Heart Organoids and Engineered Heart Tissues: Novel Tools for Modeling Human Cardiac Biology and Disease
Abstract
:1. Introduction
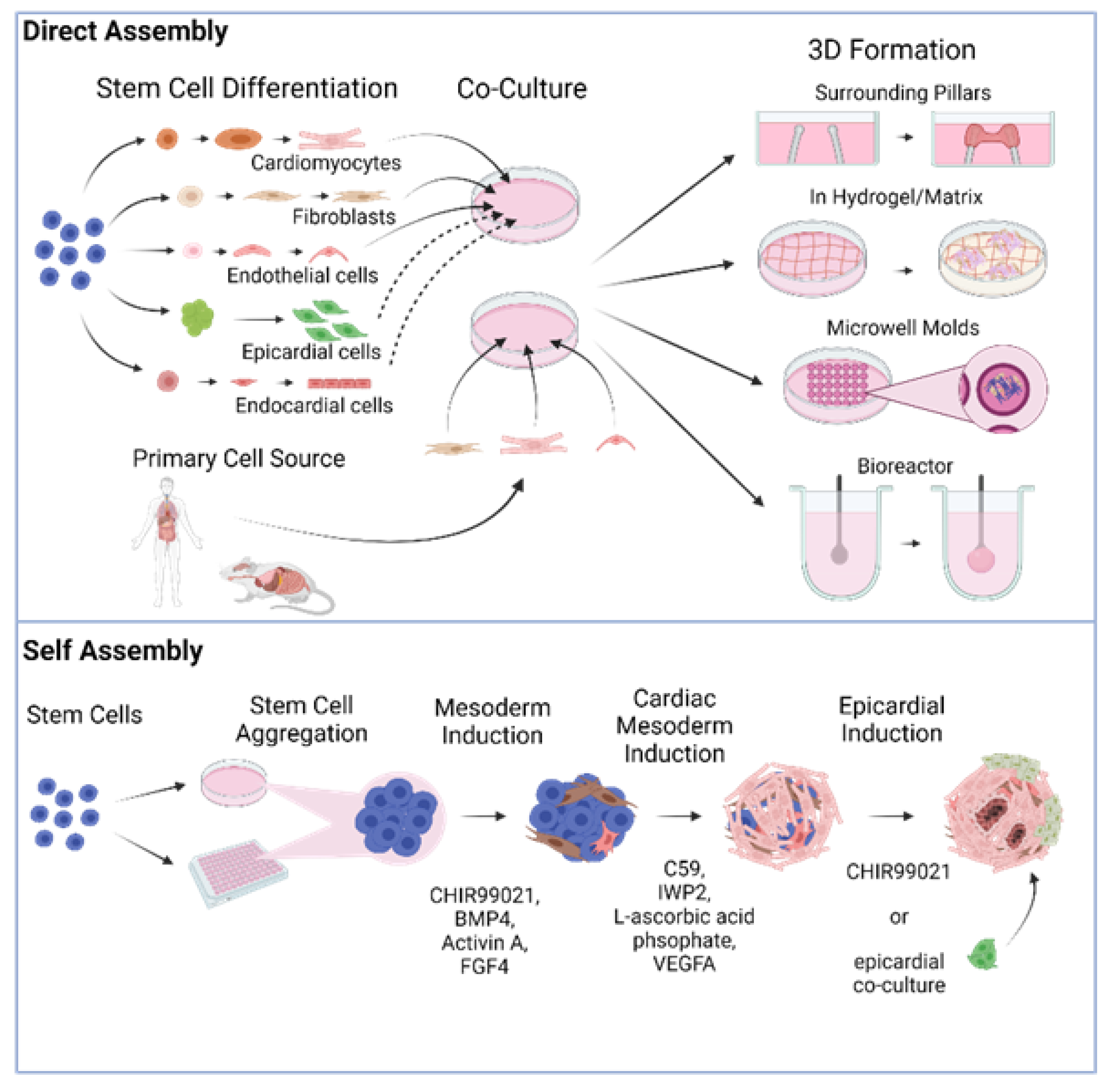
2. Directed Assembly of Human Heart Organoids
3. Self-Organizing Human Heart Organoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the american heart association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.; Yang, S.M.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nat. Cell Biol. 2017, 545, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.T.; Pollock, K.M.; Rose, M.D.; Cary, W.A.; Stewart, H.R.; Zhou, P.; Nolta, J.; Waldau, B. Generation of human vascularized brain organoids. NeuroReport 2018, 29, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.H.; Tsai, Y.-H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4, e05098. [Google Scholar] [CrossRef] [PubMed]
- Porotto, M.; Ferren, M.; Chen, Y.-W.; Siu, Y.; Makhsous, N.; Rima, B.; Briese, T.; Greninger, A.; Snoeck, H.-W.; Moscona, A. Authentic Modeling of Human Respiratory Virus Infection in Human Pluripotent Stem Cell-Derived Lung Organoids. mBio 2019, 10, e00723-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahudeen, A.A.; Choi, S.S.; Rustagi, A.; Zhu, J.; van Unen, V.; de la O, S.M.; Flynn, R.A.; Margalef-Català, M.; Santos, A.J.M.; Ju, J.; et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 2020, 588, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.; Baptista, P.M.; Brovold, M.; Moran, E.; Gaston, B.; Booth, C.; Samuel, M.; Atala, A.; Soker, S. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology 2018, 67, 750–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, H.; Deng, P.; Chen, W.; Guo, Y.; Tao, T.; Qin, J. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip 2018, 18, 3606–3616. [Google Scholar] [CrossRef]
- Mun, S.J.; Ryu, J.-S.; Lee, M.-O.; Son, Y.S.; Oh, S.J.; Cho, H.-S.; Son, M.-Y.; Kim, D.-S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef]
- Broutier, L.; Rolf, A.A.; Hindley, C.; Boj, S.F.; Clevers, H.; Koo, B.; Ortega, M.H. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]
- Dossena, M.; Piras, R.; Cherubini, A.; Barilani, M.; Dugnani, E.; Salanitro, F.; Moreth, T.; Pampaloni, F.; Piemonti, L.; Lazzari, L. Standardized GMP-compliant scalable production of human pancreas organoids. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Georgakopoulos, N.; Prior, N.; Angres, B.; Mastrogiovanni, G.; Cagan, A.; Harrison, D.; Hindley, C.J.; Arnes-Benito, R.; Liau, S.-S.; Curd, A.; et al. Long-term expansion, genomic stability and in vivo safety of adult human pancreas organoids. BMC Dev. Biol. 2020, 20, 4. [Google Scholar] [CrossRef] [Green Version]
- Spence, J.R.; Mayhew, C.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nat. Cell Biol. 2010, 470, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Serra, D.; Mayr, U.; Boni, A.; Lukonin, I.; Rempfler, M.; Meylan, L.C.; Stadler, M.B.; Strnad, P.; Papasaikas, P.; Vischi, D.; et al. Self-organization and symmetry breaking in intestinal organoid development. Nat. Cell Biol. 2019, 569, 66–72. [Google Scholar] [CrossRef]
