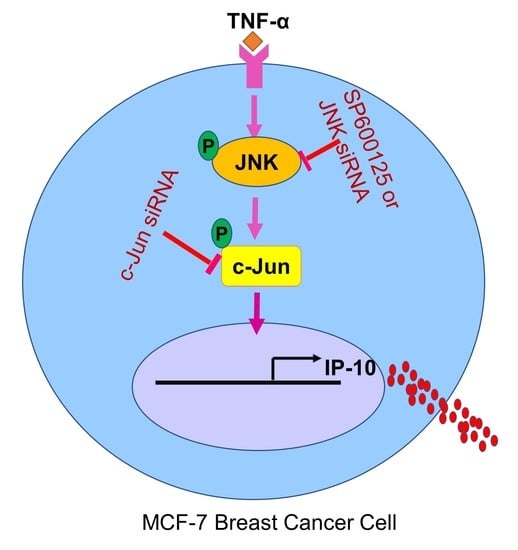
TNF-α Increases IP-10 Expression in MCF-7 Breast Cancer Cells via Activation of the JNK/c-Jun Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Stimulation
2.3. Real-Time Quantitative PCR
2.4. Small Interfering RNA (siRNA) Transfections
2.5. IP-10 Protein Determination in Cell Supernatants
2.6. Western Blotting
2.7. Immunocytofluorescence
2.8. SAPK/JNK Kinase Assay
2.9. Statistical Analysis
3. Results
3.1. TNF-α Treatment Upregulates IP-10 Gene and Protein Expression in MCF-7 Cells
3.2. JNK Inhibition Attenuates the TNFα-Induced IP-10 Production
3.3. JNK Deficiency Suppresses the TNF-α-Induced IP-10 Expression
3.4. c-Jun Deficiency Suppresses the TNF-α-Induced IP-10 Expression
3.5. Effect of the TNF-α Stimulation on the Kinase Activity of JNK in MCF-7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magesh, P.; Thankachan, S.; Venkatesh, T.; Suresh, P.S. Breast cancer fibroblasts and cross-talk. Clin. Chim. Acta Int. J. Clin. Chem. 2021, 521, 158–169. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luster, A.D.; Jhanwar, S.C.; Chaganti, R.S.; Kersey, J.H.; Ravetch, J.V. Interferon-inducible gene maps to a chromosomal band associated with a (4;11) translocation in acute leukemia cells. Proc. Natl. Acad. Sci. USA 1987, 84, 2868–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-C.; Wu, C.-L.; Su, W.-W.; Shih, K.-L.; Tarng, D.-C.; Chou, C.-T.; Chen, T.-Y.; Kor, C.-T.; Wu, H.-M. Interferon gamma-induced protein 10 is associated with insulin resistance and incident diabetes in patients with nonalcoholic fatty liver disease. Sci. Rep. 2015, 5, 10096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Rottman, J.B.; Myers, P.; Kassam, N.; Weinblatt, M.; Loetscher, M.; Koch, A.E.; Moser, B.; Mackay, C.R. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Investig. 1998, 101, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, I.; Itoh, M.; Yamada, H.; Yamamoto, K.; Tomatsu, E.; Makino, M.; Hashimoto, S.; Suzuki, A. Simultaneous measurement of serum chemokines in autoimmune thyroid diseases: Possible role of IP-10 in the inflammatory response. Endocr. J. 2015, 62, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Chalin, A.; Lefevre, B.; Devisme, C.; Pronier, C.; Carrière, V.; Thibault, V.; Amiot, L.; Samson, M. Serum CXCL10, CXCL11, CXCL12, and CXCL14 chemokine patterns in patients with acute liver injury. Cytokine 2018, 111, 500–504. [Google Scholar] [CrossRef]
- Chen, L.J.; Lv, J.; Wen, X.Y.; Niu, J.Q. CXC chemokine IP-10: A key actor in liver disease? Hepatol. Int. 2013, 7, 798–804. [Google Scholar] [CrossRef]
- Sørensen, T.L.; Trebst, C.; Kivisäkk, P.; Klaege, K.L.; Majmudar, A.; Ravid, R.; Lassmann, H.; Olsen, D.B.; Strieter, R.M.; Ransohoff, R.M.; et al. Multiple sclerosis: A study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J. Neuroimmunol. 2002, 127, 59–68. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Khoramdelazad, H.; Hassanshahi, G.; Rafatpanah, H.; Hosseini, J.; Mahmoodi, M.; Arababadi, M.K.; Derakhshan, R.; Hasheminasabzavareh, R.; Hosseini-Zijoud, S.M.; et al. Plasma levels of CXCL1 (GRO-alpha) and CXCL10 (IP-10) are elevated in type 2 diabetic patients: Evidence for the involvement of inflammation and angiogenesis/angiostasis in this disease state. Clin. Lab. 2013, 59, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Polverini, P.J. