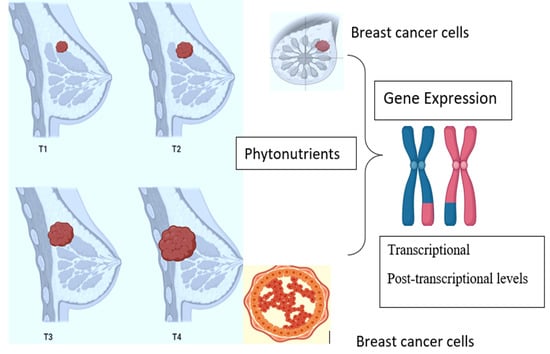
Role of Phytonutrients in Nutrigenetics and Nutrigenomics Perspective in Curing Breast Cancer
Abstract
:1. Introduction
2. Methodology
3. Genetics of Breast Cancer
4. Nutrigenomic Effects of Phytochemicals in Breast Cancer
4.1. Polyphenols
4.2. Phytosterol
4.3. Terpenoids
4.4. Alkaloids
5. Nutrigenomic Effects of Some Selected Phytochemicals in Breast Cancer
5.1. Polyphenols
5.1.1. Flavonoids
- Epigallocatechin gallate (EGCG)
- Genistein
- Quercetin
- Apigenin
- Luteolin
- Kaempferol
- Isoliquiritigenin
5.1.2. Phenolic Acids
- Curcumin
5.1.3. Lignans
- Secoisolariciresinol
5.1.4. Stilbenes
- Resveratrol
- Pterostilbene
5.1.5. Flavonolignans
- Silibinin
5.2. Terpenoids
- Thymoquinone
- Parthenolide
5.3. Saponins
- Ginsenosides
5.4. Isothiocyanates
- Benzyl Isothiocyanate
- Sulforaphane
5.5. Others
- 3,3′-Diindolylmethane
- α-Mangostin
5.6. Clinical Trials
6. Challenges in Clinical Applications
7. Authors’ Opinion
8. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- McMillon, E.; McKenna, W.; Milne, C. Guidelines on preparing a medical report for compensation purposes. Br. J. Dermatol. 1982, 106, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Momenimovahed, Z.; Salehiniya, H. Cervical cancer in Iran: Integrative insights of epidemiological analysis. BioMedicine 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Karthika, C.; Hari, B.; Rahman, H.; Akter, R.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Akhtar, M.F.; Abdel-Daim, M.M. Multiple strategies with the synergistic approach for addressing colorectal cancer. Biomed. Pharmacother. 2021, 140, 111704. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Salazar, J.D.L.G.; Pritchard, K.; Amadori, D.; Haidinger, R.; Hudis, C.A.; Khaled, H.; Liu, M.-C.; Martin, M.; Namer, M.; et al. The global breast cancer burden: Variations in epidemiology and survival. Clin. Breast Cancer 2005, 6, 391–401. [Google Scholar] [CrossRef]
- Yalaza, M.; İnan, A.; Bozer, M.J. Male breast cancer. J. Breast Health 2016, 12, 1. [Google Scholar] [CrossRef]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Manna, S.; Holz, M.K. Tamoxifen action in ER-negative breast cancer. Signal Transduct. Insights 2016, 5, S29901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembinski, R.; Prasath, V.; Bohnak, C.; Siotos, C.; Sebai, M.E.; Psoter, K.; Gani, F.; Canner, J.; Camp, M.S.; Azizi, A.; et al. Estrogen receptor positive and progesterone receptor negative breast cancer: The role of hormone therapy. Horm. Cancer 2020, 11, 148–154. [Google Scholar] [CrossRef]
- Pernas, S.; Tolaney, S.M. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833519. [Google Scholar] [CrossRef] [Green Version]
- Drăgănescu, M.; Carmocan, C.J.C. Hormone therapy in breast cancer. Chirurgia 2017, 112, 413–417. [Google Scholar] [CrossRef]
- Mehanna, J.; Haddad, F.G.; Eid, R.; Lambertini, M.; Kourie, H.R. Triple-negative breast cancer: Current perspective on the evolving therapeutic landscape. Int. J. Women Health 2019, 11, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lester, S.C.; Bose, S.; Chen, Y.-Y.; Connolly, J.L.; de Baca, M.E.; Fitzgibbons, P.L.; Hayes, D.F.; Kleer, C.; O’Malley, F.P.; Page, D.L.; et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch. Pathol. Lab. Med. 2009, 133, 1515–1538. [Google Scholar] [CrossRef]
- Bungau, S.G.; Popa, V.-C. Between religion and science some aspects concerning illness and healing in antiquity. Transylv. Rev. 2015, 24, 3–18. [Google Scholar]
- Bhattacharya, T.; Rather, G.A.; Akter, R.; Kabir, T.; Rauf, A.; Rahman, H. Nutraceuticals and bio-inspired materials from microalgae and their future perspectives. Curr. Top. Med. Chem. 2021, 21, 1. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sánchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Rui, H.J. Health-promoting components of fruits and vegetables in human health. Nutrients 2013, 4, 384S–392S. [Google Scholar]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic effects of polyphenols in cardiovascular system. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kumar, K.; Brisc, C.; Rus, M.; Nistor-Cseppento, D.C.; Bustea, C.; Aron, R.A.C.; Pantis, C.; Zengin, G.; Sehgal, A.; et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed. Pharmacother. 2021, 133, 110959. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the multifaceted therapeutic potential of withaferin A and its derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Nair, R.M.K.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. 2020, 27, 26025–26035. [Google Scholar] [CrossRef] [PubMed]