- Mithal, A.; Capilla, A.; Heinze, D.; Berical, A.; Villacorta-Martin, C.; Vedaie, M.; Jacob, A.; Abo, K.; Szymaniak, A.; Peasley, M.; et al. Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Vilar, E.; Tsai, S.-Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.C.S.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Múnera, J.O.; Sundaram, N.; Rankin, S.A.; Hill, D.; Watson, C.; Mahe, M.; Vallance, J.; Shroyer, N.F.; Sinagoga, K.L.; Zarzoso-Lacoste, A.; et al. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 2017, 21, 51–64.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michels, B.E.; Mosa, M.H.; Grebbin, B.M.; Yepes, D.; Darvishi, T.; Hausmann, J.; Urlaub, H.; Zeuzem, S.; Kvasnicka, H.M.; Oellerich, T.; et al. Human colon organoids reveal distinct physiologic and oncogenic Wnt responses. J. Exp. Med. 2019, 216, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.; Ferguson, C.; Parton, R.; Wolvetang, E.; Roost, M.S.; Lopes, S.M.C.d.S.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nat. Cell Biol. 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, K.; Wu, H.; Yoshimura, Y.; Humphreys, B.D. Human pluripotent stem cell-derived kidney organoids with improved collecting duct maturation and injury modeling. Cell Rep. 2020, 33, 108514. [Google Scholar] [CrossRef]
- Corrò, C.; Novellasdemunt, L.; Li, V.S. A brief history of organoids. Am. J. Physiol. Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nat. Cell Biol. 2018, 556, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Keung, W.; Chan, P.K.W.; Backeris, P.C.; Lee, E.K.; Wong, N.; Wong, A.O.; Wong, G.K.; Chan, C.W.Y.; Fermini, B.; Costa, K.D.; et al. Human cardiac ventricular-like organoid chambers and tissue strips from pluripotent stem cells as a two-tiered assay for inotropic responses. Clin. Pharmacol. Ther. 2019, 106, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Bursac, N.; Papadaki, M.; Cohen, R.J.; Schoen, F.J.; Eisenberg, S.R.; Carrier, R.; Vunjak-Novakovic, G.; Freed, L.E. Cardiac muscle tissue engineering: Toward an in vitro model for electrophysiological studies. Am. J. Physiol. Content 1999, 277, H433–H444. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.H.; Fink, C.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol. Bioeng. 2000, 68, 106–114. [Google Scholar] [CrossRef]
- Kelm, J.M.; Fussenegger, M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004, 22, 195–202. [Google Scholar] [CrossRef]
- Guo, X.-M.; Zhao, Y.-S.; Chang, H.-X.; Wang, C.-Y.; E, L.-L.; Zhang, X.-A.; Duan, C.-M.; Dong, L.-Z.; Jiang, H.; Li, J.; et al. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation 2006, 113, 2229–2237. [Google Scholar] [CrossRef] [Green Version]
- Radisic, M.; Fast, V.G.; Sharifov, O.F.; Iyer, R.K.; Park, H.; Vunjak-Novakovic, G. Optical mapping of impulse propagation in engineered cardiac tissue. Tissue Eng. Part A 2009, 15, 851–860. [Google Scholar] [CrossRef]
- de Lange, W.J.; Hegge, L.F.; Grimes, A.C.; Tong, C.W.; Brost, T.M.; Moss, R.L.; Ralphe, J.C. Neonatal mouse-derived engineered cardiac tissue: A novel model system for studying genetic heart disease. Circ. Res. 2011, 109, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Liau, B.; Christoforou, N.; Leong, K.; Bursac, N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials 2011, 32, 9180–9187. [Google Scholar] [CrossRef] [Green Version]
