Young and Undamaged rMSA Improves the Healthspan and Lifespan of Mice
Abstract
:1. Introduction
2. Materials and Methods
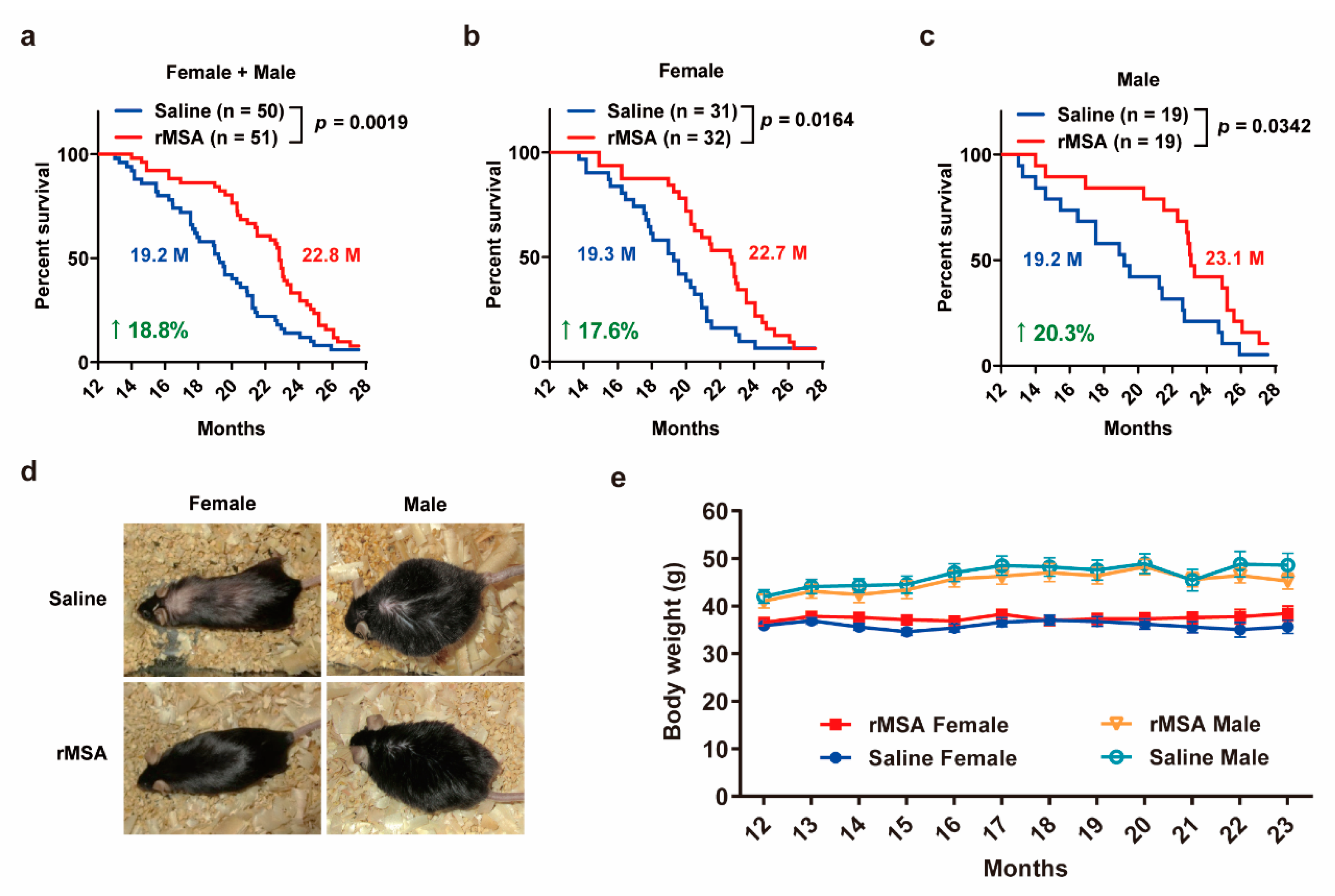
2.1. Mice and Drug Treatments
2.2. Protein Levels Determination
2.3. Grip Strength Test
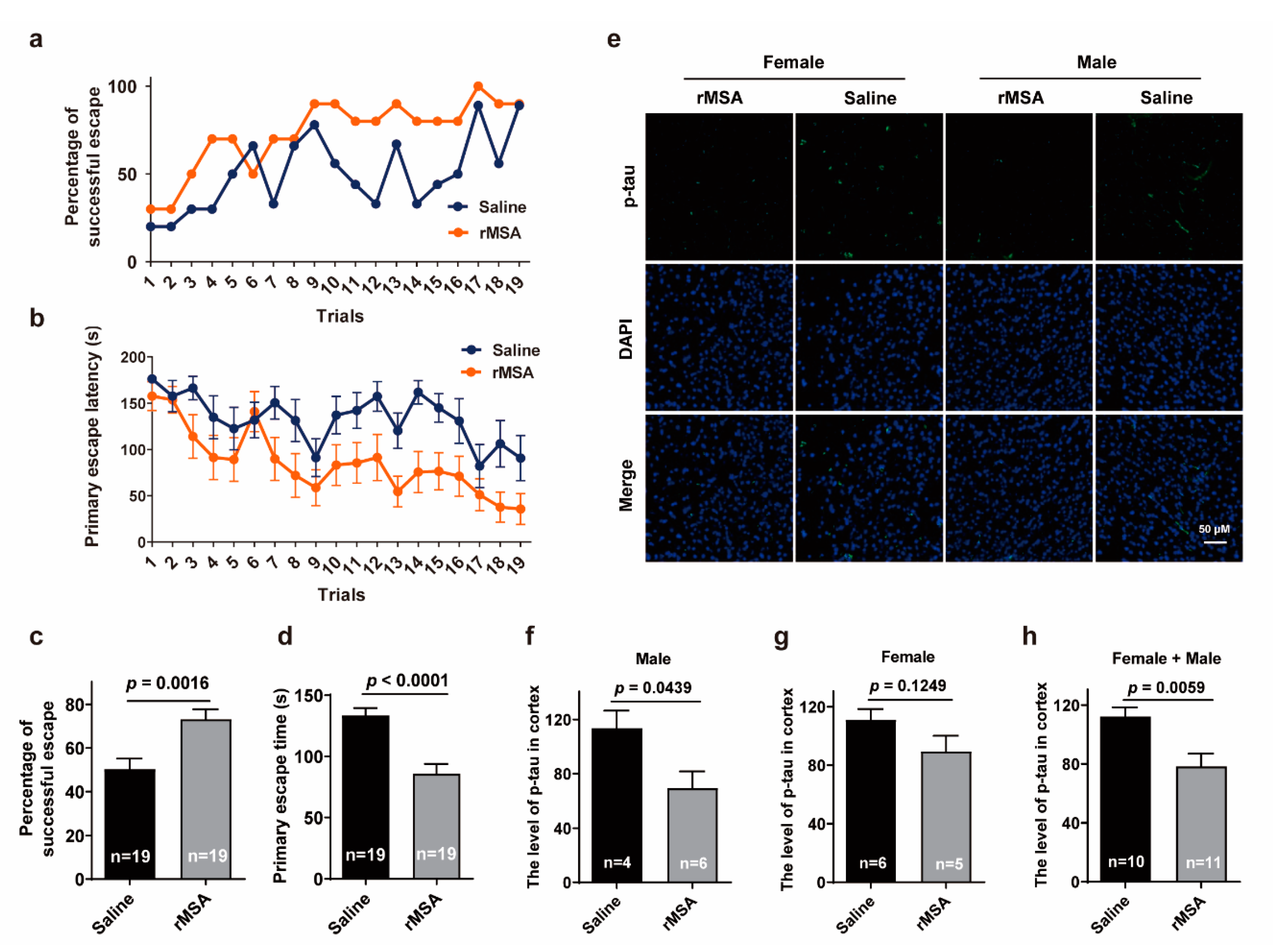
2.4. Barnes Maze Assay
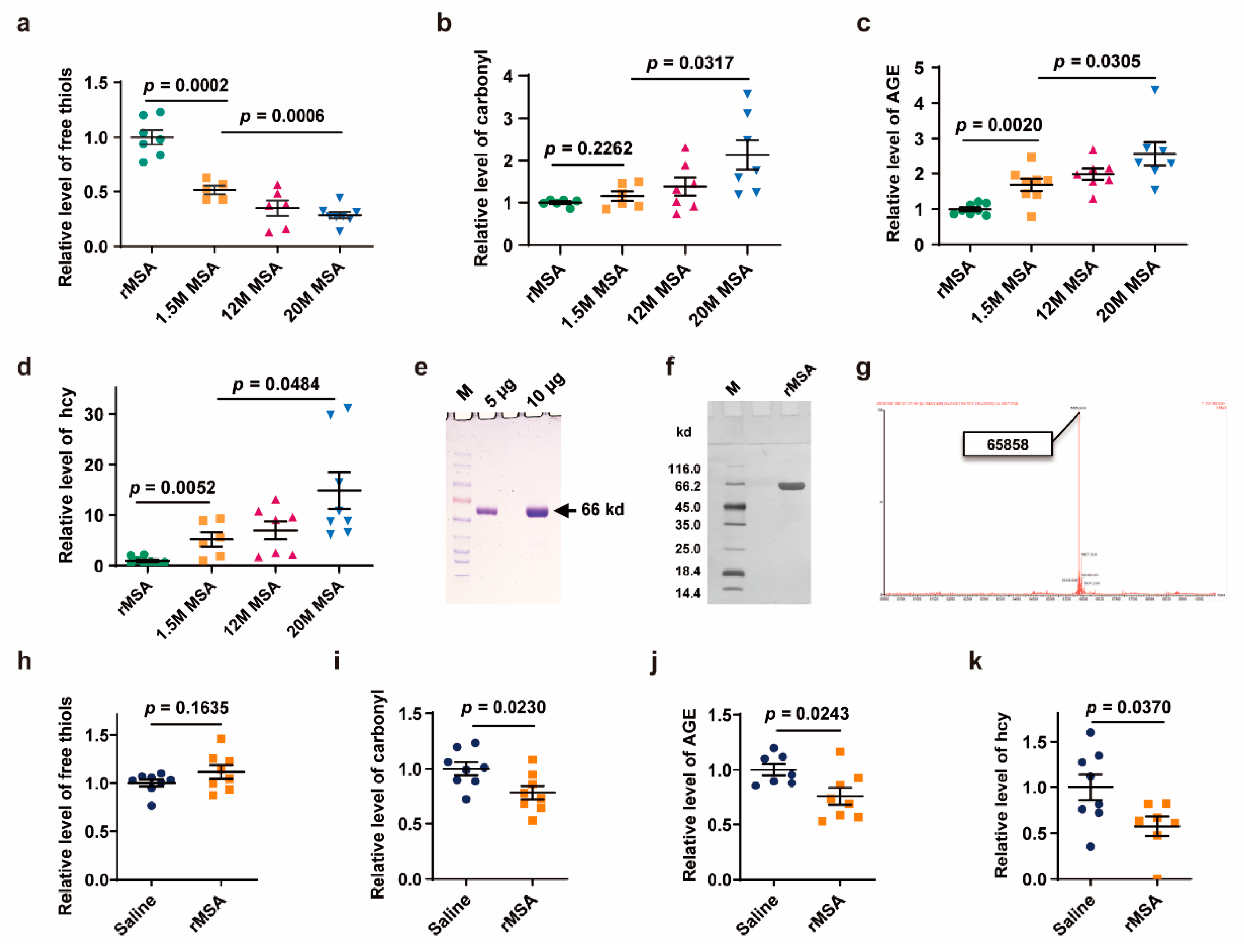
2.5. Albumin Purification
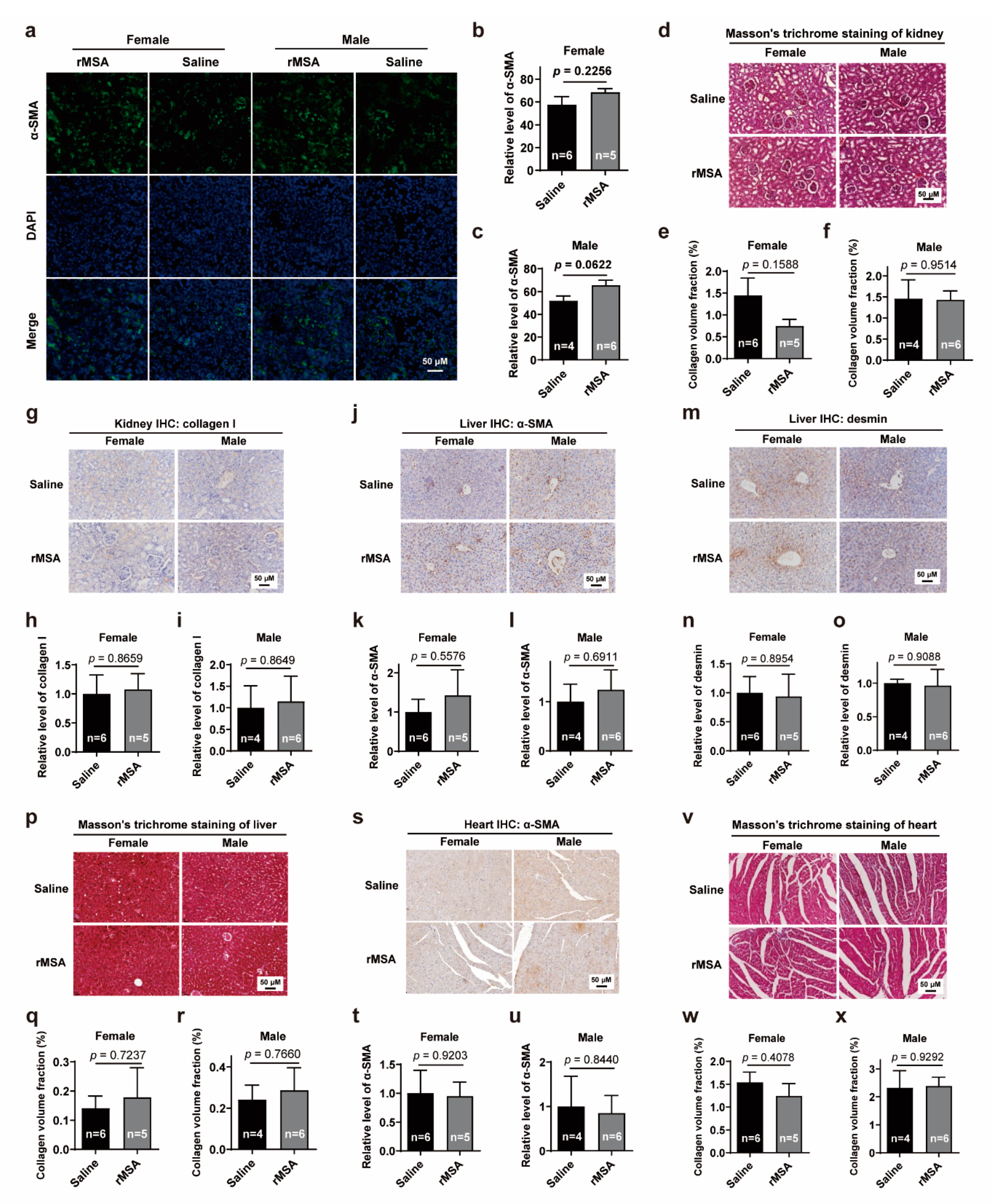
2.6. Immunofluorescence Assay
2.7. Masson’s Trichrome Staining
2.8. Toluidine Blue O Staining
2.9. Immunohistochemical Assay
2.10. Determination of Protein Damages
2.11. Statistical Analysis
3. Results
3.1. Exogenous rMSA Treatment Reduced the Damages of Endogenous Albumin
3.2. Exogenous rMSA Enhanced the Function of Skeletal Muscle in Mice
3.3. Exogenous rMSA Improved the Spatial Learning Ability and Memory of Mice
3.4. Exogenous rMSA Treatment Increased the Lifespan in Mice
3.5. Exogenous rMSA Treatment Is Safe for C57BL/6N Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSA | human serum albumin |
| AGE | advanced glycation end-product |
| hcy | homocysteine |
| rMSA | recombinant mouse serum albumin |
| p-tau | phosphorylated microtubule-associated protein tau |
| MYH7 | myosin heavy chain 7 |
| α-SMA | α-smooth muscle actin |
| COL1A1 | collagen I |
| HCP | host cell proteins |
| qRT-PCR | quantitative RT-PCR |
| ELISA | enzyme-linked immunosorbent assay |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DTNB | 5, 5′-Dithiobis-(2-nitrobenzoic acid) |
| Q-TOF | Quadrupole-Time of Flight |