- Aron, R.C.; Abid, A.; Vesa, C.; Nechifor, A.; Behl, T.; Ghitea, T.; Munteanu, M.; Fratila, O.; Andronie-Cioara, F.; Toma, M.; et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of Akkermansia muciniphila as a key gut bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef]
- Arora, A.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Sobarzo-Sanchez, E.; Bungau, S. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. 2021, 273, 119311. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bungau, S.; Bin-Jumah, M.; El-Kott, A.; Shati, A.; Aleya, L.; Alkahtani, S. Protective effects of thymoquinone and diallyl sulphide against malathion-induced toxicity in rats. Environ. Sci. Pollut. Res. 2020, 27, 10228–10235. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, G.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Zengin, G.; Bungau, S.; Munteanu, M.; Brisc, M.; et al. Elucidating the multi-targeted role of nutraceuticals: A complementary therapy to starve neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 4045. [Google Scholar] [CrossRef]
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in neurological disorders. Int. J. Mol. Sci. 2020, 21, 4424. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.; Iovan, C.; Cseppento, D.C.N.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the hormone replacement therapy and of soy isoflavones on bone resorption in postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [Green Version]
- Țiț, D.M.; Pallag, A.; Iovan, C.V.; Furău, G.; Furău, C.; Bungau, S. Somatic-vegetative symptoms evolution in postmenopausal women treated with phytoestrogens and hormone replacement therapy. Iran. J. Public Health 2017, 46, 1528–1534. [Google Scholar]
- Bumbu, A.; Bianca, P.; Tit, D.M.; Bungau, S.; Bumbu, G. The effects of soy isoflavones and hormonal replacing therapy on the incidence and evolution of postmenopausal female urinary incontinence. Farmacia 2016, 64, 419–422. [Google Scholar]
- Bungau, S.; Vesa, C.; Abid, A.; Behl, T.; Tit, D.; Purza, A.; Pasca, B.; Todan, L.; Endres, L. Withaferin A—A promising phytochemical compound with multiple results in dermatological diseases. Molecules 2021, 26, 2407. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.J. Resveratrol and neuroprotection: Impact and its therapeutic potential in Alzheimer’s disease. Front. Pharmacol. 2020, 11, 2272. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Shiovitz, S.; Korde, L.A. Genetics of breast cancer: A topic in evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef]
- Pasculli, B.; Barbano, R.; Parrella, P. Epigenetics of breast cancer: Biology and clinical implication in the era of precision medicine. Semin. Cancer Biol. 2018, 51, 22–35. [Google Scholar] [CrossRef]
- Karsli-Ceppioglu, S.; Dagdemir, A.; Judes, G.; Ngollo, M.; Penault-Llorca, F.; Pajon, A.; Bignon, Y.-J.; Bernard-Gallon, D. Epigenetic mechanisms of breast cancer: An update of the current knowledge. Epigenomics 2014, 6, 651–664. [Google Scholar] [CrossRef]
- Younas, M.; Hano, C.; Giglioli-Guivarc, N.; Abbasi, B.H. Mechanistic evaluation of phytochemicals in breast cancer remedy: Current understanding and future perspectives. RSC Adv. 2018, 8, 29714–29744. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights. Int. J. Funct. Nutr. 2020, 1, 1. [Google Scholar] [CrossRef]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and other polyphenols act as epigenetic modifiers in breast cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Hannan, M.A.; Dash, R.; Rahman, M.H.; Islam, R.; Uddin, M.J.; Sohag, A.A.M.; Rahman, M.H.; Rhim, H.J.F. Phytochemicals as a complement to cancer chemotherapy: Pharmacological modulation of the autophagy-apoptosis pathway. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Sak, K.J. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of natural stilbenes in the prevention of cancer. Oxidative Med. Cell. Longev. 2015, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grattan, B.J. Plant sterols as anticancer nutrients: Evidence for their role in breast cancer. Nutrients 2013, 5, 359–387. [Google Scholar] [CrossRef] [Green Version]