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem. Biophys. Res. Commun. 1995, 210, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Lasagni, L.; Annunziato, F.; Serio, M.; Romagnani, S. CXC chemokines: The regulatory link between inflammation and angiogenesis. Trends Immunol. 2004, 25, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Espinoza, J.A.; Torland, L.A.; Zucknick, M.; Kumar, S.; Haakensen, V.D.; Lüders, T.; Engebraaten, O.; Børresen-Dale, A.-L.; Kyte, J.A.; et al. Noninvasive profiling of serum cytokines in breast cancer patients and clinicopathological characteristics. Oncoimmunology 2018, 8, e1537691. [Google Scholar] [CrossRef] [Green Version]
- Yoshimatsu, G.; Kunnathodi, F.; Saravanan, P.B.; Shahbazov, R.; Chang, C.; Darden, C.M.; Zurawski, S.; Boyuk, G.; Kanak, M.A.; Levy, M.F.; et al. Pancreatic β-Cell-Derived IP-10/CXCL10 Isletokine Mediates Early Loss of Graft Function in Islet Cell Transplantation. Diabetes 2017, 66, 2857–2867. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, L.J.; Eriksson, E.; Hansen, D.S. CD14(+) monocytes are the main leucocytic sources of CXCL10 in response to Plasmodium falciparum. Parasitology 2020, 147, 465–470. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Jin, W.J.; Kim, H.H.; Ha, H.; Lee, Z.H. Pathogenic roles of CXCL10 signaling through CXCR3 and TLR4 in macrophages and T cells: Relevance for arthritis. Arthritis Res. Ther. 2017, 19, 163. [Google Scholar] [CrossRef]
- Ye, J.; Ma, C.; Wang, F.; Hsueh, E.C.; Toth, K.; Huang, Y.; Mo, W.; Liu, S.; Han, B.; Varvares, M.A.; et al. Specific recruitment of γδ regulatory T cells in human breast cancer. Cancer Res. 2013, 73, 6137–6148. [Google Scholar] [CrossRef] [Green Version]
- Alomar, S.Y.; Gentili, A.; Zaibi, M.S.; Kępczyńska, M.A.; Trayhurn, P. IL-1β (interleukin-1β) stimulates the production and release of multiple cytokines and chemokines by human preadipocytes. Arch. Physiol. Biochem. 2016, 122, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Narumi, S.; Yoneyama, H.; Inadera, H.; Nishioji, K.; Itoh, Y.; Okanoue, T.; Matsushima, K. TNF-alpha is a potent inducer for IFN-inducible protein-10 in hepatocytes and unaffected by GM-CSF in vivo, in contrast to IL-1beta and IFN-gamma. Cytokine 2000, 12, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, S.; Jamieson, N.B.; Lim, S.Y.; Griffiths, K.L.; Carvalho-Gaspar, M.; Al-Assar, O.; Yameen, S.; Carter, R.C.; McKay, C.J.; Spoletini, G.; et al. IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget 2014, 5, 11064–11080. [Google Scholar] [CrossRef] [Green Version]
- Al-Rashed, F.; Ahmad, Z.; Snider, A.J.; Thomas, R.; Kochumon, S.; Melhem, M.; Sindhu, S.; Obeid, L.M.; Al-Mulla, F.; Hannun, Y.A.; et al. Ceramide kinase regulates TNF-alpha-induced immune responses in human monocytic cells. Sci. Rep. 2021, 11, 8259. [Google Scholar] [CrossRef]
- Al-Roub, A.; Akhter, N.; Al-Sayyar, A.; Wilson, A.; Thomas, R.; Kochumon, S.; Al-Rashed, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. Short Chain Fatty Acid Acetate Increases TNFalpha-Induced MCP-1 Production in Monocytic Cells via ACSL1/MAPK/NF-kappaB Axis. Int. J. Mol. Sci. 2021, 22, 7683. [Google Scholar] [CrossRef]
- Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Devarajan, S.; Tuomilehto, J.; Ahmad, R. Adipose tissue expression of CCL19 chemokine is positively associated with insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, e3087. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Kochumon, S.; Shenouda, S.; Wilson, A.; Al-Mulla, F.; Ahmad, R. The Cooperative Induction of CCL4 in Human Monocytic Cells by TNF-alpha and Palmitate Requires MyD88 and Involves MAPK/NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 4658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rashed, F.; Ahmad, Z.; Thomas, R.; Melhem, M.; Snider, A.J.; Obeid, L.M.; Al-Mulla, F.; Hannun, Y.A.; Ahmad, R. Neutral sphingomyelinase 2 regulates inflammatory responses in monocytes/macrophages induced by TNF-α. Sci. Rep. 2020, 10, 16802. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Sindhu, S.; Arefanian, H.; Al Madhoun, A.; Kochumon, S.; Thomas, R.; Al-Kandari, S.; Alghaith, A.; Jacob, T.; Al-Mulla, F.; et al. Repetitive Intermittent Hyperglycemia Drives the M1 Polarization and Inflammatory Responses in THP-1 Macrophages Through the Mechanism Involving the TLR4-IRF5 Pathway. Cells 2020, 9, 1892. [Google Scholar] [CrossRef]
- Kochumon, S.; Al Madhoun, A.; Al-Rashed, F.; Thomas, R.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci. Rep. 2020, 10, 16364. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Arefanian, H.; Azim, R.; Shenouda, S.; Jacob, T.; Abu Khalaf, N.; Al-Rashed, F.; Hasan, A.; Sindhu, S.; Al-Mulla, F.; et al. Stearic Acid and TNF-alpha Co-Operatively Potentiate MIP-1alpha Production in Monocytic Cells via MyD88 Independent TLR4/TBK/IRF3 Signaling Pathway. Biomedicines 2020, 8, 403. [Google Scholar] [CrossRef]
- Kochumon, S.; Wilson, A.; Chandy, B.; Shenouda, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. Palmitate Activates CCL4 Expression in Human Monocytic Cells via TLR4/MyD88 Dependent Activation of NF-κB/MAPK/ PI3K Signaling Systems. Cell. Physiol. Biochem. 2018, 46, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Akhter, N.; Wilson, A.; Thomas, R.; Arefanian, H.; Al Madhoun, A.; Al-Mulla, F.; Ahmad, R. MIP-1alpha Expression Induced by Co-Stimulation of Human Monocytic Cells with Palmitate and TNF-alpha Involves the TLR4-IRF3 Pathway and Is Amplified by Oxidative Stress. Cells 2020, 9, 1799. [Google Scholar] [CrossRef]
- Thomas, R.; Al-Rashed, F.; Akhter, N.; Al-Mulla, F.; Ahmad, R. ACSL1 Regulates TNFalpha-Induced GM-CSF Production by Breast Cancer MDA-MB-231 Cells. Biomolecules 2019, 9, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, R.; Al-Roub, A.; Kochumon, S.; Akther, N.; Thomas, R.; Kumari, M.; Koshy, M.S.; Tiss, A.; Hannun, Y.A.; Tuomilehto, J.; et al. The Synergy between Palmitate and TNF-alpha for CCL2 Production Is Dependent on the TRIF/IRF3 Pathway: Implications for Metabolic Inflammation. J. Immunol. 2018, 200, 3599–3611. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashed, F.; Thomas, R.; Al-Roub, A.; Al-Mulla, F.; Ahmad, R. LPS Induces GM-CSF Production by Breast Cancer MDA-MB-231 Cells via Long-Chain Acyl-CoA Synthetase 1. Molecules 2020, 25, 4709. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Harris, D.P.; Bandyopadhyay, S.; Maxwell, T.J.; Willard, B.; DiCorleto, P.E. Tumor necrosis factor (TNF)-alpha induction of CXCL10 in endothelial cells requires protein arginine methyltransferase 5 (PRMT5)-mediated nuclear factor (NF)-kappaB p65 methylation. J. Biol. Chem. 2014, 289, 15328–15339. [Google Scholar] [CrossRef] [Green Version]