- Figtree, G.A.; Bubb, K.J.; Tang, O.; Kizana, E.; Gentile, C. Vascularized cardiac spheroids as novel 3D in vitro models to study cardiac fibrosis. Cells Tissues Organs 2017, 204, 191–198. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kofron, C.M.; King, M.E.; Markes, A.R.; Okundaye, A.O.; Qu, Z.; Mende, U.; Choi, B.-R. Directed fusion of cardiac spheroids into larger heterocellular microtissues enables investigation of cardiac action potential propagation via cardiac fibroblasts. PLoS ONE 2018, 13, e0196714. [Google Scholar] [CrossRef] [Green Version]
- Khademhosseini, A.; Eng, G.; Yeh, J.; Kucharczyk, P.A.; Langer, R.; Vunjak-Novakovic, G.; Radisic, M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed. Microdevices 2006, 9, 149–157. [Google Scholar] [CrossRef]
- Boudou, T.; Legant, W.R.; Mu, A.; Borochin, M.A.; Thavandiran, N.; Radisic, M.; Zandstra, P.W.; Epstein, J.A.; Margulies, K.B.; Chen, C.S. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 2012, 18, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Oltolina, F.; Zamperone, A.; Colangelo, D.; Gregoletto, L.; Reano, S.; Pietronave, S.; Merlin, S.; Talmon, M.; Novelli, E.; Diena, M.; et al. Human cardiac progenitor spheroids exhibit enhanced engraftment potential. PLoS ONE 2015, 10, e0137999. [Google Scholar]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Liao, M.-L.C.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Titmarsh, D.M.; Koenig, X.; Parker, B.L.; Ryall, J.; Quaife-Ryan, G.; Voges, H.K.; Hodson, M.; Ferguson, C.; Drowley, L.; et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci. USA 2017, 114, E8372–E8381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef] [Green Version]
- Kupfer, M.E.; Lin, W.-H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.B.; Lenz, M.; Ai, J.; Mahutga, R.R.; et al. In situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.; Vu, K.; London, N. Organoid and spheroid tumor models: Techniques and applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T.; et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Drakhlis, L.; Biswanath, S.; Farr, C.-M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Israeli, Y.; Wasserman, A.; Gabalski, M.; Ming, Y.; Ball, K.; Volmert, B.; Yang, W.; Li, B.; Zou, J.; Ni, G.; et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2020. [Google Scholar] [CrossRef]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021, 184, 3299–3317.e22. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Parker, B.L.; Quaife-Ryan, G.; Voges, H.K.; Needham, E.; Bornot, A.; Ding, M.; Andersson, H.; Polla, M.; Elliott, D.A.; et al. Drug screening in human psc-cardiac organoids identifies pro-proliferative compounds acting via the mevalonate pathway. Cell Stem Cell 2019, 24, 895–907.e6. [Google Scholar] [CrossRef]
- Buhaescu, I.; Izzedine, H. Mevalonate pathway: A review of clinical and therapeutical implications. Clin. Biochem. 2007, 40, 575–584. [Google Scholar] [CrossRef]
- Mills, R.J.; Humphrey, S.J.; Fortuna, P.R.J.; Lor, M.; Foster, S.R.; Quaife-Ryan, G.A.; Johnston, R.L.; Dumenil, T.; Bishop, C.; Rudraraju, R.; et al. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell 2021, 184, 2167–2182.e22. [Google Scholar] [CrossRef] [PubMed]