- Vedin, L.-L.; Lewandowski, S.A.; Parini, P.; Gustafsson, J.; Steffensen, K.R. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis 2009, 30, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E.J.J. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Dey, P.S.; Akter, R.; Kabir, T.; Rahman, H.; Rauf, A. Effect of natural leaf extracts as phytomedicine in curing geriatrics. Exp. Gerontol. 2021, 150, 111352. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, X.; Xu, J.; Li, C.; Yu, Y.; Wang, W.; Zhu, L. The protective effect of dietary phytosterols on cancer risk: A systematic meta-analysis. J. Oncol. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Dou, Q.P. Targeting apoptosis pathway with natural terpenoids: Implications for treatment of breast and prostate cancer. Curr. Drug Targets 2010, 11, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Palop, J.J.; Mucke, L.; Roberson, E. Quantifying biomarkers of cognitive dysfunction and neuronal network hyperexcitability in mouse models of Alzheimer’s disease: Depletion of calcium-dependent proteins and inhibitory hippocampal remodeling. In Alzheimer’s Disease and Frontotemporal Dementia; Roberson, E.D., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 670, pp. 245–262. [Google Scholar]
- Abdel-Daim, M.M.; Abo-El-Sooud, K.; Aleya, L.; Bungǎu, S.G.; Najda, A.; Saluja, R. Alleviation of drugs and chemicals toxicity: Biomedical value of antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Yokoyama, S.; Saiki, I.; Hayakawa, Y. Selective anticancer activity of Hirsutine against HER2-positive breast cancer cells by inducing DNA damage. Oncol. Rep. 2015, 33, 2072–2076. [Google Scholar] [CrossRef] [Green Version]
- Lou, C.; Takahashi, K.; Irimura, T.; Saiki, I.; Hayakawa, Y.J. Identification of Hirsutine as an anti-metastatic phytochemical by targeting NF-κB activation. Int. J. Oncol. 2014, 45, 2085–2091. [Google Scholar] [CrossRef]
- Lin, B.; Li, D.; Zhang, L. Oxymatrine mediates Bax and Bcl-2 expression in human breast cancer MCF-7 cells. Pharmazie 2016, 71, 154–157. [Google Scholar]
- Zhang, Y.; Piao, B.; Zhang, Y.; Hua, B.; Hou, W.; Xu, W.; Qi, X.; Zhu, X.; Pei, Y.; Lin, H. Oxymatrine diminishes the side population and inhibits the expression of β-catenin in MCF-7 breast cancer cells. Med. Oncol. 2010, 28, 99–107. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, W.; Serra, S.; Asa, S.; Ezzat, S. The breast cancer susceptibility FGFR2 provides an alternate mode of HER2 activation. Oncogene 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Biswas, J.; Nabavi, S.M.; Bishayee, A. Tea phytochemicals for breast cancer prevention and intervention: From bench to bedside and beyond. Semin. Cancer Biol. 2017, 46, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Han, Z.; Li, X.; Xie, H.-H.; Zhu, S.-S. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Santoro, M.; Guido, C.; Russo, A.; Aquila, S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol. Nutr. Food Res. 2012, 56, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Hong, O.-Y.; Noh, E.-M.; Jang, H.-Y.; Lee, Y.-R.; Kil Lee, B.; Jung, S.H.; Kim, J.-S.; Young-Rae, L. Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-catenin signaling pathway. Oncol. Lett. 2017, 14, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-J.; Wang, K.-L.; Chen, H.-Y.; Chiang, Y.-F.; Hsia, S.-M. Protective effects of epigallocatechin gallate (EGCG) on endometrial, breast, and ovarian cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Mirza, S.; Sharma, G.; Parshad, R.; Gupta, S.D.; Pandya, P.; Ralhan, R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J. Breast Cancer 2013, 16, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-Cachón, R.B.R.; Ascacio-Martínez, J.Á.; Barrera-Saldaña, H.A. Individual response to drug therapy: Bases and study approaches. Rev. Investig. Clin. 2012, 64, 364–376. [Google Scholar]