| K-S test | Kolmogorov–Smirnov test |
References
- Ludwig, F.C.; Elashoff, R.M. Mortality in Syngeneic Rat Parabionts of Different Chronological Age. Trans. N. Y. Acad. Sci. 1972, 34, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Egerman, M.A.; Cadena, S.M.; Gilbert, J.A.; Meyer, A.; Nelson, H.N.; Swalley, S.E.; Mallozzi, C.; Jacobi, C.; Jennings, L.L.; Clay, I.; et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015, 22, 164–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, D.; Chen, K. The essential mechanisms of aging: Irreparable damage accumulation of biochemical side-reactions. Exp. Gerontol. 2005, 40, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Losada, P.M.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Rattan, S.I. Increased molecular damage and heterogeneity as the basis of aging. Biol. Chem. 2008, 389, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Van Remmen, H.; Richardson, A.; Wehr, N.B.; Levine, R.L. Methionine oxidation and aging. Biochim. Biophys. Acta Proteins Proteom. 2005, 1703, 135–140. [Google Scholar] [CrossRef]
- Pandey, K.B.; Mehdi, M.M.; Maurya, P.K.; Rizvi, S.I. Plasma protein oxidation and its correlation with antioxidant potential during human aging. Dis. Markers 2010, 29, 31–36. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation end products: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Ostrakhovitch, E.A.; Tabibzadeh, S. Homocysteine and age-associated disorders. Ageing Res. Rev. 2019, 49, 144–164. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Serum albumin. Hepatology 1988, 8, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Garcia Martinez, R.; Caraceni, P.; Bernardi, M.; Gines, P.; Arroyo, V.; Jalan, R. Albumin: Pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013, 58, 1836–1846. [Google Scholar] [CrossRef]
- Carballal, S.; Radi, R.; Kirk, M.C.; Barnes, S.; Freeman, B.A.; Alvarez, B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry 2003, 42, 9906–9914. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radical. Bio. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Leto, S.; Yiengst, M.J.; Barrows, C.J. The effect of age and protein deprivation on the sulfhydryl content of serum albumin. J. Gerontol. 1970, 25, 4–8. [Google Scholar] [CrossRef]
- Era, S.; Kuwata, K.; Imai, H.; Nakamura, K.; Hayashi, T.; Sogami, M. Age-related change in redox state of human serum albumin. Biochim. Biophys. Acta 1995, 1247, 12–16. [Google Scholar] [CrossRef]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [Green Version]
- Chevion, M.; Berenshtein, E.; Stadtman, E.R. Human studies related to protein oxidation: Protein carbonyl content as a marker of damage. Free Radic. Res. 2000, 33, S99–S108. [Google Scholar] [PubMed]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox Signal. 2012, 17, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Jana, C.K.; Das, N.; Sohal, R.S. Specificity of age-related carbonylation of plasma proteins in the mouse and rat. Arch. Biochem. Biophys. 2002, 397, 433–439. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Liu, H.; Che, Y.; Xu, Y.; Lingling, E. Age-related variations of protein carbonyls in human saliva and plasma: Is saliva protein carbonyls an alternative biomarker of aging? Age 2015, 37, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am. J. Pathol. 1969, 56, 111–128. [Google Scholar] [PubMed]
- McLean, R.R.; Jacques, P.F.; Selhub, J.; Tucker, K.L.; Samelson, E.J.; Broe, K.E.; Hannan, M.T.; Cupples, L.A.; Kiel, D.P. Homocysteine as a predictive factor for hip fracture in older persons. N Engl. J. Med. 2004, 350, 2042–2049. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine is a protein amino acid in humans. Implications for homocysteine-linked disease. J. Biol. Chem. 2002, 277, 30425–30428. [Google Scholar] [CrossRef] [Green Version]
- Glowacki, R.; Jakubowski, H. Cross-talk between Cys34 and lysine residues in human serum albumin revealed by N-homocysteinylation. J. Biol. Chem. 2004, 279, 10864–10871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowski, H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Ferguson, S.A. Barnes maze testing strategies with small and large rodent models. J. Vis. Exp. 2014, 84, e51194. [Google Scholar] [CrossRef]
- Ellman, G.L. A colorimetric method for determining low concentrations of mercaptans. Arch. Biochem. Biophys. 1958, 74, 443–450. [Google Scholar] [CrossRef]