- Voges, H.K.; Mills, R.J.; Elliott, D.; Parton, R.; Porrello, E.R.; Hudson, J.E. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 2017, 144, 1118–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, S.S.; Miklas, J.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Massé, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods 2013, 10, 781–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Nunes, S.S. Maturation of human stem cell-derived cardiomyocytes in biowires using electrical stimulation. J. Vis. Exp. 2017, 1–8. [Google Scholar] [CrossRef]
- Leonard, A.; Bertero, A.; Powers, J.D.; Beussman, K.; Bhandari, S.; Regnier, M.; Murry, C.E.; Sniadecki, N.J. Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. J. Mol. Cell. Cardiol. 2018, 118, 147–158. [Google Scholar] [CrossRef]
- Lu, K.; Seidel, T.; Cao-Ehlker, X.; Dorn, T.; Batcha, A.M.N.; Schneider, C.M.; Semmler, M.; Volk, T.; Moretti, A.; Dendorfer, A.; et al. Progressive stretch enhances growth and maturation of 3D stem-cell-derived myocardium. Theranostics 2021, 11, 6138–6153. [Google Scholar] [CrossRef]
- Li, R.A.; Keung, W.; Cashman, T.; Backeris, P.C.; Johnson, B.V.; Bardot, E.; Wong, A.O.; Chan, P.K.; Chan, C.W.; Costa, K.D. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 2018, 163, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Pahlavan, S.; Ansari, H.; Halvaei, M.; Kostin, S.; Feiz, M.-S.; Latifi, H.; Aghdami, N.; Braun, T.; Baharvand, H. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials 2019, 192, 537–550. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.-R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Richards, D.J.; Coyle, R.C.; Tan, Y.; Jia, J.; Wong, K.; Toomer, K.; Menick, D.R.; Mei, Y. Inspiration from heart development: Biomimetic development of functional human cardiac organoids. Biomaterials 2017, 142, 112–123. [Google Scholar] [CrossRef]
- Silva, A.C.; Matthys, O.B.; Joy, D.A.; Kauss, M.A.; Natarajan, V.; Lai, M.H.; Turaga, D.; Blair, A.P.; Alexanian, M.; Bruneau, B.G.; et al. Developmental co-emergence of cardiac and gut tissues modeled by human iPSC-derived organoids. bioRxiv 2021, 2020-04. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.; et al. Revisiting cardiac cellular composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, P.; Tampakakis, E.; Jimenez, D.V.; Kannan, S.; Miyamoto, M.; Shin, H.K.; Saberi, A.; Murphy, S.; Sulistio, E.; Chelko, S.; et al. Precardiac organoids form two heart fields via Bmp/Wnt signaling. Nat. Commun. 2018, 9, 3140. [Google Scholar] [CrossRef]
- Rossi, G.; Broguiere, N.; Miyamoto, M.; Boni, A.; Guiet, R.; Girgin, M.; Kelly, R.G.; Kwon, C.; Lutolf, M.P. Capturing cardiogenesis in gastruloids. Cell Stem Cell 2021, 28, 230–240.e6. [Google Scholar] [CrossRef]
- Mjaatvedt, C.; Nakaoka, T.; Moreno-Rodriguez, R.; Norris, R.; Kern, M.; Eisenberg, C.; Turner, D.; Markwald, R. The outflow tract of the heart is recruited from a novel heart-forming field. Dev. Biol. 2001, 238, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, I.; Parsons, L.M.; Hartley, L.; Li, R.; Andrews, J.E.; Robb, L.; Harvey, R.P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2. Genes Dev. 1995, 9, 1654–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillie-Johnson, P.; Brink, S.C.V.D.; Balayo, T.; Turner, D.A.; Arias, A.M. Generation of aggregates of mouse embryonic stem cells that show symmetry breaking, polarization and emergent collective behaviour in vitro. J. Vis. Exp. 2015, 1–10, e53252. [Google Scholar] [CrossRef] [Green Version]