- Choi, E.J.; Jung, J.Y.; Kim, G.-H. Genistein inhibits the proliferation and differentiation of MCF-7 and 3T3-L1 cells via the regulation of ERα expression and induction of apoptosis. Exp. Ther. Med. 2014, 8, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Bai, Q.; Zou, L.-Y.; Zhang, Q.-Y.; Zhou, Y.; Chang, H.; Yi, L.; Zhu, J.-D.; Mi, M.-T. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosom. Cancer 2014, 53, 422–431. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- Avci, C.B.; Susluer, S.Y.; Çağlar, H.O.; Balci, T.; Aygunes, D.; Dodurga, Y.; Gunduz, C. Genistein-induced mir-23b expression inhibits the growth of breast cancer cells. Contemp. Oncol. 2015, 1, 32–35. [Google Scholar] [CrossRef]
- De La Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.; Dharmawardhane, S. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr. Cancer 2015, 68, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.J.B.; et al. Mechanistic insights and perspectives involved in nfeuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Maryam, R.; Faegheh, S.; Majid, A.-S.; Kazem, N.-K. Effect of quercetin on secretion and gene expression of leptin in breast cancer. J. Tradit. Chin. Med. 2017, 37, 321–325. [Google Scholar] [CrossRef]
- Khorsandi, L.; Orazizadeh, M.; Niazvand, F.; Abbaspour, M.R.; Mansouri, E.; Khodadadi, A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl. Med. J. 2017, 118, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.S.; Khalil, M.I.; Sami, B.M.; Alkhuriji, A.F.; Sadek, O.J. Quercetin induces apoptosis in triple-negative breast cancer cells via inhibiting fatty acid synthase and β-catenin. J. Clin. Exp. Pathol. 2017, 10, 156–172. [Google Scholar]
- Deng, X.-H.; Song, H.-Y.; Zhou, Y.-F.; Yuan, G.-Y.; Zheng, F.-J. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp. Ther. Med. 2013, 6, 1155–1158. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wang, Q.; Yang, S.; Chen, C.; Li, X.; Liu, J.; Zou, Z.; Cai, D. Quercetin inhibits angiogenesis by targeting calcineurin in the xenograft model of human breast cancer. Eur. J. Pharmacol. 2016, 781, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.-F.; He, H.-F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef]
- Cao, X.; Liu, B.; Cao, W.; Zhang, W.; Zhang, F.; Zhao, H.; Meng, R.; Zhang, L.; Niu, R.; Hao, X.; et al. Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells. Chin. J. Cancer Res. 2013, 25, 212–222. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Parra, M.R.; Stoub, H.; Gallo, K.A.; Doseff, A.I. Apigenin by targeting hnRNPA2 sensitizes triple-negative breast cancer spheroids to doxorubicin-induced apoptosis and regulates expression of ABCC4 and ABCG2 drug efflux transporters. Biochem. Pharmacol. 2020, 182, 114259. [Google Scholar] [CrossRef]
- Korga-Plewko, A.; Michalczyk, M.; Adamczuk, G.; Humeniuk, E.; Ostrowska-Lesko, M.; Jozefczyk, A.; Iwan, M.; Wojcik, M.; Dudka, J. Apigenin and Hesperidin downregulate DNA repair genes in MCF-7 breast cancer cells and augment doxorubicin toxicity. Molecules 2020, 25, 4421. [Google Scholar] [CrossRef]
- Kim, S.-H.; Hwang, K.-A.; Choi, K.-C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef]
- Lee, G.-A.; Choi, K.-C.; Hwang, K.-A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimarães, J.T.; Keating, E.; Martel, F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a flavonoid compound from Gynura medica induced apoptosis and growth inhibition in MCF-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-L.; Yu, Y.-C.; Hsia, S.-M. Perspectives on the role of isoliquiritigenin in cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jodoin, P.-M.; Porikli, F.; Konrad, J.; Benezeth, Y.; Ishwar, P. CDnet 2014: An expanded change detection benchmark dataset. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition Workshops, Columbus, OH, USA, 23–28 June 2014; pp. 387–394. [Google Scholar]
