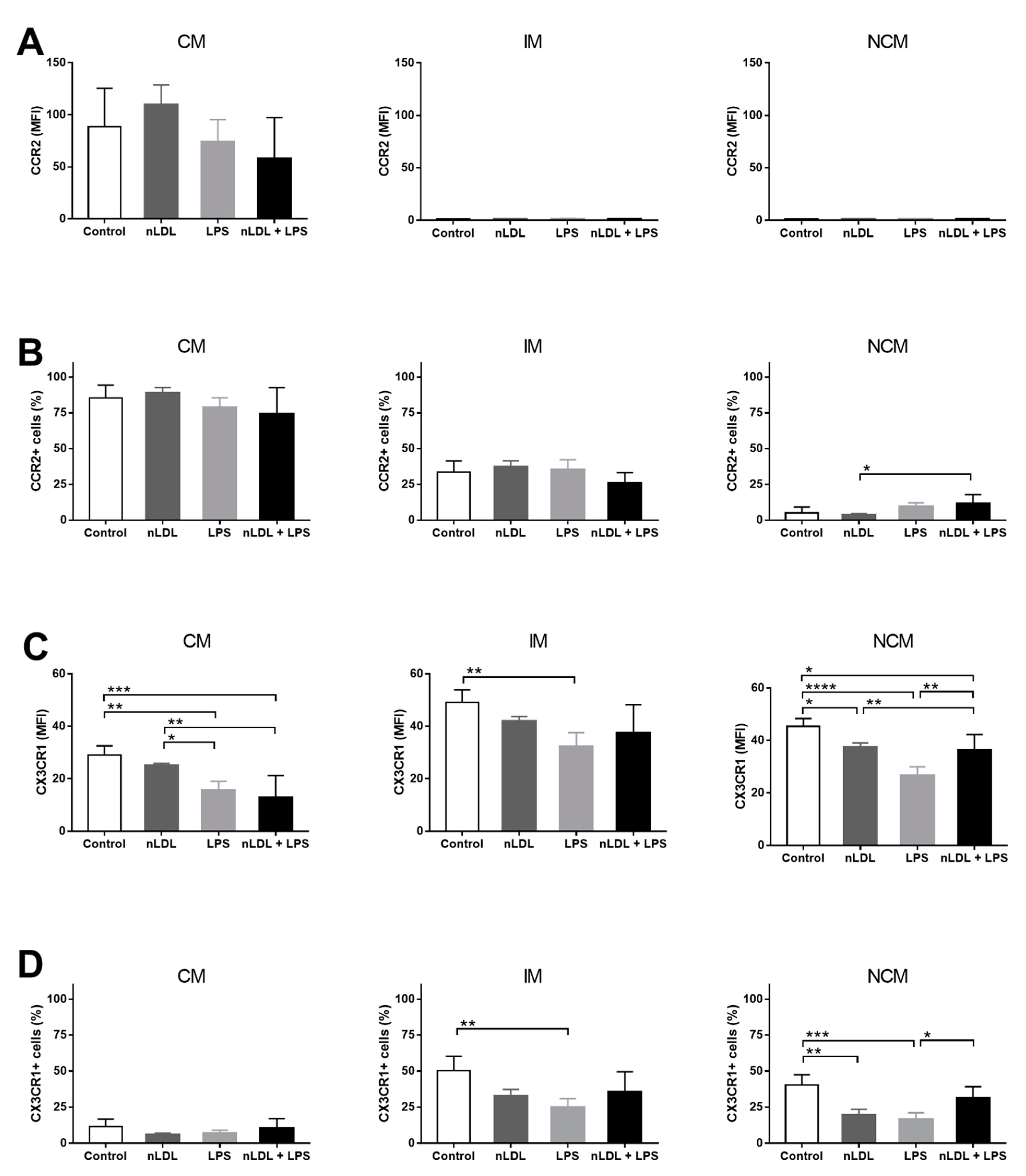
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Markin, A.; Sobenin, I.A.; Grechko, A.V.; Zhang, D.; Orekhov, A.N. Cellular Mechanisms of Human Atherogenesis: Focus on Chronification of Inflammation and Mitochondrial Mutations. Front. Pharmacol. 2020, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Uosef, A.; Villagran, M.; Zdanowski, R.; Kubiak, J.; Wosik, J.; Ghobrial, R. RhoA- and Actin-Dependent Functions of Macrophages from the Rodent Cardiac Transplantation Model Perspective—Timing Is the Essence. Biology 2021, 10, 70. [Google Scholar] [CrossRef]
- Ganesan, R.; Henkels, K.M.; Wrenshall, L.E.; Kanaho, Y.; Di Paolo, G.; Frohman, M.A.; Gomez-Cambronero, J. Oxidized LDL phagocytosis during foam cell formation in atherosclerotic plaques relies on a PLD2-CD36 functional interdependence. J. Leukoc. Biol. 2018, 103, 867–883. [Google Scholar] [CrossRef]
- Chen, C.; Khismatullin, D.B. Oxidized Low-Density Lipoprotein Contributes to Atherogenesis via Co-activation of Macrophages and Mast Cells. PLoS ONE 2015, 10, e0123088. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, R.; Francis, H.; Dervish, S.; Li, S.C.; Medbury, H.; Williams, H. Characterization of Human Monocyte Subsets by Whole Blood Flow Cytometry Analysis. J. Vis. Exp. 2018, e57941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleby, L.J.; Nausch, N.; Midzi, N.; Mduluza, T.; Allen, J.; Mutapi, F. Sources of heterogeneity in human monocyte subsets. Immunol. Lett. 2013, 152, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, R.; Barman, P.K.; Thatoi, P.K.; Tripathy, R.; Das, B.K.; Ravindran, B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015, 5, 13886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.; Mack, M.; Van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Idzkowska, E.; Eljaszewicz, A.; Miklasz, P.; Musial, W.J.; Tycinska, A.M.; Moniuszko, M. The Role of Different Monocyte Subsets in the Pathogenesis of Atherosclerosis and Acute Coronary Syndromes. Scand. J. Immunol. 2015, 82, 163–173. [Google Scholar] [CrossRef]
- Dimitrov, S.; Shaikh, F.; Pruitt, C.; Green, M.; Wilson, K.; Beg, N.; Hong, S. Differential TNF production by monocyte subsets under physical stress: Blunted mobilization of proinflammatory monocytes in prehypertensive individuals. Brain Behav. Immun. 2013, 27, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Cockx, M.; Gouwy, M.; Ruytinx, P.; Lodewijckx, I.; Van Hout, A.; Knoops, S.; Pörtner, N.; Ronsse, I.; Vanbrabant, L.; Godding, V.; et al. Monocytes from patients with Primary Ciliary Dyskinesia show enhanced inflammatory properties and produce higher levels of pro-inflammatory cytokines. Sci. Rep. 2017, 7, 14657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, Y.; Herrera, M.T.; Soldevila, G.; Garcia-Garcia, L.; Fabián, G.; Pérez-Armendariz, E.M.; Bobadilla, K.; Guzman-Beltran, S.; Sada, E.; Torres, M. High glucose concentrations induce TNF-α production through the down-regulation of CD33 in primary human monocytes. BMC Immunol. 2012, 13, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasu, M.R.; Jialal, I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E145–E154. [Google Scholar] [CrossRef] [Green Version]