- Sheng, J.; Wang, Y.; Turesky, R.J.; Kluetzman, K.; Zhang, Q.; Ding, X. Novel transgenic mouse model for studying human serum albumin as a biomarker of carcinogenic exposure. Chem. Res. Toxicol. 2016, 29, 797–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohal, R.; Ku, H.; Agarwal, S.; Forster, M.; Lal, H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994, 74, 121–133. [Google Scholar] [CrossRef]
- Conti, B.; Sanchez-Alavez, M.; Winsky-Sommerer, R.; Morale, M.C.; Lucero, J.; Brownell, S.; Fabre, V.; Huitron-Resendiz, S.; Henriksen, S.; Zorrilla, E.P.; et al. Transgenic Mice with a Reduced Core Body Temperature Have an Increased Life Span. Science 2006, 314, 825–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Abdulle, A.E.; Bourgonje, A.R.; Kieneker, L.M.; Koning, A.M.; la Bastide-van, G.S.; Bulthuis, M.; Dijkstra, G.; Faber, K.N.; Dullaart, R.; Bakker, S.; et al. Serum free thiols predict cardiovascular events and all-cause mortality in the general population: A prospective cohort study. BMC Med. 2020, 18, 130. [Google Scholar] [CrossRef]
- Yaffe, K.; Lindquist, K.; Schwartz, A.V.; Vitartas, C.; Vittinghoff, E.; Satterfield, S.; Simonsick, E.M.; Launer, L.; Rosano, C.; Cauley, J.A.; et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 2011, 77, 1351–1356. [Google Scholar] [CrossRef] [Green Version]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; Berg, E.H.V.D.; Gansevoort, R.T.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; et al. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef]
- Mehdipour, M.; Skinner, C.; Wong, N.; Lieb, M.; Liu, C.; Etienne, J.; Kato, C.; Kiprov, D.; Conboy, M.J.; Conboy, I.M. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin. Aging 2020, 12, 8790–8819. [Google Scholar] [CrossRef]