- Wang, N.; Wang, Z.; Wang, Y.; Xie, X.; Shen, J.; Peng, C.; You, J.; Peng, F.; Tang, H.; Guan, X.; et al. Dietary compound isoliquiritigenin prevents mammary carcinogenesis by inhibiting breast cancer stem cells through WIF1 demethylation. Oncotarget 2015, 6, 9854–9876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Al Basher, G.; Abdel-Daim, M.M.; Almeer, R.; Ibrahim, K.; Hamza, R.Z.; Bungau, S.; Aleya, L. Synergistic antioxidant effects of resveratrol and curcumin against fipronil-triggered oxidative damage in male albino rats. Environ. Sci. Pollut. Res. 2019, 27, 6505–6514. [Google Scholar] [CrossRef]
- Grover, M.; Behl, T.; Sachdeva, M.; Bungao, S.; Aleya, L.; Setia, D. Focus on multi-targeted role of curcumin: A boon in therapeutic paradigm. Environ. Sci. Pollut. Res. 2021, 1–15. [Google Scholar] [CrossRef]
- Kumar, U.; Sharma, U.; Rathi, G. Reversal of hypermethylation and reactivation of glutathione S-transferase pi 1 gene by curcumin in breast cancer cell line. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Xie, Z.; Wu, L.-C.; Chiu, M.; Lin, J.; Chan, K.K.; Liu, S.; Liu, Z. Reactivation of RASSF1A in breast cancer cells by curcumin. Nutr. Cancer 2012, 64, 1228–1235. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2009, 122, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Thamake, S.I.; Raut, S.L.; Ranjan, A.P.; Gryczynski, Z.; Vishwanatha, J. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology 2010, 22, 035101. [Google Scholar] [CrossRef] [PubMed]
- Delman, D.M.; Fabian, C.J.; Kimler, B.F.; Yeh, H.; Petroff, B.K. Effects of flaxseed lignan secoisolariciresinol diglucosideon preneoplastic biomarkers of cancer progression in a model of simultaneous breast and ovarian cancer development. Nutr. Cancer 2015, 67, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [Green Version]
- Akter, R.; Rahman, H.; Behl, T.; Chowdhury, A.R.; Manirujjaman, M.; Bulbul, I.J.; Elshenaw, S.E.; Tit, D.M.; Bungau, S. Prospective role of polyphenolic compounds in the treatment of neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Faisal, S.M.; Khan, M.A. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014, 38, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Medina-Aguilar, R.; Marchat, L.A.; Arechaga Ocampo, E.; Gariglio, P.; García Mena, J.; Villegas Sepúlveda, N.; Martínez Castillo, M.; López-Camarillo, C.J.O. Resveratrol inhibits cell cycle progression by targeting Aurora kinase A and Polo-like kinase 1 in breast cancer cells. Oncol. Rep. 2016, 35, 3696–3704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo-Torres, E.; Rodríguez, G.; Meneses-Morales, I.; Zarain-Herzberg, A.J. ATP2A3 gene as an important player for resveratrol anticancer activity in breast cancer cells. Mol. Carcinog. 2017, 56, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Al-Khedhairy, A.A.; Musarrat, J.J. ZnO and TiO2 nanoparticles as novel antimicrobial agents for oral hygiene: A review. J. Nanoparticle Res. 2015, 17, 1–16. [Google Scholar] [CrossRef]
- Venkatadri, R.; Muni, T.; Iyer, A.K.V.; Yakisich, J.S.; Azad, N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016, 7, e2104. [Google Scholar] [CrossRef] [Green Version]
- Wakimoto, R.; Ono, M.; Takeshima, M.; Higuchi, T.; Nakano, S. Differential anticancer activity of pterostilbene against three subtypes of human breast cancer cells. Anticancer Res. 2017, 37. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.; Portillo, M.; Martínez, J.; Milagro, F. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Wu, C.-H.; Hong, B.-H.; Ho, C.-T.; Yen, G.-C. Targeting cancer stem cells in breast cancer: Potential anticancer properties of 6-shogaol and pterostilbene. J. Agric. Food Chem. 2015, 63, 2432–2441. [Google Scholar] [CrossRef]
- Mak, K.K.; Wu, A.T.; Lee, W.H.; Chang, T.C.; Chiou, J.F.; Wang, L.S.; Wu, C.H.; Huang, C.Y.F.; Shieh, Y.S.; Chao, T.Y.; et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-κ B/microRNA 448 circuit. Mol. Nutr. Food Res. 2013, 57, 1123–1134. [Google Scholar] [CrossRef]