- Grün, J.L.; Manjarrez-Reyna, A.N.; Gómez-Arauz, A.Y.; Leon-Cabrera, S.; Rückert, F.; Fragoso, J.M.; Bueno-Hernández, N.; Islas-Andrade, S.; Meléndez-Mier, G.; Escobedo, G. High-Density Lipoprotein Reduction Differentially Modulates to Classical and Nonclassical Monocyte Subpopulations in Metabolic Syndrome Patients and in LPS-Stimulated Primary Human Monocytes In Vitro. J. Immunol. Res. 2018, 2018, 2737040. [Google Scholar] [CrossRef]
- Kopp, F.; Kupsch, S.; Schromm, A.B. Lipopolysaccharide-binding protein is bound and internalized by host cells and colocalizes with LPS in the cytoplasm: Implications for a role of LBP in intracellular LPS-signaling. Biochim. Biophys. Acta Bioenerg. 2016, 1863, 660–672. [Google Scholar] [CrossRef]
- Aw, N.H.; Canetti, E.; Suzuki, K.; Goh, J. Monocyte Subsets in Atherosclerosis and Modification with Exercise in Humans. Antioxidants 2018, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Orekhov, A.N.; Oishi, Y.; Nikiforov, N.G.; Zhelankin, A.V.; Dubrovsky, L.; Sobenin, I.A.; Kel, A.; Stelmashenko, D.; Makeev, V.J.; Foxx, K.; et al. Modified LDL Particles Activate Inflammatory Pathways in Monocyte-derived Macrophages: Transcriptome Analysis. Curr. Pharm. Des. 2018, 24, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.; Weinrich, T.W.; Woollard, K.J. Very-low and low-density lipoproteins induce neutral lipid accumulation and impair migration in monocyte subsets. Sci. Rep. 2016, 6, 20038. [Google Scholar] [CrossRef] [Green Version]
- Combadiere, C.; Potteaux, S.; Rodero, M.; Simon, T.; Pezard, A.; Esposito, B.; Merval, R.; Proudfoot, A.; Tedgui, A.; Mallat, Z. Combined Inhibition of CCL2, CX3CR1, and CCR5 Abrogates Ly6C hi and Ly6C lo Monocytosis and Almost Abolishes Atherosclerosis in Hypercholesterolemic Mice. Circulation 2008, 117, 1649–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Si, Y.; Wu, C.; Sun, L.; Ma, Y.; Ge, A.; Li, B. Lipopolysaccharide promotes lipid accumulation in human adventitial fibroblasts via TLR4-NF-κB pathway. Lipids Health Dis. 2012, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahola, A.J.; Lassenius, M.I.; Forsblom, C.; Harjutsalo, V.; Lehto, M.; Groop, P.-H. Dietary patterns reflecting healthy food choices are associated with lower serum LPS activity. Sci. Rep. 2017, 7, 6511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendyala, S.; Walker, J.M.; Holt, P.R. A High-Fat Diet Is Associated With Endotoxemia That Originates From the Gut. Gastroenterol. 2012, 142, 1100–1101.