| Reference | Cell Source | Aggregation Technique | Functional Assessment | Translational Applications |
|---|---|---|---|---|
| Mills et al., 2017 | hiPSC-CMs, Stromal cells | Heart-Dyno: Grow hCOs around two elastomeric posts | Force/contractile analysis, electrophysiology, calcium imaging, metabolic profiling | Cardiac maturation studies |
| Mills et al., 2019 | hiPSC-CMs, Stromal cells | Heart-Dyno: Grow hCOs around two elastomeric posts | Force/contractile analysis | Drug screening: Regenerative responses |
| Mills et al., 2021 | hiPSC-CMs, Stromal cells | Heart-Dyno: Grow hCOs around two elastomeric posts | Force/contractile analysis | Modeling cardiac effects of COVID-19 |
| Voges et al., 2017 | hESC-CMs, Stromal cells | Grow hCOs around two elastic exercise poles | Force/contractile analysis | Cardiac regeneration studies |
| Ronaldson-Bouchard et al., 2018 | hiPSC-CMs, Human DFs | Grow human cardiac tissues around flexible elastomeric pillars | Force/contractile analysis, electrophysiology, calcium imaging, metabolic profiling | Cardiac maturation/adult disease modeling |
| Keung et al., 2019 | hESC-CMs, Human DFs | Grow hvCOC around silicon balloon Foley catheter (custom bioreactor) | Force/contractile analysis | Drug screening: Inotropic responses |
| Kupfer et al., 2020 | hiPSCs-CMs | 3D print hChaMP, culture hiPSCs on construct, differentiate in situ | Force/contractile analysis, pressure/volume analysis, calcium imaging, optical mapping | Medical device testing/tissue grafting |
| Varzideh et al., 2019 | hESC-CPCs, hESC-MSCs, HUVECs | Culture on Matrigel-coated hydrogel | Electrophysiology, voltage-sensitive dye imaging (VSDI) | Transplantation into mice |
| Richards et al., 2020 | Human CFs, HUVECs, hADSCs | Culture in microwells containing agarose hydrogels | Calcium imaging, metabolic profiling, mechanical testing | Myocardial infarction modeling |
| Devarasetty et al., 2017 | hiPSC-CMs, Human CFs | Culture in round-bottom well plates, immobilize in hydrogel | Force/contractile analysis | Drug screening: Chronotropic responses |
| Buono et al., 2020 | hiPSC-CMs, HCMECs, Human CFs | Invert cell suspension, gravity-enforced aggregation (“Hanging Drop”) | Calcium imaging | Modeling hypertrophic cardiomyopathy |
| Silva et al., 2020 | hiPSC-mesendoderm progenitor cells | Rotary orbital suspension culture | Electrophysiology, calcium imaging | Modeling multi-tissue interactions |
| Reference | Cell Source | Aggregation Technique | Differentiation Protocol | Cell Types Observed | Stage of Development | Translational Applications |
|---|---|---|---|---|---|---|
| Andersen et al., 2018 | mESCs, hiPSCs | high-density cell seeding | D0: BMP4/ActA (40 h) | Cardiac progenitor cells, FHF cells, SHF cells, cardiomyocytes, endothelial cells, smooth muscle cells, fibroblasts | Precardiac heart field specification. No beating reported. | Discovery of CXCR4 as SHF progenitor marker in human organoids |