- Hong, B.-H.; Wu, C.-H.; Yeh, C.-T.; Yen, G.-C. Invadopodia-associated proteins blockade as a novel mechanism for 6-shogaol and pterostilbene to reduce breast cancer cell motility and invasion. Mol. Nutr. Food Res. 2013, 57, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, W.-C.; Pan, W.-L.; Law, W.-K.; Hu, J.; Ip, D.T.-M.; Waye, M.M.Y.; Ng, T.-B.; Wan, D.C.-C. Silibinin, a novel chemokine receptor type 4 antagonist, inhibits chemokine ligand 12-induced migration in breast cancer cells. Phytomedicine 2014, 21, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Han, J.; Jeon, M.; You, D.; Lee, J.; Kim, H.J.; Bae, S.; Nam, S.J.; Lee, J.E. Silibinin inhibits triple negative breast cancer cell motility by suppressing TGF-β2 expression. Tumor Biol. 2016, 37, 11397–11407. [Google Scholar] [CrossRef]
- Oh, S.-J.; Jung, S.P.; Han, J.; Kim, S.; Kim, J.S.; Nam, S.J.; Lee, J.E.; Kim, J.-H. Silibinin inhibits TPA-induced cell migration and MMP-9 expression in thyroid and breast cancer cells. Oncol. Rep. 2013, 29, 1343–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadeh, M.M.; Motamed, N.; Ranji, N.; Majidi, M.; Falahi, F. Silibinin-induced apoptosis and downregulation of microRNA-21 and microRNA-155 in MCF-7 human breast cancer cells. J. Breast Cancer 2016, 19, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Saracligil, B.; Ozturk, B.; Bozkurt, S.B.; Kahveci, Y.J. The effect of thymoquinone on the miRNA profile of MCF-7 breast cancer cells. Int. J. Pharm. Sci. Res. 2017, 8, 2849–2852. [Google Scholar]
- Alobaedi, O.H.; Talib, W.H.; Basheti, I.A. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac. J. Trop. Med. 2017, 10, 400–408. [Google Scholar] [CrossRef]
- Parbin, S.; Shilpi, A.; Kar, S.; Pradhan, N.; Sengupta, D.; Deb, M.; Rath, S.K.; Patra, S.K. Insights into the molecular interactions of thymoquinone with histone deacetylase: Evaluation of the therapeutic intervention potential against breast cancer. Mol. BioSyst. 2015, 12, 48–58. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Jeong, D.; Kim, S.J. Ginsenoside Rh2 epigenetically regulates cell-mediated immune pathway to inhibit proliferation of MCF-7 breast cancer cells. J. Ginseng Res. 2018, 42, 455–462. [Google Scholar] [CrossRef]
- Pengyue, Z.; Tao, G.; Hongyun, H.; Liqiang, Y.; Yihao, D. Breviscapine confers a neuroprotective efficacy against transient focal cerebral ischemia by attenuating neuronal and astrocytic autophagy in the penumbra. Biomed. Pharmacother. 2017, 90, 69–76. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, W.; Kanimozhi, G.; Brindha, G.R.; Tian, D. Ginsenoside Rg1 induces apoptotic cell death in triple-negative breast cancer cell lines and prevents carcinogen-induced breast tumorigenesis in Sprague Dawley rats. Evid. Based Complement. Altern. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Diao, H.; Lin, Z.; Gao, J.; Zhao, Y.; Zhang, W.; Wang, Q.; Lin, J.; Zhang, D.; Jin, Y.; et al. Benzyl isothiocyanate induces apoptosis and inhibits tumor growth in canine mammary carcinoma via downregulation of the cyclin B1/Cdk1 pathway. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.; Lim, G.; Li, Y.; Shah, R.; Lim, R.; Paholak, H.J.; McDermott, S.P.; Sun, L.; Tsume, Y.; Bai, S.; et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017, 394, 52–64. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Cheng, J.; Sun, M.; Yang, P.; Zhao, F.; Zhang, J.; Duan, X.; Fu, X.; Zhang, J.; et al. Biomechanically, structurally and functionally meticulously tailored polycaprolactone/silk fibroin scaffold for meniscus regeneration. Theranostics 2020, 10, 5090–5106. [Google Scholar] [CrossRef]
- Kuran, D.; Pogorzelska, A.; Wiktorska, K. Breast cancer prevention—Is there a future for sulforaphane and its analogs? Nutrients 2020, 12, 1559. [Google Scholar] [CrossRef]