- Shytikov, D.; Balva, O.; Debonneuil, E.; Glukhovskiy, P.; Pishel, I.M. Aged Mice Repeatedly Injected with Plasma from Young Mice: A Survival Study. BioRes. Open Access 2014, 3, 226–232. [Google Scholar] [CrossRef]
- Boada, M.; López, O.L.; Olazarán, J.; Núñez, L.; Pfeffer, M.; Paricio, M.; Lorites, J.; Piñol-Ripoll, G.; Gámez, J.E.; Anaya, F.; et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: Primary results of the AMBAR Study. Alzheimer’s Dement. 2020, 16, 1412–1425. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Ju, A.; Li, B.; Zhang, S.; Gong, Y.; Ma, B.; Jiang, Y.; Liu, H.; Fu, Y.; Luo, Y. Young and Undamaged rMSA Improves the Healthspan and Lifespan of Mice. Biomolecules 2021, 11, 1191. https://doi.org/10.3390/biom11081191
Tang J, Ju A, Li B, Zhang S, Gong Y, Ma B, Jiang Y, Liu H, Fu Y, Luo Y. Young and Undamaged rMSA Improves the Healthspan and Lifespan of Mice. Biomolecules. 2021; 11(8):1191. https://doi.org/10.3390/biom11081191
Chicago/Turabian StyleTang, Jiaze, Anji Ju, Boya Li, Shaosen Zhang, Yuanchao Gong, Boyuan Ma, Yi Jiang, Hongyi Liu, Yan Fu, and Yongzhang Luo. 2021. "Young and Undamaged rMSA Improves the Healthspan and Lifespan of Mice" Biomolecules 11, no. 8: 1191. https://doi.org/10.3390/biom11081191
APA StyleTang, J., Ju, A., Li, B., Zhang, S., Gong, Y., Ma, B., Jiang, Y., Liu, H., Fu, Y., & Luo, Y. (2021). Young and Undamaged rMSA Improves the Healthspan and Lifespan of Mice. Biomolecules, 11(8), 1191. https://doi.org/10.3390/biom11081191