- Chin, B.Y.; Choi, M.E.; Burdick, M.D.; Strieter, R.M.; Risby, T.H.; Choi, A.M. Induction of apoptosis by particulate matter: Role of TNF-alpha and MAPK. Am. J. Physiol. 1998, 275, L942–L949. [Google Scholar] [CrossRef]
- Zhao, X.W.; Zhou, J.P.; Bi, Y.L.; Wang, J.Y.; Yu, R.; Deng, C.; Wang, W.K.; Li, X.Z.; Huang, R.; Zhang, J.; et al. The role of MAPK signaling pathway in formation of EMT in oral squamous carcinoma cells induced by TNF-α. Mol. Biol. Rep. 2019, 46, 3149–3156. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Fan, W.; Yang, G.; Yu, M.X. The clinical significance of PR, ER, NF- kappa B, and TNF- alpha in breast cancer. Dis. Markers 2014, 2014, 494581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Guo, S.; Stiles, J.K. The emerging role of CXCL10 in cancer (Review). Oncol. Lett. 2011, 2, 583–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.M.; Heusey, H.L.; Griffith, L.G.; Lauffenburger, D.A.; Wells, A. IP-10 (CXCL10) Can Trigger Emergence of Dormant Breast Cancer Cells in a Metastatic Liver Microenvironment. Front. Oncol. 2021, 11, 1838. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Choi, J.; Ahn, S.S.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Inhibitory Effect of Alisma canaliculatum Ethanolic Extract on NF-κB-Dependent CXCR3 and CXCL10 Expression in TNFα-Exposed MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulligan, A.M.; Raitman, I.; Feeley, L.; Pinnaduwage, D.; Nguyen, L.T.; O’Malley, F.P.; Ohashi, P.S.; Andrulis, I.L. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Pellikainen, J.M.; Ropponen, K.M.; Kataja, V.V.; Kellokoski, J.K.; Eskelinen, M.J.; Kosma, V.-M. Expression of Matrix Metalloproteinase (MMP)-2 and MMP-9 in Breast Cancer with a Special Reference to Activator Protein-2, HER2, and Prognosis. Clin. Cancer Res. 2004, 10, 7621–7628. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Miller, B.S.; Rosenzweig, S.A.; Bhat, N.R. Activation of C-jun N-terminal kinase/stress-activated protein kinase in primary glial cultures. J. Neurosci. Res. 1996, 46, 114–121. [Google Scholar] [CrossRef]
- Pereira, A.M.; Tudor, C.; Kanger, J.S.; Subramaniam, V.; Martin-Blanco, E. Integrin-dependent activation of the JNK signaling pathway by mechanical stress. PLoS ONE 2011, 6, e26182. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, C.; Shamoon, B.; Shyamala, V.; Williams, L.T. Tumor necrosis factor alpha-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 1997, 16, 1080–1092. [Google Scholar] [CrossRef] [Green Version]
- Wicovsky, A.; Müller, N.; Daryab, N.; Marienfeld, R.; Kneitz, C.; Kavuri, S.; Leverkus, M.; Baumann, B.; Wajant, H. Sustained JNK Activation in Response to Tumor Necrosis Factor Is Mediated by Caspases in a Cell Type-specific Manner. J. Biol. Chem. 2007, 282, 2174–2183. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Zhang, Y.-P.; Ying, W.; Yao, X.-X. Interleukin-1β upregulates matrix metalloproteinase-13 gene expression via c-Jun N-terminal kinase and p38 MAPK pathways in rat hepatic stellate cells. Mol. Med. Rep. 2013, 8, 1861–1865. [Google Scholar] [CrossRef]