- Lee, G.-A.; Hwang, K.-A.; Choi, K.-C. Inhibitory effects of 3,3′-diindolylmethane on epithelial-mesenchymal transition induced by endocrine disrupting chemicals in cellular and xenograft mouse models of breast cancer. Food Chem. Toxicol. 2017, 109, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lv, M.; Wang, Y.; Zhang, J.J.P. Development of novel application of 3,3′-diindolylmethane: Sensitizing multidrug resistance human breast cancer cells to γ-irradiation. Pharm. Biol. 2016, 54, 3164–3168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Ali, S.; Ahmed, A.; Ali, A.S.; Raz, A.; Sakr, W.A.; Rahman, K.W. 3,3′-diindolylmethane Enhances the effectiveness of Herceptin against HER-2/neu-expressing breast cancer cells. PLoS ONE 2013, 8, e54657. [Google Scholar] [CrossRef] [Green Version]
- Kurose, H.; Shibata, M.-A.; Iinuma, M.; Otsuki, Y.J.J. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with α-mangostin extracted from mangosteen pericarp. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhupal, M.; Gupta, M.K.; Tripathy, D.R.; Kumar, M.; Yi, D.K.; Nanda, S.S.; Chowdhury, D. Recent advances in pharmaceutical applications of natural carbohydrate polymer gum tragacanth. Nat. Polym. Pharm. Appl. 2019, 49–86. [Google Scholar] [CrossRef]
- Willenbacher, E.; Khan, S.Z.; Mujica, S.C.A.; Trapani, D.; Hussain, S.; Wolf, D.; Willenbacher, W.; Spizzo, G.; Seeber, A. Curcumin: New insights into an ancient ingredient against cancer. Int. J. Mol. Sci. 2019, 20, 1808. [Google Scholar] [CrossRef] [Green Version]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef] [Green Version]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.-A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.-R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.R.; Heckler, C.E.; Guido, J.J.; Peoples, A.R.; Gewandter, J.S.; Ling, M.; Vinciguerra, V.P.; Anderson, T.; Evans, L.; Wade, J.; et al. Oral curcumin for radiation dermatitis: A URCC NCORP study of 686 breast cancer patients. Support. Care Cancer 2017, 26, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Suzuki, S.; Pagano, I.S.; Morimoto, Y.; Franke, A.A.; Ehya, H. Cytology in nipple aspirate fluid during a randomized soy food intervention among premenopausal women. Nutr. Cancer 2013, 65, 1116–1121. [Google Scholar] [CrossRef] [Green Version]
- Pop, E.A.; Fischer, L.M.; Coan, A.D.; Gitzinger, M.; Nakamura, J.; Zeisel, S.H. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women. Menopause 2008, 15, 684–692. [Google Scholar] [CrossRef] [Green Version]
- Clinton, S. Pilot Study of Curcumin for Women with Obesity and High Risk for Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01975363 (accessed on 16 June 2021).
- Phase II Study of Curcumin vs. Placebo for Chemotherapy-Treated Breast Cancer Patients Undergoing Radiotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT01740323 (accessed on 16 June 2021).
- Gemcitabine Hydrochloride and Genistein in Treating Women with Stage IV Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00244933?term=genistein&cond=breast+cancer (accessed on 21 March 2021).
- Khan, S.A.; Chatterton, R.T.; Michel, N.; Bryk, M.; Lee, O.; Ivancic, D.; Heinz, R.; Zalles, C.M.; Helenowski, I.B.; Jovanovic, B.D.; et al. Soy isoflavone supplementation for breast cancer risk reduction: A randomized phase II trial. Cancer Prev. Res. 2012, 5, 309–319. [Google Scholar] [CrossRef] [Green Version]
- SFX-01 in the Treatment and Evaluation of Metastatic Breast Cancer (STEM). Available online: https://clinicaltrials.gov/ct2/show/NCT02970682 (accessed on 11 June 2021).
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z.J.C. Toxic phytochemicals and their potential risks for human cancer. Cancer Prev. Res. 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Daim, M.M.; Abushouk, A.I.; Donia, T.; Alarifi, S.; Alkahtani, S.; Aleya, L.; Bungau, S. The nephroprotective effects of allicin and ascorbic acid against cisplatin-induced toxicity in rats. Environ. Sci. Pollut. Res. 2019, 26, 13502–13509. [Google Scholar] [CrossRef] [PubMed]





| IUPAC Name | Structure | Phytochemical Usual Name/Natural Sources |
|---|---|---|
| Polyphenols | ||
| [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate |  | Epigallocatechin gallate/ Green Tea |
| 5,7-dihydroxy-3-(4-hydroxyphenyl) chromen-4-one |  | Genistein/Soybean, Soy based products |