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zawada, A.M.; Fell, L.H.; Untersteller, K.; Seiler, S.; Rogacev, K.S.; Fliser, D.; Ziegler-Heitbrock, L.; Heine, G.H. Comparison of two different strategies for human monocyte subsets gating within the large-scale prospective CARE FOR HOMe Study. Cytom. Part A 2015, 87, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Saja, M.F.; Baudino, L.; Jackson, W.; Cook, H.T.; Malik, T.H.; Fossati-Jimack, L.; Ruseva, M.; Pickering, M.C.; Woollard, K.J.; Botto, M. Triglyceride-Rich Lipoproteins Modulate the Distribution and Extravasation of Ly6C/Gr1low Monocytes. Cell Rep. 2015, 12, 1802–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merah-Mourah, F.; Cohen, S.O.; Charron, D.; Mooney, N.; Haziot, A. Identification of Novel Human Monocyte Subsets and Evidence for Phenotypic Groups Defined by Interindividual Variations of Expression of Adhesion Molecules. Sci. Rep. 2020, 10, 4397. [Google Scholar] [CrossRef] [PubMed]
- Calvano, J.E.; Agnese, D.; Um, J.Y.; Goshima, M.; Singhal, R.; Coyle, S.M.; Reddell, M.T.; Kumar, A.; Calvano, S.E.; Lowry, S.F. Modulation of the Lipopolysaccharide Receptor Complex (CD14, TLR4, MD-2) and Toll-Like Receptor 2 in Systemic Inflammatory Response Syndrome-Positive Patients With and Without Infection: Relationship to Tolerance. Shock. 2003, 20, 415–419. [Google Scholar] [CrossRef]
- Ott, L.W.; Resing, K.A.; Sizemore, A.W.; Heyen, J.W.; Cocklin, R.R.; Pedrick, N.M.; Woods, H.C.; Chen, J.Y.; Goebl, M.G.; Witzmann, F.A.; et al. Tumor Necrosis Factor-alpha- and interleukin-1-induced cellular responses: Coupling proteomic and genomic information. J. Proteome. Res. 2007, 6, 2176–2185. [Google Scholar] [CrossRef] [Green Version]
- E Zhang, D.; Hetherington, C.J.; Gonzalez, D.A.; Chen, H.M.; Tenen, D. Regulation of CD14 expression during monocytic differentiation induced with 1 alpha,25-dihydroxyvitamin D. J. Immunol. 1994, 153, 3276–3284. [Google Scholar]
- Yieh, L.; Sanchez, H.B.; Osborne, T.F. Domains of transcription factor Sp1 required for synergistic activation with sterol regulatory element binding protein 1 of low density lipoprotein receptor promoter. Proc. Natl. Acad. Sci. USA 1995, 92, 6102–6106. [Google Scholar] [CrossRef] [Green Version]
- Junker, F.; Gordon, J.; Qureshi, O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front. Immunol. 2020, 11, 1393. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.R.; Weigel, C.; Scoville, S.D.; Chan, W.K.; Chatman, K.; Nemer, M.M.; Mao, C.; Young, K.A.; Zhang, J.; Yu, J.; et al. Epigenetic and Posttranscriptional Regulation of CD16 Expression during Human NK Cell Development. J. Immunol. 2017, 200, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Cui, H.; Li, Q.; Zhong, H.; Yu, J.; Li, P.; He, X. Upregulation of microRNA-218 reduces cardiac microvascular endothelial cells injury induced by coronary artery disease through the inhibition of HMGB. J. Cell. Physiol. 2020, 235, 3079–3095. [Google Scholar] [CrossRef] [PubMed]
- Devêvre, E.F.; Renovato-Martins, M.; Clément, K.; Sautes-Fridman, C.; Cremer, I.; Poitou, C. Profiling of the Three Circulating Monocyte Subpopulations in Human Obesity. J. Immunol. 2015, 194, 3917–3923. [Google Scholar] [CrossRef]
- Guerville, M.; Boudry, G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am. J. Physiol. Liver Physiol. 2016, 311, G1–G15. [Google Scholar] [CrossRef] [Green Version]