- Johnson, G.L.; Nakamura, K. The c-jun kinase/stress-activated pathway: Regulation, function and role in human disease. Biochim. Biophys. Acta 2007, 1773, 1341–1348. [Google Scholar] [CrossRef] [Green Version]
- Hah, Y.-S.; Kang, H.-G.; Cho, H.-Y.; Shin, S.-H.; Kim, U.-K.; Park, B.-W.; Lee, S.-I.; Rho, G.-J.; Kim, J.-R.; Byun, J.-H. JNK signaling plays an important role in the effects of TNF-α and IL-1β on in vitro osteoblastic differentiation of cultured human periosteal-derived cells. Mol. Biol. Rep. 2013, 40, 4869–4881. [Google Scholar] [CrossRef]
- Schütze, S.; Wiegmann, K.; Machleidt, T.; Krönke, M. TNF-induced activation of NF-kappa B. Immunobiology 1995, 193, 193–203. [Google Scholar] [CrossRef]
- McDonald, P.P.; Bald, A.; Cassatella, M.A. Activation of the NF-κB Pathway by Inflammatory Stimuli in Human Neutrophils. Blood 1997, 89, 3421–3433. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shi, W.; Zhao, C.; Zhang, D.; Liang, P.; Wang, G.; Lu, L. Triptolide sensitizes human breast cancer cells to tumor necrosis factor-α-induced apoptosis by inhibiting activation of the nuclear factor-κB pathway. Mol. Med. Rep. 2016, 13, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, J.-J.; Kennedy, N.J.; Lamb, J.A.; Flavell, R.A.; Davis, R.J. c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol. Cell. Biol. 2003, 23, 2871–2882. [Google Scholar] [CrossRef] [Green Version]
- Whitmarsh, A.J.; Davis, R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef]
- García-Tuñón, I.; Ricote, M.; Ruiz, A.A.; Fraile, B.; Paniagua, R.; Royuela, M. Influence of IFN-gamma and its receptors in human breast cancer. BMC Cancer 2007, 7, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghoobi, H.; Azizi, H.; Oskooei, V.K.; Taheri, M.; Ghafouri-Fard, S. Assessment of expression of interferon γ (IFN-G) gene and its antisense (IFNG-AS1) in breast cancer. World J. Surg. Oncol. 2018, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Simons, D.L.; Lu, X.; Tu, T.Y.; Avalos, C.; Chang, A.Y.; Dirbas, F.M.; Yim, J.H.; Waisman, J.; Lee, P.P. Breast cancer induces systemic immune changes on cytokine signaling in peripheral blood monocytes and lymphocytes. EBioMedicine 2020, 52, 102631. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochumon, S.; Al-Sayyar, A.; Jacob, T.; Hasan, A.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. TNF-α Increases IP-10 Expression in MCF-7 Breast Cancer Cells via Activation of the JNK/c-Jun Pathways. Biomolecules 2021, 11, 1355. https://doi.org/10.3390/biom11091355
Kochumon S, Al-Sayyar A, Jacob T, Hasan A, Al-Mulla F, Sindhu S, Ahmad R. TNF-α Increases IP-10 Expression in MCF-7 Breast Cancer Cells via Activation of the JNK/c-Jun Pathways. Biomolecules. 2021; 11(9):1355. https://doi.org/10.3390/biom11091355
Chicago/Turabian StyleKochumon, Shihab, Amnah Al-Sayyar, Texy Jacob, Amal Hasan, Fahd Al-Mulla, Sardar Sindhu, and Rasheed Ahmad. 2021. "TNF-α Increases IP-10 Expression in MCF-7 Breast Cancer Cells via Activation of the JNK/c-Jun Pathways" Biomolecules 11, no. 9: 1355. https://doi.org/10.3390/biom11091355