| 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one |  | Quercetin/Apple, Grapes, Onion, Berries |
| 5,7-dihydroxy-2-(4-hydroxyphenyl) chromen-4-one |  | Apigenin/Grapefruit, Chamomile, Parsley, Celery |
| 2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one |  | Luteolin/Parsley, Celery, Thyme |
| 3,5,7-trihydroxy-2-(4-hydroxyphenyl) chromen-4-one |  | Kaempferol/Fruits and Vegetables |
| (E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl) prop-2-en-1-one |  | Isoliquiritigenin/Licorice, Soybeans |
| (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl) hepta-1,6-diene-3,5-dione |  | Curcumin/Turmeric |
| (2R,3R)-2,3-bis[(4-hydroxy-3-methoxyphenyl) methyl] butane-1,4-diol |  | Secoisolariciresinol / Flax Seeds, Sesame Seeds, Sunflower Seeds |
| 5-[(E)-2-(4-hydroxyphenyl) ethynyl] benzene-1,3-diol |  | Resveratrol/Grapes, Wine, Blueberries, Cranberries, Mulberries |
| 4-[(E)-2-(3,5-dimethoxyphenyl) ethynyl] phenol |  | Pterostilbene/Blueberries |
| (2R,3R)-3,5,7-trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one |  | Silibinin/Milk Thistle Seeds |
| Terpinoids | ||
| 2-methyl-5-propan-2-ylcyclohexa-2,5-diene-1,4-dione |  | Thymoquinone/Black Cumin |
| (1S,2R,4R,7E,11S)-4,8-dimethyl-12-methylidene-3,14-dioxatricyclo [9.3.0.02,4] tetradec-7-en-13-one |  | Parthenolide/Feverfew |
| Saponins | ||
| (3S,5R,8R,9R,10R,14R,17S)-17-(2-hydroxy-6-methylhept-5-en-2-yl)-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol |  | Ginsenosides/Ginseng |
| Isotiociantes | ||
| 1-isothiocyanato-4-methylsulfinylbutane |  | Sulforaphane/Broccoli |
| Iso-thiocyanato-methyl benzene |  | Benzyl isothiocyanate/Cruciferous Vegetables |
| Others | ||
| 3-(1H-indol-3-ylmethyl)-1H-indole |  | 3,3′-Diindolylmethane/Cabbage |
| 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-9H-xanthen-9-one |  | α-Mangostin/Mangosteen |
| Phytochemicals | Type of the Study/ No. Participants | Details of Breast Cancer | Outcomes | Ref. |
|---|---|---|---|---|
| Curcumin | Phase I/40 | Advanced and metastatic | Dose range study | [134] |
| Phase II/29 | BC | Prevention | [139] | |
| Phase II/35 | High risk | Dose range study | [140] | |
| Phase I/686 | BC | Did not significantly reduce radiation dermatitis | [138] | |
| Phase II/30 | BC | Reducing fatigue in patients with chemotherapy undergoing radiotherapy | [140] | |
| Phase II/150 | Metastatic | Superior to the paclitaxel–placebo combination | [135] | |
| Genistein (with Gemcitabine) | Phase II/17 | Stage IV | No effect | [141] |
| Genistein | Phase II/126 | BC | No effect | [142] |
| Phase I | BC | Dose range study | [138] | |
| Sulphoraphane/isoti | Phase II/60 | Metastatic | Anti-tumor activity and prolonged disease stabilization | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, T.; Dutta, S.; Akter, R.; Rahman, M.H.; Karthika, C.; Nagaswarupa, H.P.; Murthy, H.C.A.; Fratila, O.; Brata, R.; Bungau, S. Role of Phytonutrients in Nutrigenetics and Nutrigenomics Perspective in Curing Breast Cancer. Biomolecules 2021, 11, 1176. https://doi.org/10.3390/biom11081176
Bhattacharya T, Dutta S, Akter R, Rahman MH, Karthika C, Nagaswarupa HP, Murthy HCA, Fratila O, Brata R, Bungau S. Role of Phytonutrients in Nutrigenetics and Nutrigenomics Perspective in Curing Breast Cancer. Biomolecules. 2021; 11(8):1176. https://doi.org/10.3390/biom11081176
Chicago/Turabian StyleBhattacharya, Tanima, Soumam Dutta, Rokeya Akter, Md. Habibur Rahman, Chenmala Karthika, Hechanur Puttappa Nagaswarupa, Hanabe Chowdappa Ananda Murthy, Ovidiu Fratila, Roxana Brata, and Simona Bungau. 2021. "Role of Phytonutrients in Nutrigenetics and Nutrigenomics Perspective in Curing Breast Cancer" Biomolecules 11, no. 8: 1176. https://doi.org/10.3390/biom11081176
APA StyleBhattacharya, T., Dutta, S., Akter, R., Rahman, M. H., Karthika, C., Nagaswarupa, H. P., Murthy, H. C. A., Fratila, O., Brata, R., & Bungau, S. (2021). Role of Phytonutrients in Nutrigenetics and Nutrigenomics Perspective in Curing Breast Cancer. Biomolecules, 11(8), 1176. https://doi.org/10.3390/biom11081176