| Lee et al., 2020 | mESCs | round-bottom, low-attachment well plate | D0-D9: FGF4D9-D15: FGF4, BMP4, BIO, LIF | Cardiac progenitor cells, FHF cells, SHF cells, cardiomyocytes (atrial), cardiomyocytes (ventricular), smooth muscle cells, endothelial cells | Atrioventricular chamber specification. Representing embryonic day E7.5-E9.5. Beating after 10 days. | |
| Drakhlis et al., 2021 | hESCs | round-bottom, low-attachment well plate with centrifugation, embedded in Matrigel | D0: CHIR99021 (24 h)D3: IWP2 (48 h) | Cardiac progenitor cells, cardiomyocytes, mesenchymal cells, endothelial cells, endocardial cells, liver anlagen | Cardiac mesoderm and foregut endoderm specification. Beating after 7–10 days. | NKX2.5 knockout recapitulates in vivo congenital heart defects |
| Rossi et al., 2021 | mESCs | round-bottom, low-attachment well plate | D0: CHIR99021 (72 h)D4: bFGF, VEGF165, L-ascorbic acid phosphate (48 h) | Cardiac progenitor cells, FHF cells, SHF cells, cardiomyocytes, endothelial cells, endodermal cells | Cardiac crescent and gut-like tube. Beating after 6 days. | |
| Lewis-Israeli et al., 2021 | hiPSCs,hESCs | round-bottom, low-attachment well plate with centrifugation | D0: CHIR99021/BMP4/ActA (24 h)D2: Wnt C-59 (48 h)D7: CHIR99021 (1 h) | Cardiac progenitor cells, FHF cells, SHF cells, cardiomyocytes (atrial), cardiomyocytes (ventricular), epicardial cells, endocardial cells, endothelial cells, cardiac fibroblasts | Heart field and atrioventricular specification and chamber formation. Fetal-like heart organoids transcriptomically similar to human fetal gestation day 57–67. Beating after 6 days. | Modeling pregestational diabetes-induced congenital heart disease |
| Hofbauer et al., 2021 | hiPSCs,hESCs | round-bottom, low-attachment well plate with centrifugation | D0: CHIR99021, ROCKi (36–40 h)D2: VEGF-A (96 h, with medium change every 48 h)D8.5: co-culture with epicardial aggregates | Cardiac progenitor cells, FHF cells, cardiomyocytes (atrial), cardiomyocytes (ventricular), endothelial cells, epicardial cells, endocardial cells, fibroblasts | First heart field specification and chamber formation. Beating after 7 days. | Cardiac injury model |
| Direct Assembly | Self-Assembly | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis-Israeli, Y.R.; Wasserman, A.H.; Aguirre, A. Heart Organoids and Engineered Heart Tissues: Novel Tools for Modeling Human Cardiac Biology and Disease. Biomolecules 2021, 11, 1277. https://doi.org/10.3390/biom11091277
Lewis-Israeli YR, Wasserman AH, Aguirre A. Heart Organoids and Engineered Heart Tissues: Novel Tools for Modeling Human Cardiac Biology and Disease. Biomolecules. 2021; 11(9):1277. https://doi.org/10.3390/biom11091277
Chicago/Turabian StyleLewis-Israeli, Yonatan R., Aaron H. Wasserman, and Aitor Aguirre. 2021. "Heart Organoids and Engineered Heart Tissues: Novel Tools for Modeling Human Cardiac Biology and Disease" Biomolecules 11, no. 9: 1277. https://doi.org/10.3390/biom11091277
APA StyleLewis-Israeli, Y. R., Wasserman, A. H., & Aguirre, A. (2021). Heart Organoids and Engineered Heart Tissues: Novel Tools for Modeling Human Cardiac Biology and Disease. Biomolecules, 11(9), 1277. https://doi.org/10.3390/biom11091277