- Hersoug, L.; Møller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Lepper, P.M.; Schumann, C.; Triantafilou, K.; Rasche, F.M.; Schuster, T.; Frank, H.; Schneider, E.M.; Triantafilou, M.; von Eynatten, M. Association of Lipopolysaccharide-Binding Protein and Coronary Artery Disease in Men. J. Am. Coll. Cardiol. 2007, 50, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Zasłona, Z.; Pålsson-McDermott, E.M.; Menon, D.; Haneklaus, M.; Flis, E.; Prendeville, H.; Corcoran, S.E.; Peters-Golden, M.; O’Neill, L.A.J. The Induction of Pro–IL-1β by Lipopolysaccharide Requires Endogenous Prostaglandin E2Production. J. Immunol. 2017, 198, 3558–3564. [Google Scholar] [CrossRef] [Green Version]
- Haraldsen, G.; Kvale, D.; Lien, B.; Farstad, I.N.; Brandtzaeg, P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J. Immunol. 1996, 156, 2558–2565. [Google Scholar] [PubMed]
- Lim, J.H.; Um, H.J.; Park, J.-W.; Lee, I.-K.; Kwon, T.K. Interleukin-1β promotes the expression of monocyte chemoattractant protein-1 in human aorta smooth muscle cells via multiple signaling pathways. Exp. Mol. Med. 2009, 41, 757–764. [Google Scholar] [CrossRef]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, F.; Wang, Y.; Gisterå, A.; Roy, J.; Paulsson-Berne, G.; Hedin, U.; Lerman, A.; Hansson, G.K.; Herrmann, J.; et al. Inflammasome-Driven Interleukin-1α and Interleukin-1β Production in Atherosclerotic Plaques Relates to Hyperlipidemia and Plaque Complexity. JACC Basic Transl. Sci. 2019, 4, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Teh, Y.C.; Ding, J.L.; Ng, L.G.; Chong, S.Z. Capturing the Fantastic Voyage of Monocytes Through Time and Space. Front. Immunol. 2019, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Ancuta, P.; Rao, R.; Moses, A.; Mehle, A.; Shaw, S.K.; Luscinskas, F.W.; Gabuzda, D. Fractalkine Preferentially Mediates Arrest and Migration of CD16+ Monocytes. J. Exp. Med. 2003, 197, 1701–1707. [Google Scholar] [CrossRef] [Green Version]
- Han, K.H.; Tangirala, R.K.; Green, S.R.; Quehenberger, O. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1983–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.H.; Irvine, H.; Vedel, S.; Raungaard, B.; Beck-Nielsen, H.; Handberg, A. Elevated Atherosclerosis-Related Gene Expression, Monocyte Activation and Microparticle-Release Are Related to Increased Lipoprotein-Associated Oxidative Stress in Familial Hypercholesterolemia. PLoS ONE 2015, 10, e0121516. [Google Scholar] [CrossRef]
- Geng, S.; Chen, K.; Yuan, R.; Peng, L.; Maitra, U.; Diao, N.; Chen, C.; Zhang, Y.; Hu, Y.; Qi, C.-F.; et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat. Commun. 2016, 7, 13436. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Bowman, J.D.; Surani, S.; Horseman, M. Endotoxin, Toll-like Receptor-4, and Atherosclerotic Heart Disease. Curr. Cardiol. Rev. 2017, 13, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Grin, P.; Dwivedi, D.J.; Chathely, K.M.; Trigatti, B.; Dino, L.; Prat, A.; Seidah, N.; Liaw, P.C.; Fox-Robichaud, A.E. Low-density lipoprotein (LDL)-dependent uptake of Gram-positive lipoteichoic acid and Gram-negative lipopolysaccharide occurs through LDL receptor. Sci. Rep. 2018, 8, 10496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vreugdenhil, A.C.; Snoek, A.P.; van ‘t Veer, C.; Greve, J.W.; Buurman, W.A. LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction. J. Clin. Investig. 2001, 107, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| LDL-C | |||
|---|---|---|---|
| <100 mg/dL | >100 mg/dL | p-Value | |
| Sex (F/M) | 28/37 | 41/44 | 0.2650 |
| Age (years) | 47.2 ± 1.056 | 49.49 ± 1.031 | 0.1913 |
| BMI (kg/m2) | 28.22 ± 1.172 | 28.05 ± 0.9035 | 0.9155 |
| Waist circumference (cm) | 91.58 ± 2.94 | 96.73 ± 2.002 | 0.1696 |
| Body fat (%) | 31.87 ± 1.696 | 31.91 ± 2.614 | 0.9909 |
| Cholesterol (mg/dL) | 166.7 ± 7.079 | 220.7 ± 4.723 | <0.0001 |
| Triglycerides (mg/dL) | 242.6 ± 41.08 | 186.4 ± 11.14 | 0.0769 |
| LDL-C (mg/dL) | 76.53 ± 4.156 | 128.7 ± 3.653 | <0.0001 |
| HDL-C (mg/dL) | 42.13 ± 3.44 | 47.06 ± 2.226 | 0.2332 |
| Insulin (µU/L) | 14.43 ± 1.057 | 13.52 ± 0.8046 | 0.5208 |
| Glucose (mmol/L) | 5.104 ± 0.2653 | 5.346 ± 0.2502 | 0.5675 |
| HOMA-IR (a.u.) | 3.299 ± 0.3261 | 3.126 ± 0.1866 | 0.6290 |
| CRP (mg/L) | 5.095 ± 0.4755 | 5.012 ± 0.6004 | 0.6328 |
| Castelli’s risk index II | 1.966 ± 0.1827 | 2.892 ± 0.1294 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manjarrez-Reyna, A.N.; Martínez-Reyes, C.P.; Aguayo-Guerrero, J.A.; Méndez-García, L.A.; Esquivel-Velázquez, M.; León-Cabrera, S.; Vargas-Alarcón, G.; Fragoso, J.M.; Carreón-Torres, E.; Pérez-Méndez, O.; et al. Native Low-Density Lipoproteins Act in Synergy with Lipopolysaccharide to Alter the Balance of Human Monocyte Subsets and Their Ability to Produce IL-1 Beta, CCR2, and CX3CR1 In Vitro and In Vivo: Implications in Atherogenesis. Biomolecules 2021, 11, 1169. https://doi.org/10.3390/biom11081169
Manjarrez-Reyna AN, Martínez-Reyes CP, Aguayo-Guerrero JA, Méndez-García LA, Esquivel-Velázquez M, León-Cabrera S, Vargas-Alarcón G, Fragoso JM, Carreón-Torres E, Pérez-Méndez O, et al. Native Low-Density Lipoproteins Act in Synergy with Lipopolysaccharide to Alter the Balance of Human Monocyte Subsets and Their Ability to Produce IL-1 Beta, CCR2, and CX3CR1 In Vitro and In Vivo: Implications in Atherogenesis. Biomolecules. 2021; 11(8):1169. https://doi.org/10.3390/biom11081169
Chicago/Turabian StyleManjarrez-Reyna, Aarón N., Camilo P. Martínez-Reyes, José A. Aguayo-Guerrero, Lucia A. Méndez-García, Marcela Esquivel-Velázquez, Sonia León-Cabrera, Gilberto Vargas-Alarcón, José M. Fragoso, Elizabeth Carreón-Torres, Oscar Pérez-Méndez, and et al. 2021. "Native Low-Density Lipoproteins Act in Synergy with Lipopolysaccharide to Alter the Balance of Human Monocyte Subsets and Their Ability to Produce IL-1 Beta, CCR2, and CX3CR1 In Vitro and In Vivo: Implications in Atherogenesis" Biomolecules 11, no. 8: 1169. https://doi.org/10.3390/biom11081169